Abstract
The central regulator of adipogenesis, PPARγ, is a nuclear receptor that is linked to obesity and metabolic diseases. Here we report that MKRN1 is an E3 ligase of PPARγ that induces its ubiquitination, followed by proteasome-dependent degradation. Furthermore, we identified two lysine sites at 184 and 185 that appear to be targeted for ubiquitination by MKRN1. Stable overexpression of MKRN1 reduced PPARγ protein levels and suppressed adipocyte differentiation in 3T3-L1 and C3H10T1/2 cells. In contrast, MKRN1 depletion stimulated adipocyte differentiation in these cells. Finally, MKRN1 knockout MEFs showed an increased capacity for adipocyte differentiation compared with wild-type MEFs, with a concomitant increase of PPARγ and adipogenic markers. Together, these data indicate that MKRN1 is an elusive PPARγ E3 ligase that targets PPARγ for proteasomal degradation by ubiquitin-dependent pathways, and further depict MKRN1 as a novel target for diseases involving PPARγ.
Keywords: PPARγ, MKRN1, ubiquitination, adipocyte differentiation
Excessive body fat in obese individuals is considered a major cause of insulin resistance, cardiovascular diseases, and diabetes.1 In obesity, adipocytes alter the status of energy homeostasis by the generation of adipokines, demonstrating that adipocytes have active roles in energy homeostasis and body composition, in addition to storage of triglycerides.2 Adipokines secreted by obese adipocytes such as resistin and TNF-α impair insulin actions in peripheral tissues, whereas adiponectin from lean adipose tissue improves insulin sensitivity in liver and muscle.2, 3 Therefore, a better understanding of adipocyte biology and mechanisms that direct adipocyte differentiation is being rapidly sought.4
Adipocyte differentiation from pre-adipocytes is controlled by a number of transcriptional cascades, including peroxisome-proliferator-activated receptorγ (PPARγ), the CCAAT/enhancer-binding protein (C/EBP) family, and adipocyte determination and differentiation-dependent factor.5 Loss- and gain-of-function studies have demonstrated that PPARγ is a necessary and sufficient transcriptional regulator to convert adipocytes from their precursors.6, 7 Activation of PPARγ by its ligands induces PPARγ binding to its responsive elements in the promoter, and increases the expressions of lipid synthesis- and differentiation-related genes, including CD36, lipoprotein lipase (LPL), fatty acid-binding protein (aP2), and C/EBPα.8 Upstream transcriptional events that induce the expression of PPARγ are C/EBPβ, C/EBPδ, Krox-20, KLF5, EBF, ZNF453, TCF7L2, and ADD1/SREBP1c transcription factors.4, 5, 9 In contrast, inhibitory transcriptional regulators such as KLF2, GATA2/3, IRF, and β-catenin/TCF have been shown to suppress the expression of PPARγ and adipocyte differentiation.5, 9, 10, 11, 12, 13
PPARγ protein is also post-translationally regulated by several modifications.14 Phosphorylation on serine 122 in the AF1 region of PPARγ is modified by ERK1/2 and P38/JNK kinases activated from growth factors, cytokines, and stress signals, and leads to inhibition of PPARγ activity and adipocyte differentiation.14, 15 Sumoylation on lysine residue 395 targets PPARγ to NCoR corepressor and prevents repression of inflammatory genes.16 A short-lived PPARγ protein has been shown to be polyubiquitinated and degraded in a proteasome-dependent manner.17, 18 Recent RNAi-based screening has shown that ubiquitin ligase Siah2 probably regulates PPARγ coregulator (NCoR, RXRα), thereby inhibiting PPAR activity in mature 3T3-L1 adipocytes.19 Another report also showed that ubiquitin ligase NEDD4-1 ubiquitinates PPARγ protein.20 These data indicate that various post-translational modifications are integral players responsible for the physiological effects of PPARγ. Therefore, the identification and elucidation of the mechanisms of post-translational modification will help us to understand the physiological functions of PPARγ, adipocyte biology, and further applications to therapeutic interventions.
Makorin Ring Finger Protein 1 (MKRN1) is known to act as an E3 ubiquitin ligase, targeting various substrates, such as hTERT, p53, and FADD.21, 22, 23, 24, 25 It may also function as a regulator of nuclear receptors, including androgen and retinoic acid receptors, independent of E3 ligase activities.26 In this study, we report that MKRN1 may mediate PPARγ2 ubiquitination and proteasome-dependent degradation. The physiological function of MKRN1 in negative regulation of PPARγ2 protein was further demonstrated using MKRN1 knockout MEFs, 3T3-L1, and C3H10T1/2 cell lines, indicating that MKRN1 is a potential new therapeutic target in PPARγ-related diseases.
Results
PPARγ2 protein levels are regulated by MKRN1
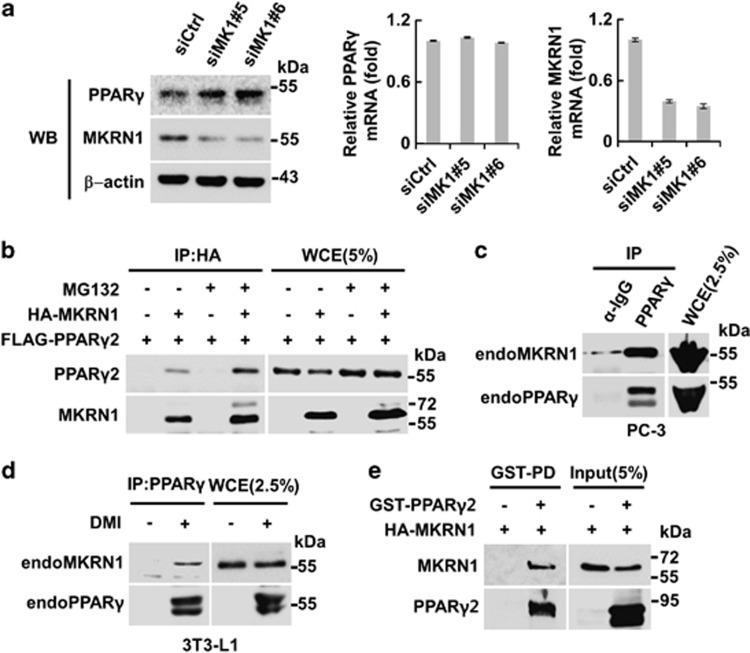
In previous studies, MKRN1 was shown to repress the transcriptional activities of nuclear receptors such as androgen and retinoic acid receptors.26 This finding prompted us to investigate whether MKRN1 also has regulatory effects on the transcriptional activities of PPARγ, a nuclear receptor responsible for adipocyte differentiation.27 The effects of MKRN1 on PPARγ2 were examined by co-transfecting H1299 human lung cancer cells with plasmids expressing PPARγ2 and PPRE-luciferase reporter in the presence or absence of troglitazone, an agonist of PPARγ2. The expression of PPARγ2 stimulated reporter gene expression, and this induction was further increased in the presence of troglitazone. Interestingly, MKRN1 co-expression with PPARγ2 was able to suppress the transcriptional activities to basal levels, implicating a negative regulatory role of MKRN1 on PPARγ2 activities (Supplementary Figure 1a). To analyze the effects of MKRN1 more critically, we used MKRN1 depleted in H1299 followed by luciferase reporter assays. Two independent MKRN1 siRNA #5 and #6 (siMK1#5 and #6, respectively) suppressed levels of endogenous MKRN1 up to 90% in H1299 (Supplementary Figure 1b). The data indicated that, under depletion of MKRN1, PPARγ2 activities with or without troglitazone were doubled compared with those of the control siRNA (Supplementary Figure 1b). To analyze the effects of MKRN1 on PPARγ in a more physiological context, we employed a PC-3 human prostate cancer cell line, which endogenously expresses PPARγ.28 On ablation of MKRN1, we observed increased levels of PPARγ protein levels, with no significant changes in mRNA levels (Figure 1a). Overall, these observations indicate that MKRN1 may negatively regulate PPARγ in post-translation.
Figure 1.
MKRN1 directly interacts with PPARγ. (a) Effects of MKRN1 depletion on PPARγ protein and mRNA levels. PC-3 cells were transfected with the indicated siRNAs for 48 h and analyzed by western blotting or by qRT-PCR. (b) Interaction between exogenous MKRN1 and PPARγ2. HEK293T cells were transfected with the plasmids expressing 3 × FLAG-PPARγ2 (FLAG-PPARγ2) and HA-MKRN1 plasmids with or without 10 μM MG132 for 6 h. The transfected cells were then harvested and WCE were immunoprecipitated using α-HA antibodies, followed by western blotting using α-FLAG and α-HA antibodies. Cell lysates (5% input) were used for western blotting. (c and d) Interactions between endogenous (endo) MKRN1 and PPARγ. PC-3 cell lysates were immunoprecipitated using α-PPARγ or α-IgG antibodies followed by western blotting using α-PPARγ and α-MKRN1 antibodies. Cell lysates (2.5% input) were used for western blotting (c). WCE of 3T3-L1 undifferentiated or differentiated into adipocytes using DMI (dexamethasone+3-isobutyl-1-methylxanthine+insulin) were immunoprecipitated using α-PPARγ antibodies and detected as above. Cell lysates (2.5% input) were used for western blotting (d). (e) Direct interaction between PPARγ2 and MKRN1 in vitro. In vitro translated MRKN1 was incubated with or without recombinant protein GST-PPARγ2, followed by GST pulldown using glutathione–Sepharose. The proteins were detected using α-HA and α-PPARγ antibodies. Input (5%) were used for western blotting
MKRN1 functions as an E3 ligase of PPARγ
The ability of MKRN1 to regulate PPARγ activities through post-translational regulation led us to examine the interactions between MKRN1 and PPARγ2. Two proteins that are exogenously expressed in 293T cells interact with each other (Figure 1b, Supplementary Figure 2a). Interactions between endogenous PPARγ and MKRN1 were further observed using extracts of PC-3 or 3T3-L1 cells differentiated into adipocytes by DMI (a cocktail of dexamethasone, 3-isobutyl-1-methylxanthine, and insulin) treatment (Figures 1c and d). Finally, employing GST-pulldown assays using recombinant GST- PPARγ2 and MKRN1 translated in vitro, we were able to show that the two proteins interact directly (Figure 1e). Domain analyses using a truncated mutant of MKRN1 showed that the C-terminal region of MKRN1 was responsible for its interaction with PPARγ2. On the other hand, N-terminal region of PPARγ2 was required for its interaction with MKRN1 (Supplementary Figures 2b and c).
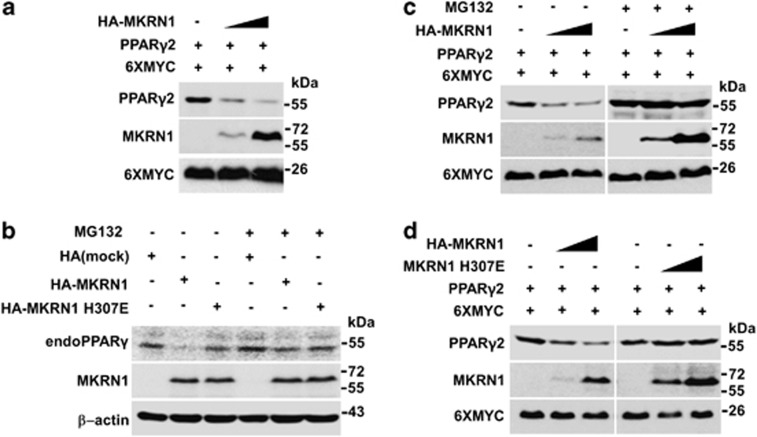
As MKRN1 is known as an E3 ligase, its ability to induce degradation of PPARγ2 was tested.21, 22, 23, 24, 25 With an increase of MKRN1 expressed in H1299 cells, the protein levels of PPARγ2 decreased (Figure 2a). Transient overexpression of MRKN1 in differentiated 3T3-L1 adipocytes suppressed the accumulation of endogenous PPARγ, suggesting that MKRN1 induces PPARγ destabilization. However, the addition of proteasome inhibitor reverted the destabilization of PPARγ. As expected, MKRN1 E3 ligase-defective mutant H307E did not have significant effects on PPARγ stability (Figure 2b). Treatment with MG132, a proteasome inhibitor, blocked MKRN1-mediated degradation of PPARγ2, indicating that proteasome-dependent degradation is required in these processes (Figure 2c). When H307E, a MKRN1 E3 ligase-defective point mutant, was employed, it was able to bind to, but incapable of inducing degradation of, PPARγ2, indicating that E3 ligase activities of MKRN1 are involved in the degradation mechanism (Figure 2d, Supplementary Figure 3a). Using cycloheximide (CHX), an inhibitor of protein translation, MKRN1 was shown to reduce the half-life of PPARγ2 significantly, whereas H307E was not (Supplementary Figure 3b). These observations suggest that the ablation of MKRN1 increased the stability and thus the half-life of endogenous PPARγ protein in PC-3 cells (Supplementary Figure 3c).
Figure 2.
MKRN1 decreases PPARγ2 protein stability through its E3 ligase function. (a) PPARγ2 degradation by MKRN1. H1299 cells were transfected with the plasmids expressing PPARγ2 (0.4 μg), 6XMYC (0.3 μg) and increasing concentrations of HA-MKRN1 (0.2 and 0.4 μg), followed by western blotting. 6XMYC was used as a transfection control. (b) Degradation of endogenous PPARγ by MKRN1. 3T3-L1 cells, differentiated with DMI for 2 days, were transfected with the plasmids expressing HA-MKRN1 and HA-MKRN1 H307E using a microporator in the presence or absence MG132. Cells were harvested 24 h after transfection and lysed, detected by western blotting using the indicated antibodies. (c) Effects of MG132 on MKRN1-mediated PPARγ2 degradation. H1299 cells, transfected with the plasmids as indicated above, were treated with 10 μM MG132 for 6 h. The proteins were detected by western blotting. (d) Effects of MKRN1 H307E mutant on PPARγ2 degradation. H1299 cells were transfected with the plasmids expressing PPARγ2 (0.4 μg), increasing amount of HA-MKRN1 or HA-MKRN1 H307E (0.2 and 0.4 μg). WCE were detected by western blotting, as described above
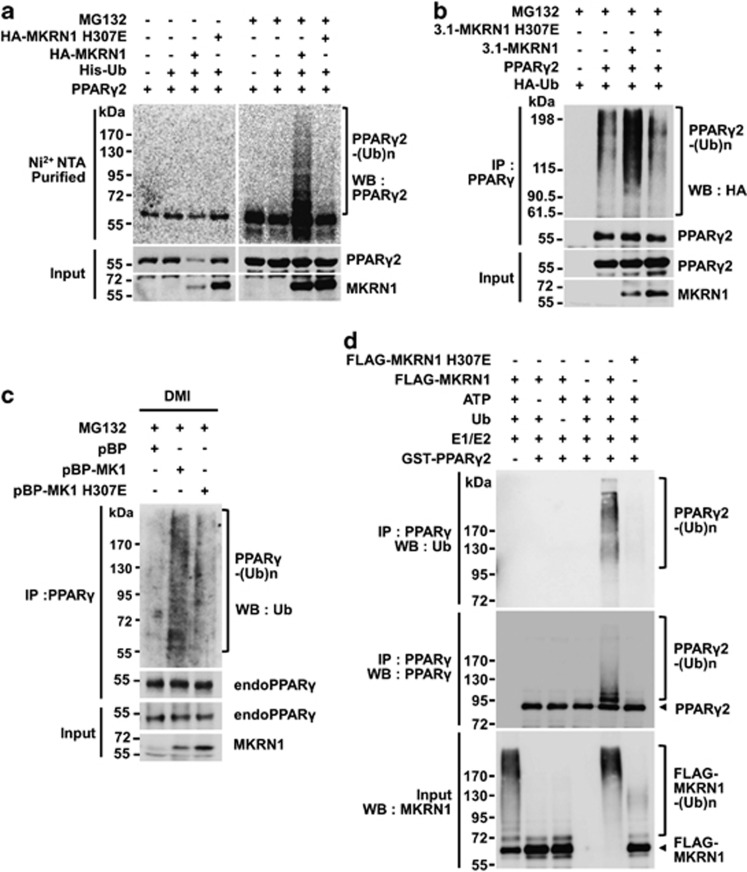
To further confirm the activities of MKRN1 on PPARγ2, ubiquitination analyses were carried out under denaturation conditions. His-Ub pulldown assays using Ni2+-NTA followed by immunoblotting of PPARγ2 showed that there was an increase of PPARγ2 ubiquitination in the presence of MKRN1 under treatment with MG132 (Figure 3a). On the other hand, the H307E mutant was not able to induce ubiquitination, suggesting that the E3 ligase activities of MKRN1 are required for PPARγ2 ubiquitination (Figure 3a). The ubiquitination analyses were extended by first immunoprecipitating PPARγ2, followed by immunoblotting to detect HA-Ub. These analyses also confirmed that only MKRN1 was able to induce PPARγ2 ubiquitination, but not H307E (Figure 3b). We detected residual amounts of ubiquitinated PPARγ in the absence of MKRN1. Based on these data, we cannot exclude the possibility that other E3 ligases are involved in the ubiquitination of PPARγ.20 The levels of endogenous PPARγ ubiquitinated were further detected using a whole-cell lysate of differentiated 3T3-L1. The results showed that in the presence of stably expressed MKRN1, there was an increase of ubiquitinated endogenous PPARγ in differentiated 3T3-L1 cells, implicating MKRN1 as an E3 ligase of PPARγ (Figure 3c). Finally, using a cell-free ubiquitination assay system, MKRN1 was shown to induce ubiquitination of recombinant PPARγ2, whereas MKRN1 H307E was not (Figure 3d). GST was used as a control (Supplementary Figure 7a). We observed a similar effect of MKRN1 on PPARγ1 ubiqutination and degradation, suggesting that both PPARγ1 and PPARγ2 are substrates of MKRN1 (Supplementary Figures 4a–c).
Figure 3.
MKRN1 mediates ubiquitination of PPARγ2 through E3 ligase activity. (a) Ubiquitination of PPARγ2 by MKRN1 and MKRN1 H307E mutants. H1299 cells were transfected with the indicated plasmid in the presence or absence of MG132. His-Ub conjugated proteins were pulled-down using Ni2+-NTA beads and detected using α-PPARγ or α-HA antibodies. (b) PPARγ2 ubiquitination by active MKRN1 E3 ligase. H1299 cells, transfected as indicated, were immunoprecipitated with monoclonal α-PPARγ antibodies, followed by western blotting as described above. (c) Ubiquitination of endogenous PPARγ by MKRN1. 3T3-L1 stable cell lines expressing pBabe-Puro-MKRN1 (pBP-MK1) and pBabe-Puro-MKRN1 H307E (pBP-MK1 H307E) were differentiated by treatment with DMI. Cells were treated with MG132 for 6 h before harvest and lysed, followed by immunoprecipitation using α-ubiquitin antibodies and western blotting. (d) Ubiquitination of PPARγ2 by MKRN1 in vitro. Purified recombinant GST-PPARγ2 was incubated with E1, E2, ubiquitin (Ub) and ATP in the absence and presence of FLAG-MKRN1 wild-type or H307E mutant as indicated. Reactions were boiled in 1% SDS to disrupt protein interaction, followed by immunoprecipitation using α-PPARγ antibodies. Ubiquitination of PPARγ was analyzed by western blotting using α-Ub or α-PPARγ antibodies. For input, ubiquitin reactions were directly subjected to western blotting using α-MKRN1 antibodies
Previous reports indicated that the presence of PPARγ2 agonist expedites the degradation process of PPARγ2.17 To verify whether PPARγ2 agonist facilitates MKRN1-dependent degradation processes, troglitazone was included in the assays. On treatment with troglitazone, there was an increase in MKRN1 and PPARγ2 interaction, with more efficient MKRN1-dependent degradation of PPARγ2 (Supplementary Figures 5a and b). In agreement with these data, PPARγ2 ubiquitination was further increased by the agonist (Supplementary Figure 5c). Finally, using wild type or MKRN1 null MEFs, we showed that the presence of PPARγ2 agonists such as rosiglitazone, troglitazone, or GW7845 stimulated PPARγ2 destabilization in wild-type MEFs, whereas these effects were not clearly observed in MKRN1 knockout MEFs (Supplementary Figure 5d). These results suggest that agonist-stimulated destabilization of PPARγ2 requires the presence of MKRN1. These agonists did not affect MKRN1 levels, as shown in Supplementary Figure 5d. In summary, on interacting with PPARγ, MKRN1 was able to induce the ubiquitination and degradation of PPARγ, and these MKRN1-mediated processes were facilitated by the presence of a PPARγ agonist.
Two lysines of PPARγ located at the putative nucleus localization signal (NLS) are targeted for ubiquitination by MKRN1
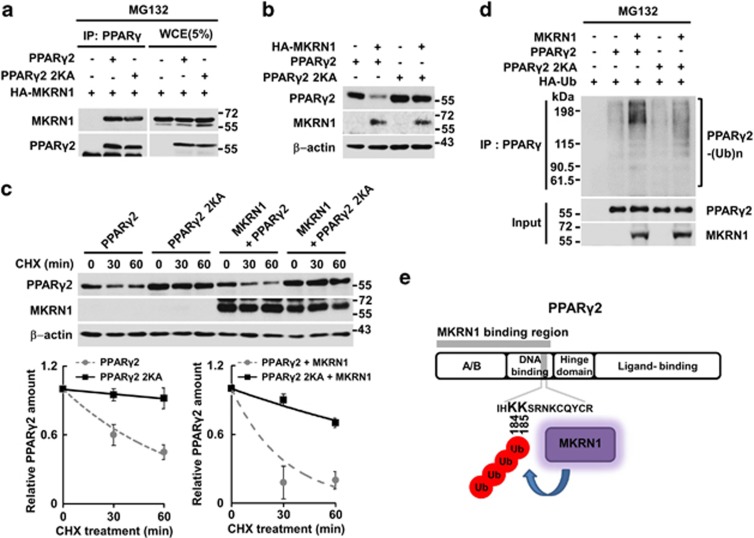
PPARγ is a nuclear receptor with a putative NLS region located between amino-acid regions 181 and 224.29, 30 Our immunocytochemistry analyses showed that ∼80% of H1299 cells exhibited the nucleus-only localization of PPARγ2, as previously reported (Supplementary Figure 6).31 Positively charged lysine sites in the NLS are commonly considered to have important roles in the nuclear localization of various proteins.29, 30, 31, 32 When two lysines that are consecutively located at 184 and 185 sites in the NLS region were mutated to alanines (2KA), ∼30% of the cells showed nuclear localization of PPARγ2, whereas the rest displayed nuclear/cytosol localization (Supplementary Figure 6). However, when the two lysines were mutated to arginines (2KR), no significant changes of PPARγ2 localization were observed (Supplementary Figure 6). On co-expression of MKRN1 and PPARγ2 or 2KR, we observed the nuclear/cytoplasmic localization of PPARγ2 in 70% of cells expressing both proteins (Supplementary Figure 6). These observations suggest that MKRN1 might mask the NLS function of PPARγ2 in a way that blocks its localization into the nucleus. As expected, 2KA was able to bind to MKRN1 as the wild type (Figure 4a). One interesting observation was that this protein was protected from MKRN1-mediated ubiquitination and degradation, indicating that these two lysines might be targeted for ubiquitination by MKRN1 (Figures 4b–d). Overall, MKRN1-mediated ubiquitination of PPARγ2 at lysines 184 and 185 located at NLS may be necessary for its ubiquitination and subsequent degradation (Figure 4e). A cell-free ubiquitination system was employed to further confirm whether lysines 184 and 185 are actually targeted for ubiquitination by MKRN1. The 2KA mutant was not ubiquitinated by MKRN1, suggesting that these two sites are indeed targeted for ubiquitination (Supplementary Figure 7b). As MKRN1 was able to induce translocation of both wild-type PPARγ2 and 2KR mutants into the cytoplasm, interaction between the two proteins seems to be required for translocation of PPARγ by MKRN1, rather than the ubiquitination process.
Figure 4.
Two lysine residues of PPARγ2 (184 and 185) are targeted for ubiquitination by MKRN1. (a) Interaction between PPARγ2 point mutant (2KA) and MKRN1. HEK293 T cells were transfected with the plasmids expressing PPARγ2 WT and PPARγ2 2KA mutant alone or with HA-MKRN1. WCE were immunoprecipitated with α-HA antibodies followed by western blotting using α-HA or α-PPARγ antibodies. Cell lysates (5% input) were used for western blotting. (b) Effects of MKRN1 on protein stability of PPARγ2 2KA mutant. H1299 cells transfected with plasmids expressing the indicated proteins were detected by western blotting, as indicated above (Figure 2a). (c) Half-life of PPARγ2 2KA mutant in the presence of MKRN1. H1299 cells were transfected with plasmids expressing indicated proteins followed by treatment with 50 μg/ml CHX for up to 60 min. WCE were perceived by western blotting and their levels measured as in Supplementary Figure 3b. (d) PPARγ2 2KA ubiquitination by MKRN1. H1299 cells were transfected with the plasmids expressing MKRN1, PPARγ2, PPARγ2 2KA, or HA-Ub. WCE were immunoprecipitated with monoclonal α-PPARγ antibodies, followed by western blotting, as described in Figure 3b. (e) Schematic of PPARγ2 ubiquitination target sites
MKRN1 suppresses adipocyte differentiation
As the forced expression of PPARγ in fibroblastic cells can induce adipogenesis, we investigated the physiological effects of MKRN1 in adipocyte differentiation. The role of MKRN1 as a negative regulator of PPARγ was analyzed by adipocyte differentiation from 3T3-L1 pre-adipocytes stably overexpressing MKRN1 or H307E. Overexpression of MKRN1 using a retrovirus suppressed the adipogenesis of 3T3-L1 induced by DMI treatment, as assessed using Oil-Red-O staining and morphological analysis (Figure 5a and Supplementary Figure 8a). Overexpressed MKRN1 consistently suppressed the expressions of adipogenic markers, such as aP2, C/EBPα, and CD36, as well as PPARγ (Figures 5b and c). Overexpressed H307E did not exhibit any noticeable effects on adipogenes, which corroborates the data shown above (Figures 5a–c and Supplementary Figure 8a). To further confirm whether overexpressed MKRN1 could suppress adipocyte differentiation by specifically targeting PPARγ for destabilization, we performed rescue experiments by expressing PPARγ in the presence of MKRN1. We observed that overexpression of PPARγ was able to overcome MKRN1-dependent suppression of adipocyte differentiation, suggesting that MKRN1 could target PPARγ as its substrate (Figure 6). The effects of MKRN1 on adipocyte differentiation were further confirmed under MKRN1-depleted conditions. To maximize the effects of MKRN1 depletion using shMKRN1 #1 and #3 on adipocyte differentiation, we applied DI instead of DMI, which is known to induce less-efficient adipocyte differentiation.33, 34 In both stable cell lines, we were able to observe effective differentiation of pre-adipocytes with greater expressions of PPARγ, aP2, and its other targets (Figures 5d and e and Supplementary Figure 8b). Consistently, the real-time reverse-transcription PCR (qRT-PCR) data indicated that the adipogenic markers, including PPARγ, aP2, and CD36, were all increased under MKRN1 depletion (Figure 5f). The effects of MKRN1 on adipocyte differentiation were also observed in mesenchymal C3H10T1/2 cells. MKRN1 or pBabe empty vector was stably overexpressed and stimulated into adipocytes with the addition of DMI and PPARγ agonists, GW7845. Consistent with the effects in 3T3-L1 cells, C3H10T1/2 cells stably expressing MKRN1 decreased PPARγ protein expression, resulting in less lipid accumulation compared with control cells (Supplementary Figures 9a and b). Gene expression analyses also showed decreased expression of PPARγ and its target aP2 mRNA in cells stably expressing MKRN1 (Supplementary Figure 9c). To address the roles of endogenous MKRN1 in C3H10T1/2 cells, stable cells expressing shMKRN1 #3 or GFP were generated. Compared with control cells, stable C3H10T1/2 cells expressing shMKRN1 #3 exhibited markedly increased lipid accumulation and expression of adipocyte markers (Supplementary Figures 10a–c).
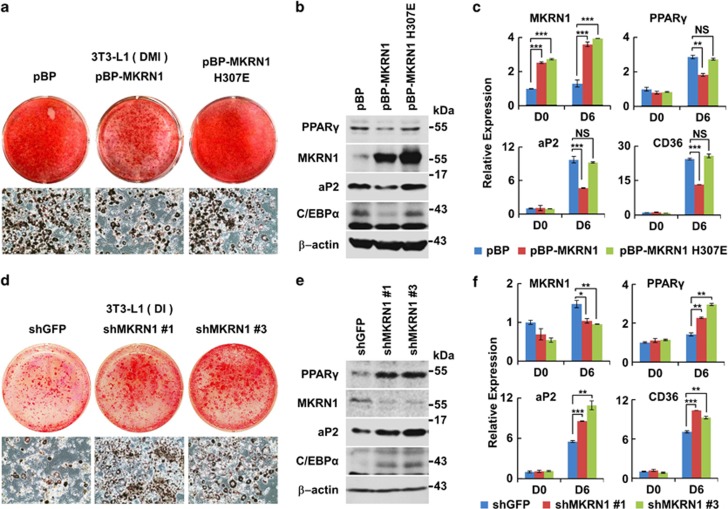
Figure 5.
MKRN1 reduces PPARγ protein levels and inhibits adipocyte differentiation in 3T3-L1 cells. (a) Effects of MKRN1 overexpression on 3T3-L1 adipocyte differentiation. Stable 3T3-L1 cell lines overexpressing MKRN1 (pBP-MKRN1) or its H307E mutant (pBP-MKRN1 H307E) using retroviral vectors were treated with DMI for 6 days and their adipocyte differentiation was detected using Oil-Red-O staining. 3T3-L1 cells retrovirally transduced with pBabe-puro empty vector (pBP) were used as control cells. (b) Effects of MKRN1 overexpression on the levels of PPARγ and its target proteins. The WCE described above were analyzed using antibodies against the proteins indicated. (c) Levels of adipocyte differentiation markers in 3T3-L1 cells expressing MKRN1. mRNA levels of cells indicated above were measured by qRT-PCR analysis using primers targeted for MKRN1, PPARγ, aP2 and CD36 mRNAs. Data are presented as mean±S.D.; n=3 with **P<0.01 and ***P<0.001 compared with each lane. (d) Effects of MKRN1 depletion on adipogenesis of 3T3-L1. Stable cell lines constitutively depleted of MKNR1 were constructed using a lentivirus expressing shRNA for GFP or mouse MKRN1 (shGFP, shMKRN1 #1, shMKRN1 #3, respectively). The adipogeneses of these three cell lines were induced using DI (dexamethasone+insulin) for 6 days. Cells were stained and detected as described above. (e) MKRN1 knockdown increases the levels of adipogenic proteins. WCE from above were analyzed as above. (f) Effects of MKRN1 depletion on mRNA levels of the adipogenic markers. The mRNA of adipogenic markers were analyzed as described above. Data are presented mean±S.D.; n=3 with *P<0.05, **P<0.01 and ***P<0.001 compared with each lane
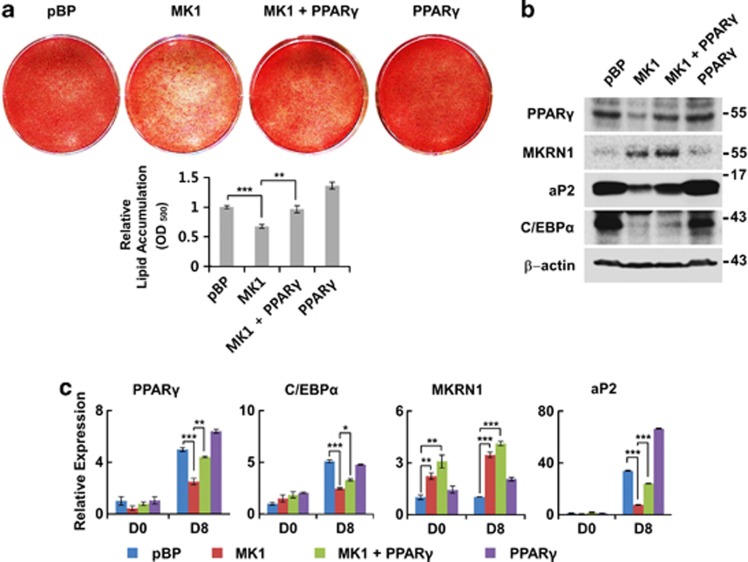
Figure 6.
MKRN1-dependent suppression of adipogenesis was rescued by the expression of PPARγ in 3T3-L1 cells. (a) Effects of overexpressed PPARγ on MKRN1-dependent suppression of adipocyte differentiation. Stable cell lines retrovirally overexpressing MKRN1 (MK1), PPARγ or MKRN1+PPARγ (MK1+PPARγ) were treated with DMI for 6 days, and adipocyte differentiation was detected using Oil-Red-O staining. 3T3-L1 cells transfected with retrovirus containing pBabe-puro empty vector (pBP) were used as control cells. Oil-Red-O staining extractions were performed as indicated above. (b and c) Expression patterns of PPARγ and its targets. WCE from above were detected using antibodies against proteins indicated (b). mRNA levels of adipogenic markers in 3T3-L1 stable cell lines were analyzed by qRT-PCR method using indicated primers (c). Data are presented as mean±S.D.; n=3 with *P<0.05, **P<0.01 and ***P<0.001 compared with each lane
To further confirm the effects of MKRN1 in adipogenesis shown above, potentials of MKRN1 knockout or wild-type MEFs for adipocyte differentiation were compared. Similar to the increased differentiation in MKRN1-silenced 3T3-L1 and C3H10T1/2 cells, primary MKRN1−/− MEFs showed robust increases in lipid accumulation and morphological differentiation when compared with WT MEFs (Figures 7a–c). There were also significant increases in protein and mRNA levels of PPARγ, aP2, C/EBPα, and CD36 in the MKRN1−/− cells (Figures 7d and e). As expected, the expressions of C/EBPβ and C/EBPδ were not affected in the MKRN1−/− cells, suggesting that there are PPARγ-specific effects of MKRN1 in adipogenesis (Figures 7d and e). Confirming these observations, the ubiquitination status of endogenous PPARγ was weaker under MKRN1−/− MEFs, suggesting that MKRN1 is necessary for PPARγ ubiquitination (Figure 7f). Overall, our data demonstrate that MKRN1 can negatively control adipocyte differentiation by suppressing PPARγ function post-translationally.
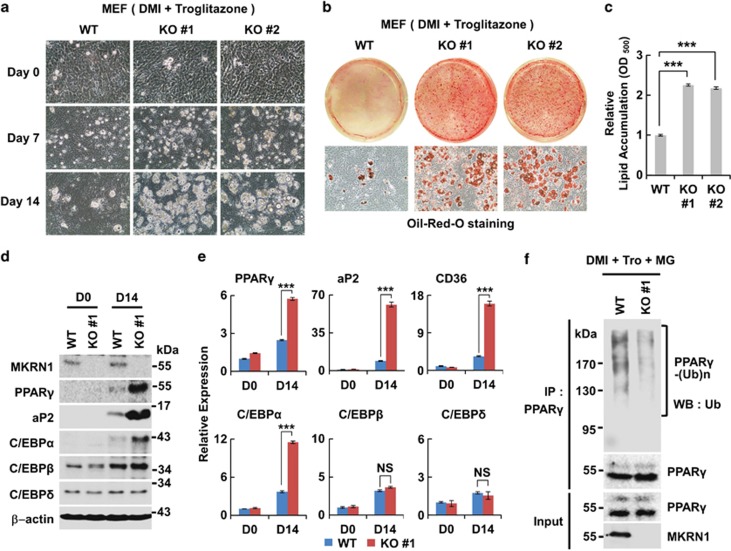
Figure 7.
MKRN1 knockout MEFs exhibit the increased potential for adipocyte differentiation. (a and b) Adipogenesis of MKRN1 knockout MEFs. MKRN1 knockout (KO #1 and KO #2) and wild-type MEFs, cultured in medium containing DMI and troglitazone from 0 to 14 days, were photographed as described above (a). Differentiated MEF cells were detected using Oil-Red-O staining (b). (c) The Oil-Red-O dye was extracted using isopropanol and measured at 500 nm. Data represent mean±S.D.; n=3 with ***P<0.001. See Materials and methods for details. (d) Protein levels of PPARγ and its target proteins in wild type and MKRN1 knockout MEFs. MKRN1 knockout MEFs displayed increased levels of PPARγ with its target proteins. WCE of MKRN1 knockout and wild-type MEFs were analyzed as described in Figure 5b. (e) mRNA levels of PPARγ and its target proteins in wild type and MKRN1 knockout MEFs. MKRN1 knockout MEFs increase in mRNA levels of the adipogenic markers. The mRNA of adipogenic markers and upstream of PPARγ were analyzed by qRT-PCR analysis as described in Figure 5c. Data are mean±S.D.; n=3 with ***P<0.001 compared with each lane. (f) Reduction of endogenous PPARγ ubiquitinationin MKRN1 knockout MEFs. MKRN1 WT and knockout MEFs were differentiated to adipocytes using DMI and troglitazone (DMI+tro) for 14 days. Subsequently, WT and knockout MEFs were treated with 10 μM MG132 (MG) for 6 h before harvest and lysed, followed by immunoprecipitation using α-PPARγ antibodies and western blotting under denaturating conditions
Discussion
The combined trends of increased caloric intake and decreased physical exercise have contributed to the increasing prevalence of obesity and related insulin resistance in industrialized populations. Insulin resistance is associated with increased risks of type 2 diabetes and cardiovascular disease. At the crossroads of obesity, insulin resistance, and cardiovascular disease lies the nuclear receptor PPARγ.35, 36, 37, 38 Therefore, better understanding of PPAR biology is of scientific and therapeutic importance.
As PPARγ functions as a master regulator of adipogenesis and is a critical factor associated with various metabolic diseases, amounts of PPARγ should be tightly controlled at the transcriptional and post-translational levels.5, 8, 35 Transcriptional pathways that control the expression of PPARγ have been extensively studied;7, 16 but the stability of short-lived PPARγ, which is probably controlled by post-translational modification, remains incompletely understood. Previous studies showed that PPARγ is polyubiquitinated and degraded in a proteasome-dependent manner.17 Ubiquitin ligase Siah2 was recently identified for its inhibitory actions on PPARγ activity, further suggesting that ubiquitin ligase is an important regulator of PPARγ activity and adipogenesis. However, Siah2 does not appear to directly interact with PPARγ, but instead a nuclear receptor corepressor, NCoR, has been suggested as a target of Siah2, indicating that another E3 ligase(s) exists for ubiquitination of PPARγ.19
In this study, we propose MKRN1 as a direct E3 ligase-targeting PPARγ. MKRN1 reduces PPARγ protein levels through direct interaction. Two lysine residues at 184 and 185 in the DNA-binding domain of PPARγ are critical sites for MKRN1-mediated ubiquitination. Interestingly, these two lysine residues are conserved in mammals, birds, reptiles, amphibians, and fishes, implying an evolutionarily conserved role for MKRN1 in PPARγ regulation. Overexpression and knockdown studies indicate that MKRN1 is a negative regulator of PPARγ, decreasing PPARγ protein levels. MEFs isolated from MKRN1−/− embryos further demonstrate the regulatory roles of MKRN1 in PPARγ-mediated adipogenesis. Thus, our results demonstrate that MKRN1 is a novel regulator of PPARγ, catalyzing polyubiquitination and subsequent degradation of PPARγ.
Previous studies have implicated that an elusive E3 ligase responsible for PPARγ degradation is tightly linked to PPARγ activation on ligand binding.17 Therefore, we investigated whether the regulatory actions of MKRN1 could be affected by PPARγ activation. The regulatory activities of MKRN1 on PPARγ appear to be influenced by ligand binding, as treatment with troglitazone enhanced the association between the two proteins and increased the ubiquitination of PPARγ, confirming previous findings (Supplementary Figures 5a–d).17, 19 How does PPARγ activation by agonists facilitate MKRN1 and PPARγ interaction and subsequently increase the degradation of PPARγ? One may speculate that the activation of PPARγ causes a conformational shift toward the higher incidence of MKRN1 binding with PPARγ, followed by cytoplasmic relocation and degradation. How the regulatory functions of MKRN1 act on PPARγ in a ligand-dependent manner remains an intriguing question for future studies.
In this study, we identified the functions of MKRN1 in post-translational modification of PPARγ and adipocyte differentiation. Interestingly, however, MKRN1 is expressed in multiple tissues, suggesting functions in other cell types. Whether MKRN1 leads to the suppression of PPARγ in non-adipose tissues and similar modulations of other nuclear receptor families associated with metabolic disease are intriguing questions. Further studies are necessary to evaluate the effects of MKRN1 in non-adipose tissues and other targets for degradation.
Our results also suggest that MKRN1 could be a novel therapeutic target for obesity and diabetes. Further studies of MKRN1 activities and cellular and pathological effects in vivo are of great importance for finding novel therapeutic approaches to obesity-related diseases. The generation and characterization of tissue-specific knockout mice will be required to determine the roles of MKRN1 in obesity and related metabolic diseases. It will be interesting to explore whether MKRN1 and its mechanisms can be exploited to develop new therapeutic interventions for the treatment of obesity and metabolic diseases in the future.
Materials and Methods
Plasmids
pcDNA3.1-PPARγ2 was generated from pBabe-puro-PPARγ2, which was described previously.34 pcDNA3.1-PPARγ2 2KA and pcDNA3.1-PPARγ2 2KR mutants were produced using site-directed mutagenesis methods, as provided by the manufacturer (Takara Bio, Shiga, Japan). The primers used for these constructs are as follows: 2KA-5′-AACTGCCGGATCCACGCAGCAGCAAGAAATAAATGTCAG-3′ (forward), 2KA-5′-CTGACATTTATTTCTTGCTGCTGCGTGGATCCGGCAGTT-3′ (reverse), 2KR-5′-AACTGCCGGATCCACAGGAGGAGTAGAAATAAATGTCAG-3′ (forward), 2KR-5′-CTGACATTTATTTCTACTCCTCCTGTGGATCCGGCAGTT-3′ (reverse). MKRN1 constructs (pcDNA3-HA-MKRN1 WT/H307E, pcDNA3.1-MKRN1 WT/H307E, pcDNA3-FLAG-MKRN1 WT/H307E) were described previously.22 pcDNA3-HA-MKRN1 WT and H307E mutant were subcloned into pBabe-Puro. pcDNA3-His-Ub and pHM6-HA-Ub was previously described.39, 40 pTK-PPREx3-luc was kindly provided by HW Lee (Yonsei University, Seoul, Korea).
Antibodies and chemicals
Western blotting and immunoprecipitation data analysis were performed using the following antibodies: PPARγ (mouse sc-7273X, rabbit sc-7196X, Santa Cruz Biotechnology, Santa Cruz, CA, USA), C/EBPα (rabbit sc-61X, Santa Cruz Biotechnology), C/EBPβ (rabbit sc-150X, Santa Cruz Biotechnology), C/EBPδ (rabbit sc-636X, Santa Cruz Biotechnology), aP2 (goat sc-18661, Santa Cruz Biotechnology), HA (mouse sc-7392, rabbit sc-805, Santa Cruz Biotechnology), FLAG (mouse F3165, rabbit F7425; Sigma-Aldrich, St. Louis, MO, USA), GFP (sc-8334, Santa Cruz Biotechnology), β-actin (A5316; Sigma-Aldrich), normal mouse IgG (sc-2025; Santa Cruz Biotechnology), normal goat IgG (sc-2028; Santa Cruz Biotechnology), myc (sc-40, Santa Cruz Biotechnology), MKRN1 (A300-990A; Bethyl Laboratories, Montgomery, AL, USA), and HRP-conjugated-α-UbFK2 (PW0150, Enzo Life Sciences, Farmingdale, NY, USA). CHX (C4859), N-ethylmaleimide (NEM, E3876) and dimethyl sulfoxide (DMSO, D8418), dexamethasone (D1756), IBMX (I5879), Troglitazone (T2573), Rosiglitzaone (R2408), and Oil-Red-O (O0625) were purchased from Sigma-Aldrich. Insulin (11 376 497 001) was purchased from Roche (Mannheim, Germany). GW7845 was kindly provided by T Willson (GlaxoSmithKline, QC, Canada).
Transfection
H1299 and PC-3 cells were transfected using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). Polyethyleneimine (PEI, Sigma-Aldrich) was used for the transfection of HEK293T cells. 3T3-L1 cells were transfected using a microporator (Neon Transfection System, Invitrogen). Lipofectamin RNAiMax (Invitrogen) was used for siRNA transfection. MKRN1 siRNAs were previously described (the siRNA sequences are as follows: #5- 5′GGCGAAGCTGAGTCAAGAA-3′ and #6- 5′-GGATCCTCTCCAACTGCAA-3′).22
Cell culture and adipocyte differentiation
H1299 (human lung carcinoma cell line) and HEK293T (human kidney cancer cell line) were cultured in DMEM containing 10% FBS (Gibco, Invitrogen). Adipocytes were differentiated from 3T3-L1 pre-adipocyte cells, mesenchymal C3H10T1/2 cells, or mouse MEFs. Briefly, 3T3-L1 cells were maintained in DMEM with 10% bovine serum for 3–5 days, and confluent cells differentiated in DMEM containing 10% FBS, dexamethasone (1 μM), 3-isobutyl-1-methylxanthine (IBMX; 520 μM), and insulin (1 μg/ml) (DMI mixture) for 2 days. The cells were then cultured in DMEM containing 10% FBS and insulin (5 μg/ml) for 2 additional days. MEFs and C3H10T1/2 cells were cultured in DMEM with 10% FBS for 3–5 days, and confluent cells differentiated in DMEM containing 10% FBS, dexamethasone (1 μM), 3-isobutyl-1-methylxanthine (IBMX; 520 μM), insulin (10 μg/ml; (DMI mixture) and TZD (1 μM, Troglitazone and GW9544) for 2–3 days. The cells were further cultured in DMEM containing 10% FBS, insulin (10 μg/ml), and TZD (1 μM) for an additional 6–8 days. Differentiated cells were detected using Oil-Red-O staining, as previously described (O0625, Sigma-Aldrich).41 The stained cells were photographed using CKX41SF (Olympus, Tokyo, Japan). The dye from Oil-Red-O staining were extracted using isopropanol, followed by optical density measurement at 500 nm using Benchmark Plus (Bio-Rad Inc., US).
Generation of primary MEFs
MKRN1 knockout MEFs were generated as previously described using MKRN1 C57BL/6J mice.25 Briefly, at E13.5 day, embryos were removed from pregnant female mice and ground using blades. Ground embryos were incubated in 3 ml of trypsin-EDTA 37 °C for 20 min, transferred to 150-mm culture dishes, and incubated at 37 °C in 20 ml of complete media. After 8 h, the medium was changed to fresh DMEM containing 10% FBS. Once the cells were 70–80% confluent, they were maintained at 1 : 3 ratios.
Generation of MKRN1 overexpression and knockdown stable cell lines
All short-hairpin RNA (shRNA) constructs were purchased from Sigma-Aldrich. The sequences are as follows: mouse MKRN1#1-5′-GCGAGATGTTGCTTATGCTTT-3′, MKRN1#2-5′-CCAGAGGTCACAGCACATAAA-3′, MKRN1#3-5′-GAGTGGGACTTGTTTCACGAT-3′. 3T3-L1 cells stably expressing control shRNA (shGFP) or mouse MKRN1 shRNA (shMKRN1#1, shMKRN1#3) were established using the manufacturer's instructions. HEK293T cells were transfected with pLKO.1-shGFP or shMKRN1#1, #3, and lentiviral packaging vectors. At 48 h post transfection, viral supernatants were harvested, filtrated, and added to 3T3-L1 or C3H10T1/2 cells. Transduced cells were selected by puromycin treatment for 7 days. MKRN1 overexpression cell lines were produced using pBABE-puro-MKRN1 and pBABE-puro-MKRN1 H307E mutant plasmids, and the packaging cell line Phoenix E, as previously described.34
In vitro ubiquitination assay
FLAG-MKRN1 was transiently expressed in HEK293T cells, purified by immunoprecipitation using anti-FLAG M2 affinity gel (Sigma-Aldrich, A2220), and eluted by adding FLAG peptide (Sigma-Aldrich, F3290) according to the manufacturer's instructions. Bacterially expressed recombinant GST-PPAR2 was incubated with 200 ng E1 (UBE1, E-305, Boston Biochem, Cambridge, MA, USA), 500 ng E2 (UbcH5c, Boston Biochem, E2-627), 10 μg Ubiquitin (Sigma-Aldrich, U6235), 2 mM ATP (Fermentas, Vilnius, Lithuania, R0441) in the absence and presence of FLAG-MKRN1 wild type or H307E mutant in 50 μl of reaction buffer (40 mM Tris-HCl, pH 7.6, 50 mM NaCl, and 1 mM DTT) for 3 h at 37 °C. After incubation, reactions were boiled in 1% SDS to disrupt any protein interactions, diluted with immunoprecipitation buffer to 0.1% SDS, and immunoprecipitated using α-PPARγ mouse monoclonal antibodies (Sigma-Aldrich, 7273X), followed by western blotting using α-Ub-HRP or α-PPARγ mouse antibodies (Sigma-Aldrich, 7273X). Reactions were directly subjected to western blotting using α-MKRN1 antibodies to see MKRN1 self-ubiquitination as a control.
RT-PCR analysis
RNAs were extracted using Trizol (Invitrogen) according to the manufacturer's directions. cDNA was synthesized from total RNA using Reverse Transcriptase M-MLV (Takara Bio), amplified, and then analyzed using the QuantiTect SYBR Green PCR Kit and real-time PCR (Qiagen) with the following primers: human and mouse MKRN1; 5′-GAGCAGGTTCAGAGGACTGG-3′ (forward) and 5′-CACTCTCCCACTGCAGCATA-3′ (reverse), mouse PPARγ; 5′-CCATTCTGGCCCACCAAC-3′ (forward), 5′-AATGCGAGTGGTCTTCCATCA-3′ (reverse), human PPARγ; 5′-TTCAGAAATGCCTTGCAGTG-3′ (forward), 5′-CCAACAGCTTCTCCTTCTCG-3′ (reverse), C/EBPα; 5′-GCGGGCAAAGCCAAGAA-3′ (forward), 5′-GCGTTCCCGCCGTACC-3′ (reverse), aP2; 5′-CACCGCAGACGACAGGAAG-3′ (forward), 5′-GCACCTGCACCAGGGC-3′ (reverse), CD36; 5′-GGCCAAGCTATTGCGACAT-3′ (forward), 5′-CAGATCCGAACACAGCGTAGA-3′ (reverse), 36B4; 5′-AGATGCAGCAGATCCGCAT-3′ (forward), 5′-GTTCTTGCCCATCAGCACC-3′ (reverse), C/EBPβ; 5′-TGATGCAATCCGGATCAA-3′ (forward), 5′-CACGTGTGTTGCGTCAGT-3′ (reverse), and C/EBPδ; 5′-GAGAACGAGAAGCTGCATCA-3′ (forward), 5′-GGCTGGGCAGTTTTTGAA-3′ (reverse). Each gene expression level was normalized to that of the 36B4 gene in the same sample, except for those shown in Figure 1a. The qRT-PCR data shown in Figure 1a were normalized by the GAPDH gene.
Immunoprecipitation and ubiquitination assay
HEK293T cells were lysed using lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, and a protease inhibitor cocktail (2 μg/ml Aprotinin, 1 μg/ml Pepstatin A, 2 μg/ml Leupeptins, 100 μg/ml PMSF (phenylmethanesulfonylfuoride), Sigma-Aldrich). Cell lysates were incubated with antibodies for 2 h, followed by incubation with protein G Sepharose (GE Healthcare, UK) for 2 h. Immunoprecipitates were boiled in sample buffer for 5 min. In the ubiquitination assay using HA-Ub, H1299 or 3T3-L1 cells transfected with the indicated plasmids were harvested by PBS containing 10 nM NEM to prevent deubiquitination. Cells were lysed in 1% SDS by boiling for 10 min, followed by dilution to 0.1% SDS by adding lysis buffer, protease inhibitors, and NEM. Lysed samples were immunoprecipitated with α-PPARγ antibodies (sc-7273x, Santa Cruz Biotechnology), followed by western blotting. Ubiquitination assays using His-Ub were performed using 6 M guanidinium-HCl buffer (pH 8) containing 5 mM NEM. His-ubiquitin-conjugated proteins were purified by incubating with Ni2-NTA agarose beads (Qiagen), followed by washing and elution in sample buffer.22
Protein purification, in vitro translation, and in vitro binding assays
pGEX-4T-1-PPARγ2 was expressed in Escherichia coli and purified using glutathione–sepharose 4B (GE Healthcare). Bacteria expressing GST- PPARγ2 were lysed using lysis buffer (25 mM sodium phosphate buffer (pH 7), 300 mM NaCl, 20 mM β-mercaptoethanol, and 5% glycerol and protease inhibitors). GST-PPARγ2 was purified using GST Sepharose beads following the manufacturer's protocol (GE Healthcare). A TNT T7-coupled reticulocyte lysate system (L4610, Promega, Madison, WI, USA) was used to produce HA-MKRN1 protein. Translated HA-MKRN1 was incubated with GST or GST-PPARγ2 for 2 h, and then Sepharose beads were added followed by an 1-h incubation. These complexes were washed and eluted with 10 mM reduced glutathione, followed by western blotting.
Statistical analyses
All statistical analyses were performed using PRISM (GraphPad Software, San Diego, CA, USA) and results are presented as mean±S.D. The P-values were designated as follows: *P<0.05; **P<0.01; ***P<0.001.
Acknowledgments
We thank No-Joon Song and Sung-Pil Choi for their help with cell culture and adipocyte differentiation, and Dr. Peter Tontonoz (University of California at Los Angeles) for helpful discussion. This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2010-0017787), and by the Cooperative Research Program for Agricultural Science & Technology Development (2012-PJ008462).
Glossary
- 3T3-L1
murine preadipocytic line derived from NIH-3T3
- C3H10T1/2
Mesenchymal stem cell line, fibroblasts
- H1299
human lung cancer cell line
- PC-3
prostate cancer cell line
- HEK293T
human kidney cancer cell line
- MEFs
Mouse embryonic fibroblasts
- ATCC
American Type Culture Collection
- PPARγ
peroxisome-proliferator-activated receptor γ
- C/EBP, β and δ
CCAAT/enhancer-binding proteins, β and δ
- LPL
lipoprotein lipase
- aP2
fatty acid-binding protein
- MKRN1
Makorin Ring Finger Protein 1
- CD36
Cluster of Differentiation 36
- NR
nuclear receptor superfamily
- qRT-PCR
real-time reverse-transcription PCR
- DMI
A cocktail of dexamethasone, 3-isobutyl-1-methylxanthine, and insulin
- DI
A cocktail of dexamethasone and insulin, IBMX, 3-isobutyl-1-methylxanthine
- DMSO
dimethyl sulfoxide
- IP
immunoprecipitation
- NP40
Nonidet P-40
- PBS
phosphate-buffered saline
- CHX
cycloheximide
- Tro
Troglitazone
- NEM
N-ethylmaleimide
- pBP
pBABE-puro empty vector
- shRNA
short-hairpin RNA
- Ub
Ubiquitin
- WCE
whole-cell extracts
- WB
western blot
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by M Piacentini
Supplementary Material
References
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Eguchi J, Yan QW, Schones DE, Kamal M, Hsu CH, Zhang MQ, et al. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metab. 2008;7:86–94. doi: 10.1016/j.cmet.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, et al. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- Hu Y, Davies GE. Berberine increases expression of GATA-2 and GATA-3 during inhibition of adipocyte differentiation. Phytomedicine. 2009;16:864–873. doi: 10.1016/j.phymed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–138. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- van Beekum O, Fleskens V, Kalkhoven E. Posttranslational modifications of PPAR-gamma: fine-tuning the metabolic master regulator. Obesity (Silver Spring) 2009;17:213–219. doi: 10.1038/oby.2008.473. [DOI] [PubMed] [Google Scholar]
- Adams M, Reginato MJ, Shao DL, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. Journal of Biological Chemistry. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- Kilroy G, Kirk-Ballard H, Carter LE, Floyd ZE. The ubiquitin ligase Siah2 regulates PPARgamma activity in adipocytes. Endocrinology. 2012;153:1206–1218. doi: 10.1210/en.2011-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Wang P, Zhao G, Wang H, Wang M, Chen J, et al. Upregulation of SIRT1 by 17beta-estradiol depends on ubiquitin-proteasome degradation of PPAR-gamma mediated by NEDD4-1. Protein Cell. 2013;4:310–321. doi: 10.1007/s13238-013-2124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park SM, Kang MR, Oh SY, Lee TH, Muller MT, et al. Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT. Genes Dev. 2005;19:776–781. doi: 10.1101/gad.1289405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J. 2009;28:2100–2113. doi: 10.1038/emboj.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A, Lee EW, Yeh JY, Yang MR, Oh W, Moon JS, et al. MKRN1 induces degradation of West Nile Virus capsid protein by functioning as an E3 ligase. J Virol. 2010;84:426–436. doi: 10.1128/JVI.00725-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, et al. Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun. 2012;3:978. doi: 10.1038/ncomms1981. [DOI] [PubMed] [Google Scholar]
- Ko A, Shin JY, Seo J, Lee KD, Lee EW, Lee MS, et al. Acceleration of gastric tumorigenesis through MKRN1-mediated posttranslational regulation of p14ARF. J Natl Cancer Insti. 2012;104:1660–1672. doi: 10.1093/jnci/djs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omwancha J, Zhou XF, Chen SY, Baslan T, Fisher CJ, Zheng Z, et al. Makorin RING finger protein 1 (MKRN1) has negative and positive effects on RNA polymerase II-dependent transcription. Endocrine. 2006;29:363–373. doi: 10.1385/ENDO:29:2:363. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. Mppar-Gamma-2 - tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci USA. 2000;97:10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, et al. Non-DNA binding, dominant-negative, human PPAR gamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto F, Umemoto T, Motojima K, Fujiki Y. Nuclear transport of peroxisome-proliferator activated receptor &alpha. J Biochem. 2011;149:311–319. doi: 10.1093/jb/mvq144. [DOI] [PubMed] [Google Scholar]
- Umemoto T, Fujiki Y. Ligand-dependent nucleo-cytoplasmic shuttling of peroxisome proliferator-activated receptors, PPARalpha and PPARgamma. Genes Cells. 2012;17:576–596. doi: 10.1111/j.1365-2443.2012.01607.x. [DOI] [PubMed] [Google Scholar]
- Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–968. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Waki H, Choi SP, Park KM, Tontonoz P. The small molecule phenamil is a modulator of adipocyte differentiation and PPARgamma expression. J Lipid Res. 2010;51:2775–2784. doi: 10.1194/jlr.M008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Charo IF. Macrophage polarization and insulin resistance: PPARgamma in control. Cell Metab. 2007;6:96–98. doi: 10.1016/j.cmet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Tsai YS, Xu L, Smithies O, Maeda N. Genetic variations in peroxisome proliferator-activated receptor gamma expression affect blood pressure. Proc Natl Acad Sci USA. 2009;106:19084–19089. doi: 10.1073/pnas.0909657106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Chakrabarti R. Role of PPAR in cardiovascular diseases. Recent Patents Cardiovasc Drug Discov. 2006;1:193–209. doi: 10.2174/157489006777442441. [DOI] [PubMed] [Google Scholar]
- Camus S, Higgins M, Lane DP, Lain S. Differences in the ubiquitination of p53 by Mdm2 and the HPV protein E6. FEBS Lett. 2003;536:220–224. doi: 10.1016/s0014-5793(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Yang MR, Lee SR, Oh W, Lee EW, Yeh JY, Nah JJ, et al. West Nile virus capsid protein induces p53-mediated apoptosis via the sequestration of HDM2 to the nucleolus. Cell Microbiol. 2008;10:165–176. doi: 10.1111/j.1462-5822.2007.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Tang QQ, Vinson C, Lane MD. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 2004;101:43–47. doi: 10.1073/pnas.0307229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.