Abstract
The molecular basis of programmed cell death (PCD) is unknown. An important clue is provided by the Bcl-2 protein, which can protect many cell types from PCD, although it is not known where or how it acts. Nuclear condensation, DNA fragmentation and a requirement for new RNA and protein synthesis are often considered hallmarks of PCD. We show here, however, that anucleate cytoplasts can undergo PCD and that Bcl-2 and extracellular survival signals can protect them, indicating that, in some cases at least, the nucleus is not required for PCD or for Bcl-2 or survival factor protection. We propose that PCD, like the cell cycle, is orchestrated by a cytoplasmic regulator that has multiple intracellular targets.
Full text
PDF




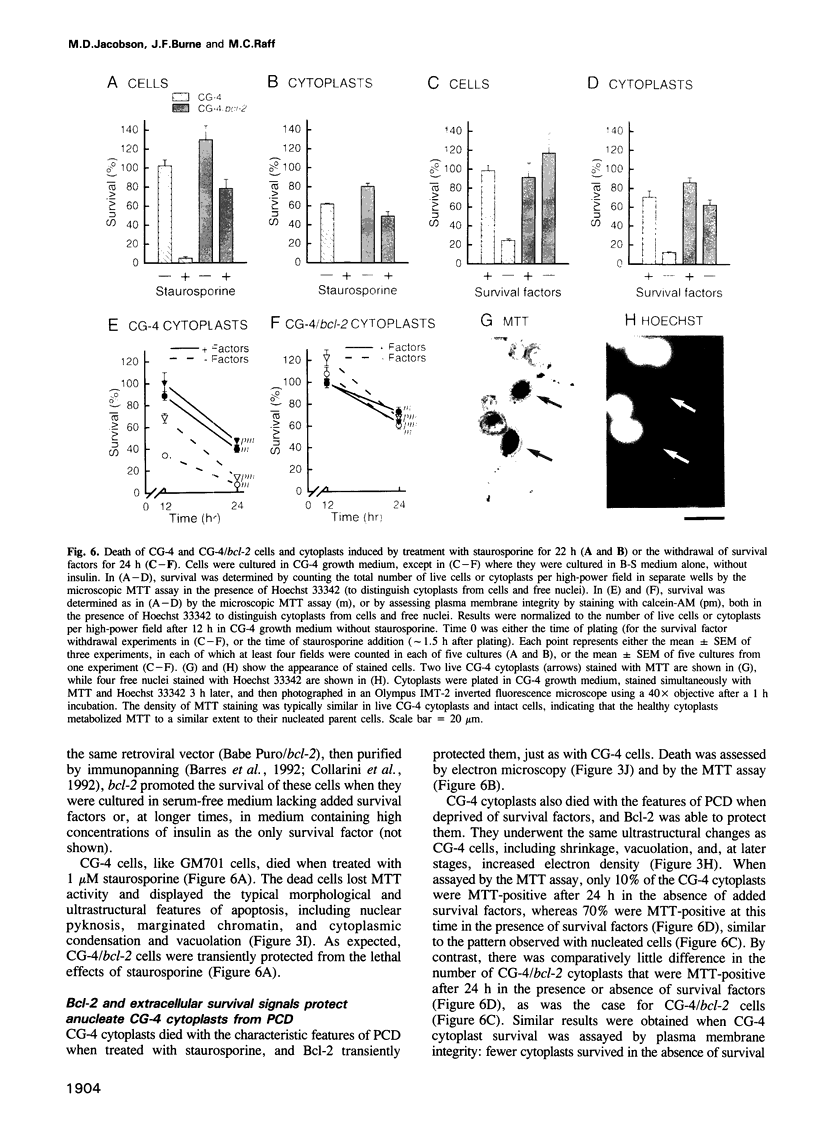
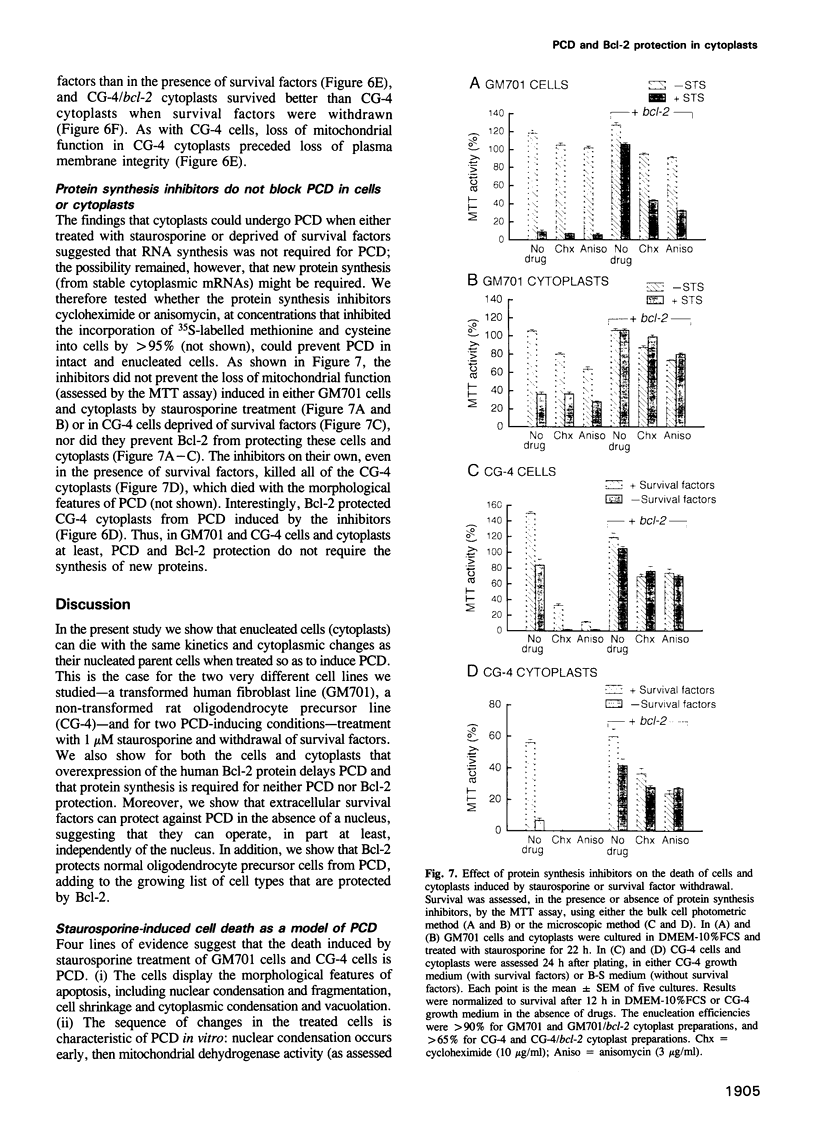





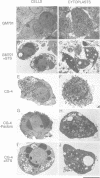
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp T. E., Wyatt S., Paterson H. F., Davies A. M. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993 Apr 23;73(2):295–307. doi: 10.1016/0092-8674(93)90230-n. [DOI] [PubMed] [Google Scholar]
- Alnemri E. S., Litwack G. Activation of internucleosomal DNA cleavage in human CEM lymphocytes by glucocorticoid and novobiocin. Evidence for a non-Ca2(+)-requiring mechanism(s). J Biol Chem. 1990 Oct 5;265(28):17323–17333. [PubMed] [Google Scholar]
- Alnemri E. S., Robertson N. M., Fernandes T. F., Croce C. M., Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Schmid R., Sendnter M., Raff M. C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993 May;118(1):283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Greene L. A. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991 Oct;115(2):461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina-Stein M., Tritton T. R. Aurintricarboxylic acid is a nonspecific enzyme inhibitor. Mol Pharmacol. 1976 Jan;12(1):191–193. [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briehl M. M., Miesfeld R. L. Isolation and characterization of transcripts induced by androgen withdrawal and apoptotic cell death in the rat ventral prostate. Mol Endocrinol. 1991 Oct;5(10):1381–1388. doi: 10.1210/mend-5-10-1381. [DOI] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collarini E. J., Kuhn R., Marshall C. J., Monuki E. S., Lemke G., Richardson W. D. Down-regulation of the POU transcription factor SCIP is an early event in oligodendrocyte differentiation in vitro. Development. 1992 Sep;116(1):193–200. doi: 10.1242/dev.116.1.193. [DOI] [PubMed] [Google Scholar]
- Colombel M., Olsson C. A., Ng P. Y., Buttyan R. Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res. 1992 Aug 15;52(16):4313–4319. [PubMed] [Google Scholar]
- Colotta F., Polentarutti N., Sironi M., Mantovani A. Expression and involvement of c-fos and c-jun protooncogenes in programmed cell death induced by growth factor deprivation in lymphoid cell lines. J Biol Chem. 1992 Sep 15;267(26):18278–18283. [PubMed] [Google Scholar]
- Cotter T. G., Glynn J. M., Echeverri F., Green D. R. The induction of apoptosis by chemotherapeutic agents occurs in all phases of the cell cycle. Anticancer Res. 1992 May-Jun;12(3):773–779. [PubMed] [Google Scholar]
- Crompton T. IL3-dependent cells die by apoptosis on removal of their growth factor. Growth Factors. 1991;4(2):109–116. doi: 10.3109/08977199109000262. [DOI] [PubMed] [Google Scholar]
- Deckwerth T. L., Johnson E. M., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993 Dec;123(5):1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Freeman R. S., Estus S., Horigome K., Johnson E. M., Jr Cell death genes in invertebrates and (maybe) vertebrates. Curr Opin Neurobiol. 1993 Feb;3(1):25–31. doi: 10.1016/0959-4388(93)90031-s. [DOI] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Garcia I., Martinou I., Tsujimoto Y., Martinou J. C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992 Oct 9;258(5080):302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- Gudas J. M., Karenlampi S. O., Hankinson O. Intracellular location of the Ah receptor. J Cell Physiol. 1986 Sep;128(3):441–448. doi: 10.1002/jcp.1041280313. [DOI] [PubMed] [Google Scholar]
- Harrigan M. T., Baughman G., Campbell N. F., Bourgeois S. Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol Cell Biol. 1989 Aug;9(8):3438–3446. doi: 10.1128/mcb.9.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Sulston J. E., Thomson J. N. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983 Jun 17;220(4603):1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- Henderson S., Huen D., Rowe M., Dawson C., Johnson G., Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner M. O., Horvitz H. R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994 Feb 25;76(4):665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y., Voyvodic J. T., Burne J. F., Raff M. C. Control of lens epithelial cell survival. J Cell Biol. 1993 May;121(4):899–908. doi: 10.1083/jcb.121.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. The cell cycle then and now. Trends Biochem Sci. 1992 Aug;17(8):281–285. doi: 10.1016/0968-0004(92)90435-c. [DOI] [PubMed] [Google Scholar]
- Kizaki H., Tadakuma T., Odaka C., Muramatsu J., Ishimura Y. Activation of a suicide process of thymocytes through DNA fragmentation by calcium ionophores and phorbol esters. J Immunol. 1989 Sep 15;143(6):1790–1794. [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988 Feb;122(2):552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Raff M. C. Analysis of the cell-cell interactions that control type-2 astrocyte development in vitro. Neuron. 1990 Apr;4(4):525–534. doi: 10.1016/0896-6273(90)90110-2. [DOI] [PubMed] [Google Scholar]
- Lin E. Y., Orlofsky A., Berger M. S., Prystowsky M. B. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1993 Aug 15;151(4):1979–1988. [PubMed] [Google Scholar]
- Lockshin R. A. Programmed cell death. Activation of lysis by a mechanism involving the synthesis of protein. J Insect Physiol. 1969 Sep;15(9):1505–1516. doi: 10.1016/0022-1910(69)90172-3. [DOI] [PubMed] [Google Scholar]
- Louis J. C., Magal E., Muir D., Manthorpe M., Varon S. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J Neurosci Res. 1992 Jan;31(1):193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- Louis J. C., Magal E., Takayama S., Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993 Jan 29;259(5095):689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J. Apoptosis: suicide, execution or murder? Trends Cell Biol. 1993 May;3(5):141–144. doi: 10.1016/0962-8924(93)90128-n. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Mesner P. W., Winters T. R., Green S. H. Nerve growth factor withdrawal-induced cell death in neuronal PC12 cells resembles that in sympathetic neurons. J Cell Biol. 1992 Dec;119(6):1669–1680. doi: 10.1083/jcb.119.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Monaghan P., Robertson D., Amos T. A., Dyer M. J., Mason D. Y., Greaves M. F. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992 Dec;40(12):1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Neilan J. G., Lu Z., Afonso C. L., Kutish G. F., Sussman M. D., Rock D. L. An African swine fever virus gene with similarity to the proto-oncogene bcl-2 and the Epstein-Barr virus gene BHRF1. J Virol. 1993 Jul;67(7):4391–4394. doi: 10.1128/jvi.67.7.4391-4394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A., González A., Gambón F., Díaz-Espada F., López-Rivas A. Apoptosis in human thymocytes after treatment with glucocorticoids. Clin Exp Immunol. 1992 May;88(2):341–344. doi: 10.1111/j.1365-2249.1992.tb03084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Ojcius D. M., Zychlinsky A., Zheng L. M., Young J. D. Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes. Exp Cell Res. 1991 Nov;197(1):43–49. doi: 10.1016/0014-4827(91)90477-c. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Tytell M., Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990 Mar;138(1):104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Owens G. P., Hahn W. E., Cohen J. J. Identification of mRNAs associated with programmed cell death in immature thymocytes. Mol Cell Biol. 1991 Aug;11(8):4177–4188. doi: 10.1128/mcb.11.8.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Cuddy M., Slabiak T., Croce C. M., Nowell P. C. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature. 1988 Nov 17;336(6196):259–261. doi: 10.1038/336259a0. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Philpott K. L., Brooks S. F. Apoptosis: the cell cycle and cell death. Curr Biol. 1993 Jun 1;3(6):391–394. doi: 10.1016/0960-9822(93)90211-6. [DOI] [PubMed] [Google Scholar]
- Ryan J. J., Danish R., Gottlieb C. A., Clarke M. F. Cell cycle analysis of p53-induced cell death in murine erythroleukemia cells. Mol Cell Biol. 1993 Jan;13(1):711–719. doi: 10.1128/mcb.13.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., Kosz L., Kay B. K. Gene activation is required for developmentally programmed cell death. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6594–6598. doi: 10.1073/pnas.87.17.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. A., Davies A. M. Inhibition of protein synthesis prevents cell death in sensory and parasympathetic neurons deprived of neurotrophic factor in vitro. J Neurobiol. 1990 Jun;21(4):630–638. doi: 10.1002/neu.480210410. [DOI] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. W., Bishop C. J., Simmons R. J. Biophysical and morphological correlates of kinetic change and death in a starved human melanoma cell line. J Cell Sci. 1981 Jun;49:119–137. doi: 10.1242/jcs.49.1.119. [DOI] [PubMed] [Google Scholar]
- Shi L., Nishioka W. K., Th'ng J., Bradbury E. M., Litchfield D. W., Greenberg A. H. Premature p34cdc2 activation required for apoptosis. Science. 1994 Feb 25;263(5150):1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Szalay M. G., Paskar L., Sahai B. M., Boyer M., Singh B., Green D. R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990 May 1;144(9):3326–3333. [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Skalka M., Matyásová J., Cejková M. Dna in chromatin of irradiated lymphoid tissues degrades in vivo into regular fragments. FEBS Lett. 1976 Dec 31;72(2):271–274. doi: 10.1016/0014-5793(76)80984-2. [DOI] [PubMed] [Google Scholar]
- Stanisic T., Sadlowski R., Lee C., Grayhack J. T. Partial inhibition of castration induced ventral prostate regression with actinomycin D and cycloheximide. Invest Urol. 1978 Jul;16(1):19–22. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev Biol. 1966 Feb;13(1):77–94. doi: 10.1016/0012-1606(66)90050-9. [DOI] [PubMed] [Google Scholar]
- Tomei L. D., Shapiro J. P., Cope F. O. Apoptosis in C3H/10T1/2 mouse embryonic cells: evidence for internucleosomal DNA modification in the absence of double-strand cleavage. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):853–857. doi: 10.1073/pnas.90.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ikegaki N., Croce C. M. Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2(1):3–7. [PubMed] [Google Scholar]
- Ucker D. S., Ashwell J. D., Nickas G. Activation-driven T cell death. I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989 Dec 1;143(11):3461–3469. [PubMed] [Google Scholar]
- Ucker D. S. Death by suicide: one way to go in mammalian cellular development? New Biol. 1991 Feb;3(2):103–109. [PubMed] [Google Scholar]
- Ucker D. S., Obermiller P. S., Eckhart W., Apgar J. R., Berger N. A., Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992 Jul;12(7):3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky S. R. The genetic program of cell death. Hypothesis and some applications: transformation, carcinogenesis, ageing. J Theor Biol. 1982 Aug 21;97(4):591–602. doi: 10.1016/0022-5193(82)90360-5. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Weissman I. L., Kim S. K. Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science. 1992 Dec 18;258(5090):1955–1957. doi: 10.1126/science.1470921. [DOI] [PubMed] [Google Scholar]
- Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993 Oct 22;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Vukmanović S., Zamoyska R. Anti-CD3-induced cell death in T cell hybridomas: mitochondrial failure and DNA fragmentation are distinct events. Eur J Immunol. 1991 Feb;21(2):419–424. doi: 10.1002/eji.1830210225. [DOI] [PubMed] [Google Scholar]
- Waring P., Egan M., Braithwaite A., Mullbacher A., Sjaarda A. Apoptosis induced in macrophages and T blasts by the mycotoxin sporidesmin and protection by Zn2+ salts. Int J Immunopharmacol. 1990;12(4):445–457. doi: 10.1016/0192-0561(90)90028-l. [DOI] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]
- Williams J. R., Little J. B., Shipley W. U. Association of mammalian cell death with a specific endonucleolytic degradation of DNA. Nature. 1974 Dec 20;252(5485):754–755. doi: 10.1038/252754a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Yuan J., Horvitz H. R. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992 Oct;116(2):309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]