Introduction
Food allergy is one of the most common chronic diseases of childhood, with widely varying estimates of prevalence worldwide and in the United States (US)1–3. Several studies have suggested that food allergy has increased dramatically in prevalence over the past several decades in developed countries4,5, and it is perceived that it has increased in the US, but data regarding trends in the US have not been systematically synthesized and evaluated6–8. In this systematic review and meta-analysis with meta-regression we aimed to (1) determine the prevalence of self-reported food allergy in children in the US, and (2) explore sources of variation in prevalence estimates, including case definition, changes over time, and racial/ethnic differences.
Systematic reviews of self-reported food allergy prevalence have previously been published, but had several limitations that we address in the current study1,2. Earlier studies have focused on broad geographic areas, did not account for differences in wording of questions, and did not account for date of survey administration in their estimates, all of which likely contributed to wide prevalence estimates. Further, we incorporate the Centers for Disease Control (CDC) sponsored surveys of the US population into our analyses, which include a wealth of data available on hundreds of thousands of children. While the gold standard for diagnosis of food allergy is the double blind, placebo controlled oral food challenge, this method has yet to be employed in epidemiologic studies in the US, and questionnaire-based data from high quality surveys is the best we have available. Thus, here we examine the full range of publically available data on self-reported food allergy in US children.
Methods
Search Methods
We conducted a systematic literature search (up to February 2012) of MEDLINE and EMBASE for reports that included descriptions of the prevalence of food allergy in the United States. Search terms included prevalence and its synonyms combined with food allergy and its synonyms, including the combination of specific foods with allergy terms. In addition, we searched the reference list of all identified relevant publications and relevant reviews, and examined publically available databases by searching the Centers for Disease Control (CDC) websites9,10 (See Supplementary Methods for detailed description of search strategy). The search was updated on November 15th 2012, with one new relevant reference identified from reviewing titles/abstracts from 1,276 items found11; however, the data that were reported in this reference had already been incorporated into the meta-analysis. In addition, two new survey estimates were identified from the CDC (of NHIS 2011 and National Health and Nutrition Examination Survey [NHANES 2009–10]12). The review protocol was not registered.
Eligibility Criteria
Our first goal was to estimate the prevalence of self-reported food allergy in the general pediatric US population. Thus, studies that did not sample from the general population, were not based in the US, did not report on individual level data, did not include estimates for children or did not include food allergy as an outcome were excluded. Included studies reported on the prevalence of food allergy overall and/or specific food allergies. All references were in English. There was no time restriction. Literature eligibility was assessed in duplicate (C.K. reviewed all of the references, and J.S., S.S. and E.M. jointly reviewed a duplicate search); discrepancies were resolved by consensus. References were first screened for eligibility by scan of titles and abstracts, followed by a full-text review. Our second goal was to assess the contribution of study and individual level covariates to variation in estimates of prevalence; specifically to assess temporal changes in food allergy prevalence, ethnic differences and changes over time, and the impact of questionnaire design on estimates of prevalence. Because only the surveys administered by the CDC were considered to be of high enough quality in terms of reporting response rate and accounting for non-response in their estimates, surveys included in meta-regression were limited to those conducted by the CDC (see details in results).
Data collection process
The following information was extracted from each reference: report characteristics (article name, authors, publication year, journal), study characteristics (study name, year(s) of study, number of participants, method for selection of participants, age of participants), participation rate when available, and diagnostic method for food allergy (self-report of diagnosis or symptoms in the past year, self-report of ever diagnosis or symptoms and/or self-report of food allergy with no time-period specified, laboratory testing [skin test or specific IgE], combination of laboratory testing and symptoms, or oral food challenge), prevalence and confidence intervals of food allergy in general and specific foods (when available), and ethnicity-specific prevalences when available. Ethnicity was defined as Non-Hispanic White (referred to as White here), Non-Hispanic Black (referred to as Black), Hispanic and other. When confidence intervals or standard errors were not published, binomial standard errors and confidence intervals were calculated from the published data, or, if the underlying data were publically available, from the data directly using appropriate survey methodology. See Supplementary Methods for details about methods of analysis for publically available data. Clarification about details of study design and results were obtained directly from the study authors by personal communication for two reports13,14.
Statistical methods
All analyses were conducted with STATA SE/11 (College Station, TX) or R (Vienna, Austria). The analysis of summary statistics, including heterogeneity, by meta-analysis was conducted with the package METAN in STATA using a random effects model. Heterogeneity was quantified using the I-squared measure15; this measure estimates the proportion of between study variation due to heterogeneity. Meta-regression is a method that explores sources of heterogeneity in meta-analysis associated with study-level covariates16. Temporal trends and differences in prevalence by method of determining food allergy (i.e., differences in questionnaire design) were analyzed by meta-regression by creating a multivariate model that included both time and category of questionnaire using the package METAREG in STATA. In addition, analyses of ethnic differences over time were also done using meta-regression, adjusting for differences in questionnaire design. For this question, both stratified analyses by ethnicity, and interactions between ethnicity and time were explored. When studies spanned multiple years of data collection, the midpoint year was used for analysis. Only one of the surveys (two administrations) included in the meta-regression reported data on specific food allergies, and thus ethnic differences and changes over time were not analyzed for specific food allergies.
Results
Study Selection and Characteristics
10,090 references were identified from the database searches. After the title/abstract screening, full text screening and searches of CDC websites for unpublished surveys, a total of eight surveys were found that met the inclusion criteria11–14,17–31, including one that was administered annually for 15 years (the National Health Interview Survey23,24), one that was administered periodically and included estimates of self-reported food allergy on four separate occasions (the National Health and Nutrition Examination Survey12,22,25,26), one that was administered twice (the National Survey of Children’s Health27) and one that was administered three times (the Sicherer et al. peanut and tree nut survey13,18,20); each separate survey date is hereafter analyzed separately. Only one survey used a methodology other than self-report; the NHANES 2005–6 survey, which measured IgE to milk, egg, peanut and shrimp22. Because data are lacking on population-level positive and negative predictive values for specific IgE, it is not currently possible to extrapolate the overall prevalence of food allergy from IgE data32, and this survey was thus not used in other analyses. All surveys were conducted cross-sectionally. These surveys included a total of 452,237 subjects and covered the period of time from 1988–2011. Search results are summarized in Figure 1 and Table 1.
Figure 1.
Search results. Abbreviations: NHIS: National Health Interview Survey, NHANES: National Health and Nutrition Examination Survey. CDC: Centers for Disease Control
Table 1.
| Study Name | Year | N | Setting | Diagnostic Criteria | Primary Data Used? |
Age Range |
Data on overall prevalence available |
Participation Rate |
Used in meta-regression |
|---|---|---|---|---|---|---|---|---|---|
| National Maternal and Infant Health Survey17 | 1991 | 8285 | Phone and Mail | Self-report of ever food allergy. Infant/toddler. | 3 | X | 90% | X | |
| National Health and Nutrition Examination Survey III25,26 | 1988–1994 | 6600 | In-person | Self-report of ever reaction to a food. | X | <18 | X | 86% | X |
| Sicherer Peanut/Tree nut18 | 1997 | 2998 | Phone | Self-report ever of peanut/tree nut allergy. | <18 | 67% | |||
| Sicherer Shellfish19 | 2002 | 3814 | Phone | Self-report ever of shellfish allergy. | <18 | 67% | |||
| Sicherer Peanut/Tree nut20 | 2002 | 3127 | Phone | Self-report ever of peanut/tree nut allergy. | <18 | 52% | |||
| National Survey of Children's Health 200321,28 | 2003 | 102166 | Phone | Self-report of food allergy in past 12 months. | X | <18 | X | 55% | X |
| Infant Feeding Practices Study II31 | 2005–6 | 2441 | Self-report ever. Infant/toddler. | <1 | X | 76% | |||
| Sicherer Peanut/Tree nut13 | 2008 | 2915 | Phone | Self-report ever of peanut/tree nut allergy. | <18 | 42% | |||
| National Health Interview Survey 1997–201123,24,29,30 | 1997–2011 | 179,774 | In-person | Self-report of food allergy in past 12 months. | X | <18 | X | ~80% | X |
| National Survey of Children's Health 200724,27 | 2007 | 91393 | Phone | Self-report of food allergy in past 12 months. | X | <18 | X | 47% | X |
| National Health and Nutrition Examination Surveys 2007–8, 2009–1012,25* | 2007–2010 | 7002 | In-person | Self-report of food allergy – no time frame defined | X | <18 | X | >85% | X |
| Gupta14 | 2009–2010 | 38480 | Internet | Self-report of food allergy – current. | <18 | X | Not published |
Seven surveys reported on the prevalence of self-reported food allergy overall in the United States 11–14,17,20–31, providing 23 point estimates. There were several ways of identifying food allergy by self-report. Surveys generally fell into one of two categories with respect to type of question used to ascertain self-reported food allergy: (1) questions pertaining to ever having had diagnosis or symptoms of food allergy and/or those that did not specify a time-frame 12–14,17,20,25,26, and (2) questions pertaining to current symptoms or diagnosis of food allergy 11,23–30. Studies were further split into those that were limited to the infant/toddler period17,31 and those that were not.
For specific food allergies, self-reported data were available for peanut, tree nut and shellfish allergy. For peanut, data were available from 4 surveys and 7 survey administrations12–14,18,20,22,25,31. For tree nuts, 3 surveys and 6 survey administrations 12–14,18,20,25 and for shellfish 3 surveys and 4 survey administrations12,14,19,22,25. (See Supplementary Figures 1, 2 and 3).
Results of individual studies and synthesis of results for the systematic review
Figure 2 shows the point estimates and confidence intervals for the included surveys in the systematic review, stratified by method of identifying food allergy and presented by year of study. There was substantial heterogeneity between these estimates (I2 =97.4%, p<0.001), and so we are unable to present a summary estimate.
Figure 2.
Forest plot of food allergy prevalence in children in the US, by category of food allergy definition, ordered by year of survey; surveys done repeatedly are included separately by year of survey. Abbreviations: ES: Estimate, NMIHS: National Maternal and Infant Health Study, NHANES: National Health and Nutrition Examination Survey, NHIS: National Health Interview Survey, NSCH: National Survey of Children’s Health, IFPS II: Infant Feeding Practices Study II.
Assessment of quality
Two surveys that reported national prevalence were deemed to be of insufficient quality to be included in the meta-regression: the Infant Feeding Practices Survey II (IFPS II) used a consumer panel for sampling and was thus inadequately representative of the U.S. population31, and the Gupta survey did not report response rate and was an internet survey14, and thus had high potential for selection bias22,32. Details of response rates and design for each study are included in Table 1. All remaining surveys were conducted by the CDC. This left 5 surveys and 20 survey administrations covering 395,220 children for meta-regression of prevalence.
Results of Meta-regression
Method of identifying food allergy as a source of heterogeneity
Even when restricted to surveys conducted by the CDC, there was too much heterogeneity to calculate a summary measure of food allergy prevalence (I2 =91%, p<0.001). We then investigated the sources of this heterogeneity, and identified the question type and year of survey as significant sources of heterogeneity in the estimates. Specifically, 51% of between study variability was explained by method of identifying food allergy alone, while 88% of the variability was explained by the combination of method and year of survey administration. For example, compared to estimates of prevalence of self-reported current food allergy, the prevalence of self-reported history of food allergy ever was considerably higher, even after adjusting for year of study (difference: 2.5 percentage points between current and ever/time undefined food allergy, 95% CI: 1.5–3.4%, p<0.001 for all children).
Changes over time
The estimated prevalence of self-reported food allergy among children in the US increased significantly over the period from 1988–2011. After adjusting for method of defining food allergy, the self-reported prevalence of food allergy among children was estimated to have increased by 1.2 percentage points per decade during this time (95% CI: 0.7–1.6, see Figure 3). For example, within the NHIS survey, estimates of current food allergy increased from 3.4% (95% CI: 3.0–3.8%) in 2000 to 4.6% (95% CI: 4.2–5.1%) in 2010.
Figure 3.
Prevalence of food allergy over time. Individual dots reflect individual survey dates. The size of the dots is proportionate to the number of subjects surveyed.
Relationship between race/ethnicity and food allergy prevalence
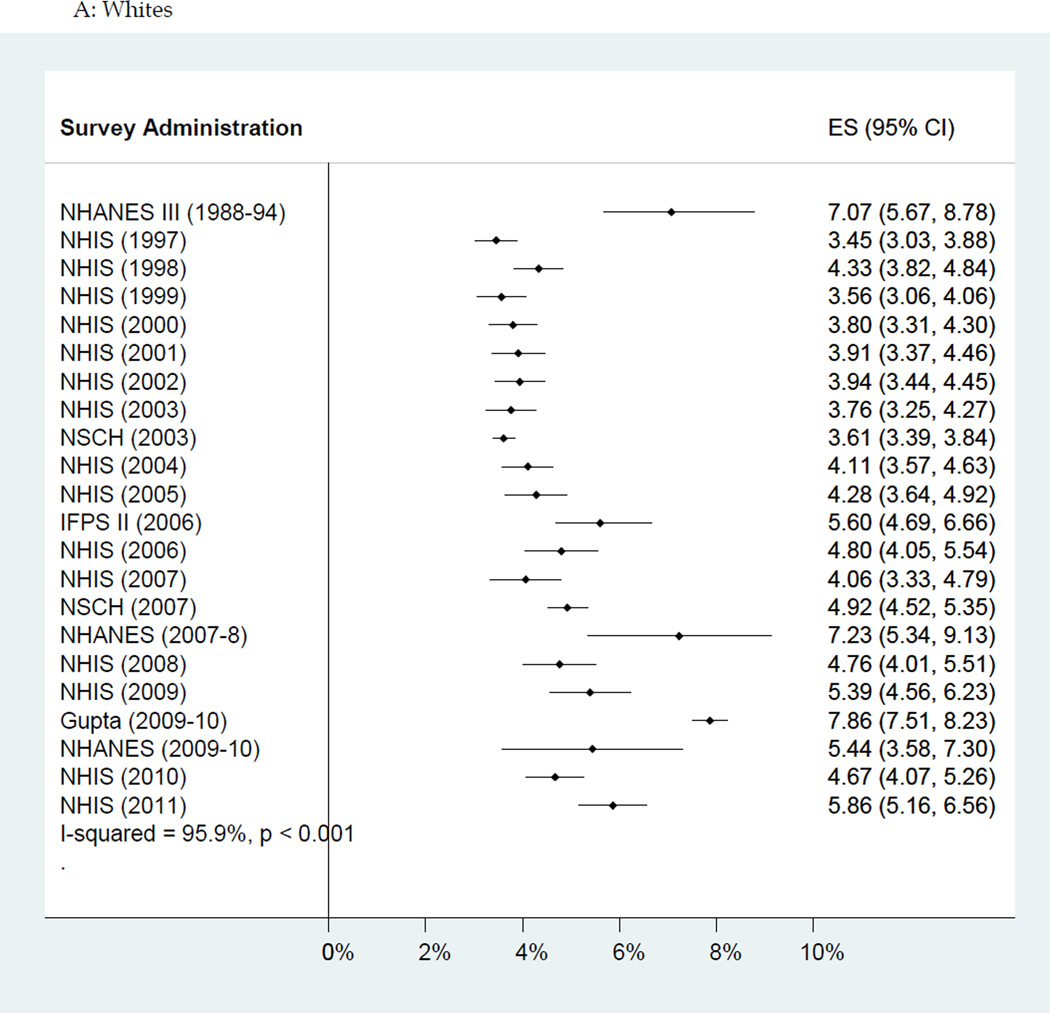
The rate of increase in estimated food allergy prevalence varied significantly by race/ethnicity (see Figure 4); the estimated increase in food allergy prevalence per decade among Black children was 2.1 percentage points (95% CI: 1.5–2.7%) compared to 1.2 percentage points among Hispanics (95% CI: 0.7–1.7%) and 1.0 percentage points (95% CI: 0.4–1.6%) among Whites (p=0.01 for comparison of trends between Blacks and Whites, and p=0.04 for comparison between Blacks and Hispanics). As an example of this trend, Blacks tended to report less food allergy than Whites until approximately the late 2000s, when Blacks began to report food allergy more frequently in nearly all surveys.
Figure 4.
Forest plot of self-report of current food allergy by ethnicity, ordered by time; surveys done repeatedly are included separately by year of survey. A: Non-Hispanic Whites. B: Non- Hispanic Blacks. C: Hispanics. Abbreviations: ES: Estimate, NHANES: National Health and Nutrition Examination Survey, NHIS: National Health Interview Survey, NSCH: National Survey of Children’s Health, IFPS II: Infant Feeding Practices Study II.
Sensitivity Analyses
To assess the effect of excluding surveys that did not meet the quality criteria, we also did analyses that included the three survey administrations that had been excluded in the primary analyses14,22,31 (the IFPS II survey, the Gupta survey and NHANES 2005–6). For the NHANES 2005–6 survey, definitions of allergy according to Liu et al. were used. The overall estimate of change over time did not change appreciably: instead of an estimate of 1.2 percentage point increase/decade, the estimate was 1.3 percentage points/decade (95% CI: 0.5–2.2). Between ethnicity differences were more marked: in this analysis Blacks had a 3.5 percentage point increase per decade (95% CI: 2.4–4.6%), compared to 1.4 percentage points (95% CI: 0.9–1.9%) among Hispanics (95% CI: 0.9–1.9) and 1.1 percentage points (95% CI: 0.4–1.9%) among Whites (p=0.002 for comparison of trends between Blacks and Hispanics, and p<0.001 for comparison between Blacks and Whites).
Because the questionnaire structure for NHANES III was distinctive from the other surveys in that it asked about history of reactions to foods but did not use the words “food allergy”, we also did a sensitivity analysis excluding NHANES III from the meta-regression. These analyses yielded similar results as the main analyses: in this analysis, change over time was 1.4 percentage points/decade (95% CI: 0.9–1.8), the rate of increase among Blacks was 2.2 percentage points (95% CI: 1.4–2.9%) compared to 1.3 percentage points among Hispanics (95% CI: 0.8–1.8%) and 1.2 percentage points (95% CI: 0.7–1.8%) among Whites (p=0.03 for comparison between Blacks and Hispanics and p=0.01 for comparison of trends between Blacks and Whites).
Discussion
In this study, we identified twenty-seven surveys that asked about food allergy in the United States. Due to substantial heterogeneity in survey design, we were unable to arrive at a point estimate for current prevalence of self-reported food allergy in US children, although it appears that the prevalence of self-reported food allergy is between three and six percent. However, when we examined temporal trends by race/ethnicity using the 20 surveys administered by the CDC, we did find clear evidence of increasing rates of self-reported food allergy among children, with significant racial/ethnic disparities in the rate of increase. The combined data suggest that the rate of self-reported prevalence of food allergy has increased by more than 1 percentage point per decade. Interestingly, the data show that the rate of increase of self-reported food allergy was twice as high among Blacks as among Whites.
The very few systematic reviews of self-reported food allergy prevalence previously conducted had important limitations that we were able to address here1–3,33. First, previous studies analyzed data from a wide geographical range, although it is well known that substantial differences in the rate of allergy exist between regions of the world34. By restricting our analyses to the US, we are able to more meaningfully assess trends over time and differences between sub-populations. Second, previous systematic reviews did not account for differences in wording of questions, a factor that contributed considerably to heterogeneity here, and prevented us from presenting a summary measure of self-reported prevalence. Third, they did not account for date of survey administration in their estimates, another large source of heterogeneity, and here we clearly demonstrate significant changes over time in prevalence. Finally, previous systematic reviews did not include the CDC and FDA sponsored surveys of the US population, although there is a wealth of data available in these surveys. Here we examined the full range of publically available data and because we took a comprehensive approach to identifying sources of data, both published and unpublished, our analysis is also unlikely to be influenced by publication bias. Our results showing increases in self-reported food allergy prevalence over time are consistent with more geographically localized studies of peanut allergy in the US35, and with longitudinal data on peanut allergy in the United Kingdom36 and Canada37, although in the United Kingdom, increases over the 2000s were not seen36. Together, these data point to an alarming increase in this disease, one that could cause more and more societal impact if current trends continue. Moreover, although much attention is given to racial/ethnic disparities in asthma, relatively little research has been focused on racial/ethnic disparities in food allergy, despite the rapid increase in self-report of this disease among Blacks.
Because the data are all based on self-report, we cannot distinguish between true increases in food allergy prevalence, increased recognition of true food allergy, and/or greater awareness resulting in over diagnosis of food allergy, a phenomenon that is clearly documented38. None of the studies included oral food challenges, the gold standard for diagnosis of food allergy39. Previous analyses have shown that self-report of food allergy overestimates the true rate of clinical disease40–43. Nonetheless, although food challenges are the gold standard for diagnosis of food allergy, they are not feasible in large epidemiologic studies for both practical and potential ethical reasons, and so questionnaires are the best data that we currently have and are useful for examining changes over time.
Here we show that the nature of what was being estimated (current allergy vs. history of allergy/no timeframe defined) was a substantial source of heterogeneity in estimates of food allergy prevalence. We did not have enough data to examine the effects of further variation in specific wording on prevalence estimates. Questions varied substantially, from wording designed to identify the symptoms consistent with an IgE mediated food allergy (such as done by Sicherer, Gupta and in NHANES III), to much more general questions, such as those asking for doctor diagnosis of “food or GI/digestive allergy” (such as asked in NHIS and NCHS) to simply “do you have any food allergies?” (NHANES 2007–8 and NHANES 2009–10). In general, the data point to the need for clarity about what we are estimating when we report prevalence, and to the need for the development of validated and easily administered methods that can accurately estimate clinical disease.
Although it is known that there are large disparities in asthma prevalence and severity by race/ethnicity, the role of race/ethnicity as a risk factor for food allergy has not been well established. Here we show that while the current prevalence of self-reported food allergy may be higher among Blacks than Whites or Hispanics, this has not always been the case. We demonstrate a more rapid increase in the rate of self-reported food allergy among Blacks over the past several decades than among Whites or Hispanics. Racial/ethnic disparities have also been seen in the few studies done that looked at specific food allergies 13,20.
In general, Blacks have higher total IgE levels than White and Hispanic Americans and genetic risk factors have been implicated in these differences. A higher proportion African Ancestry has been associated with higher risk of sensitization to foods among young children, even among those self-identified as Black44–47. Consistent with this observation, the estimated differences in food allergy between Blacks and Whites are substantially higher for IgE than for self-report. Specifically, in NHANES 2005–6, based on IgE, food allergy prevalence was 8.4% among Black children, compared to 2.5% among Whites and 4.2% among Hispanics [data not shown]22. At the same time, in NHIS, self-reported food allergy prevalence was 3.9–4.6% among Blacks, 4.3–4.8% among Whites and 3.1–2.5% among Hispanics29. Thus, despite this apparent genetic predisposition towards higher total and specific IgE levels, before the mid-2000s Blacks reported food allergy at rates equal to or lower than Whites. This suggests that either there are biologic differences in how specific IgE and clinical symptoms relate between racial/ethnic groups, or that recognition of food allergy has lagged among Blacks. However, because we do not have food-specific IgE data from multiple time points, we cannot distinguish between lagging recognition of food allergy among Blacks and a real increase in the rate of allergic disease, perhaps as a result of heightened susceptibility to some changing environmental factor48. Because of the limitations of meta-regression, which is a study-level analysis, we are not able to assess how much of the apparent racial/ethnic disparities are due to socio-economic factors. Both Whites and Hispanics also showed an increase in food allergy prevalence, although at a slower rate than Blacks, suggesting that whatever is driving this increase is not isolated to Blacks. Further investigation should be focused on substantiating the disparity in rate of increase of food allergy in Blacks, and in examining whether increases can be explained by other changes, such as access to care, dietary changes or other environmental factors. These investigations will not only identify at risk populations, but also help elucidate the mechanisms of food allergy.
After accounting for differences in survey questionnaire design in this comprehensive synthesis of the available data on self-reported food allergy in US children, we find a marked increase of self-reported food allergy of approximately 1 percentage point per decade that shows no signs of abating. Whether the apparent rise in prevalence of food allergy is real not only has important implications for our understanding of the pathophysiology of food allergy, but also for whether we should be allocating resources preferentially to prevention and treatment or to improving the accuracy of diagnosis. In addition, Blacks have had a disproportionate increase in self-reported food allergy, highlighting an underappreciated and emerging health disparity. From this wealth of survey data, it is clear that that more parents believe that their children have food allergies than ever, and that disparities are growing. Whether this represents a true biological increase, and if so, why, are urgent questions to answer.
Supplementary Material
Acknowledgments
Funding Source: The study described was made possible in part by Grant Numbers 1KL2RR025006-01(to CAK) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, and by Grant 1K23AI103187-01 (to CAK). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overviewtranslational.asp.
Abbreviations
- CDC
Centers for Disease Control
- CI
Confidence Interval
- ES
Estimate
- NHANES
National Health and Nutrition Examination Survey
- NHIS
National Health Interview Survey
- NMIHS
National Maternal and Infant Health Study
- NSCH
National Survey of Children’s Health
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no financial disclosures relevant to this manuscript.
Conflict of Interest: Jessica Savage has received research support from the American Academy of Allergy, Asthma and Immunology and the Food Allergy and Anaphylaxis Network. Robert Wood discloses consulting with the Asthma and Allergy Foundation of America, royalties from Up-to-Date, and service on the Medical Advisory Board to the Food Allergy and Anaphylaxis Network. Elizabeth Matsui discloses receipt of a monetary award from ThermoFisher. Corinne Keet, Shannon Seopaul and Roger Peng have no pertinent disclosures.
Contributor’s Statement:
Corinne A. Keet: Dr. Keet conceptualized and designed the study, performed data collection, carried out the analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
Jessica H. Savage: Dr. Savage contributed to the design of the study, performed data collection and analysis, critically reviewed the manuscript and approved the final manuscript as submitted.
Shannon Seopaul: Ms. Seopaul performed data collection and analysis, critically reviewed the manuscript and approved the final manuscript as submitted.
Roger D. Peng: Dr. Peng contributed to the design of the study, advised on the analyses, created figures, critically reviewed the manuscript, and approved the final manuscript as submitted.
Robert A. Wood: Dr. Wood contributed to the design of the study, critically reviewed the manuscript and approved the final manuscript as submitted.
Elizabeth C. Matsui: Dr. Matsui contributed to the design of the study, performed data collection and analysis, contributed to the analysis of the study, critically reviewed the manuscript and approved the final manuscript as submitted.
Contributor Information
Corinne A. Keet, Email: ckeet1@jhmi.edu, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, and Graduate Student, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Baltimore, MD.
Jessica H. Savage, Email: jessicahsavage@gmail.com, Brigham and Women's Hospital, Division of Rheumatology, Immunology, and Allergy, Boston, MA.
Shannon Seopaul, Email: sseopau1@jhmi.edu, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, Baltimore, MD.
Roger D. Peng, Email: rpeng@jhsph.edu, Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD.
Robert A. Wood, Email: rwood@jhmi.edu, Johns Hopkins University School of Medicine, Division of Allergy and Immunology, Department of Pediatrics, Baltimore, MD.
Elizabeth C. Matsui, Email: ematsui@jhmi.edu, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, Baltimore, MD.
References
- 1.Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: A meta-analysis. Journal of Allergy and Clinical Immunology. 2007;120(3):638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: A systematic review. JAMA - Journal of the American Medical Association. 2010;303(18):1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 3.Greenhawt MWC, Conte ML, Doucet M, Engler A, Carmargo CA. Racial and Ethnic Disparity in Food Allergy in the United States: A Systematic Review. Journal of Allergy and Clinical Immunology: In Practice. 2013 Jul;1(4):378–386. doi: 10.1016/j.jaip.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Shoshan M, Turnbull E, Clarke A. Food allergy: temporal trends and determinants. Curr Allergy Asthma Rep. 2012 Aug;12(4):346–372. doi: 10.1007/s11882-012-0274-3. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007 Jan;62(1):91–96. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007 Sep;120(3):491–503. doi: 10.1016/j.jaci.2007.07.015. quiz 504-495. [DOI] [PubMed] [Google Scholar]
- 7.Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011 Mar;22(2):155–160. doi: 10.1111/j.1399-3038.2011.01145.x. [DOI] [PubMed] [Google Scholar]
- 8.Katz Y. Food allergy epidemic: can we reverse the trend? Isr Med Assoc J. 2012 Jan;14(1):5–6. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [Accessed 2/15/2012];Data & Statistics. 2012. 2012 http://www.cdc.gov/datastatistics/
- 10.Centers for Disease Control and Prevention. [Accessed Feburary 15, 2012];National Center for Health Statistics. 2012 http://www.cdc.gov/nchs/.
- 11.Branum AM, Simon AE, Lukacs SL. Among children with food allergy, do sociodemographic factors and healthcare use differ by severity? Matern Child Health J. 2012 Apr;16(Suppl 1):S44–S50. doi: 10.1007/s10995-012-1009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Journal of Allergy and Clinical Immunology. 2013 Nov;132(5):1216–1219. e5. doi: 10.1016/j.jaci.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, sesame allergy: 11-year follow-up. Journal of Allergy and Clinical Immunology. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton SC, Adams JL, Suttorp MJ, Shekelle PG. Meta-regression Approaches: What, Why, When and How? AHRQ Technical Reviews. 2004 Mar; Report No: 04-0033. [PubMed] [Google Scholar]
- 17.Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114(1):27–32. doi: 10.1542/peds.114.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Sicherer SH, Munoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. Journal of Allergy and Clinical Immunology. 1999;103(4):559–562. doi: 10.1016/s0091-6749(99)70224-1. [DOI] [PubMed] [Google Scholar]
- 19.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. Journal of Allergy and Clinical Immunology. 2004;114(1):159–165. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. Journal of Allergy and Clinical Immunology. 2003;112(6):1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 21.Victorino CC, Gauthier AH. The social determinants of child health: variations across health outcomes - a population-based cross-sectional analysis. BMC Pediatrics. 2009;9:53. doi: 10.1186/1471-2431-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: Results from the National Health and Nutrition Examination Survey 2005–2006. Journal of Allergy and Clinical Immunology. 2010;126(4):798–806. e713. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. Vital Health Stat 10. 2010 Dec;(247):1–82. [PubMed] [Google Scholar]
- 24.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124(6):1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 25.CDC/National Center for Health Statistics. [Accessed 8/27/2012];About the National Health and Nutrition Examination Survey. 2011 http://www.cdc.gov/nchs/nhanes.htm.
- 26.Keet CA, Matsui EC, Savage JH, et al. Potential mechanisms for the association between fall birth and food allergy. Allergy. 2012 Jun;67(6):775–782. doi: 10.1111/j.1398-9995.2012.02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services. Design and Operation of the National Survey of Children's Health 2007. DHHS Publication No. (PHS) 2012-1331. 2012 Jun; 2012.
- 28.Blumberg SJOL, Frankel MR, Osborn L, Srinath KP, Giambo P. Design and Operation of the National Survey of Children's Health, 2003. Vital Health Stat 1. 2005;(43) [PubMed] [Google Scholar]
- 29.CDC/National Center for Health Statistics. [Accessed August 22, 2012];About the National Health Interview Survey. 2012 http://www.cdc.gov/nchs/nhis/about_nhis.htm.
- 30.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Stat. 2011;10(250) [PubMed] [Google Scholar]
- 31.Luccioli S, Ross M, Labiner-Wolfe J, Fein SB. Maternally reported food allergies and other food-related health problems in infants: Characteristics and associated factors. Pediatrics. 2008;122(SUPPL. 2):S105–S112. doi: 10.1542/peds.2008-1315n. [DOI] [PubMed] [Google Scholar]
- 32.Keet CA, Wood RA, Matsui EC. Limitations of reliance on specific IgE for epidemiologic surveillance of food allergy. J Allergy Clin Immunol. 2012 Nov;130(5):1207–1209. e1210. doi: 10.1016/j.jaci.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuidmeer L, Goldhahn K, Rona RJ, et al. The prevalence of plant food allergies: A systematic review. Journal of Allergy and Clinical Immunology. 2008;121(5):1210–1218. e1214. doi: 10.1016/j.jaci.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Venter C, Arshad SH. Epidemiology of food allergy. Pediatr Clin North Am. 2011 Apr;58(2):327–349. ix. doi: 10.1016/j.pcl.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Rinaldi M, Harnack L, Oberg C, Schreiner P, St Sauver J, Travis LL. Peanut allergy diagnoses among children residing in Olmsted County, Minnesota. J Allergy Clin Immunol. 2012 Oct;130(4):945–950. doi: 10.1016/j.jaci.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venter C, Hasan Arshad S, Grundy J, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010 Jan;65(1):103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Shoshan M, Kagan RS, Alizadehfar R, et al. Is the prevalence of peanut allergy increasing? A 5-year follow-up study in children in Montreal. J Allergy Clin Immunol. 2009 Apr;123(4):783–788. doi: 10.1016/j.jaci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011 Apr;158(4):578–583. e571. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010 Dec;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987 May;79(5):683–688. [PubMed] [Google Scholar]
- 41.Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011 Mar;127(3):668–676. e661–e662. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 42.Eggesbo M, Botten G, Halvorsen R, Magnus P. The prevalence of CMA/CMPI in young children: the validity of parentally perceived reactions in a population-based study. Allergy. 2001 May;56(5):393–402. doi: 10.1034/j.1398-9995.2001.056005393.x. [DOI] [PubMed] [Google Scholar]
- 43.Host A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990 Nov;45(8):587–596. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 44.Salari K, Choudhry S, Tang H, et al. Genetic admixture and asthma-related phenotypes in Mexican American and Puerto Rican asthmatics. Genet Epidemiol. 2005 Jul;29(1):76–86. doi: 10.1002/gepi.20079. [DOI] [PubMed] [Google Scholar]
- 45.Torgerson DG, Gignoux CR, Galanter JM, et al. Case-control admixture mapping in Latino populations enriches for known asthma-associated genes. J Allergy Clin Immunol. 2012 Jul;130(1):76–82. e12. doi: 10.1016/j.jaci.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet. 2009 Jun;125(5–6):565–579. doi: 10.1007/s00439-009-0649-2. [DOI] [PubMed] [Google Scholar]
- 47.Flores C, Ma SF, Pino-Yanes M, et al. African ancestry is associated with asthma risk in African Americans. PLoS One. 2012;7(1):e26807. doi: 10.1371/journal.pone.0026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc. 2010 Nov;11(9):617–628. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.