Abstract
Purpose of review
Kidney transplantation remains the optimal treatment for children with end-stage renal disease; yet, in the United States, profound differences in access to transplant persist, with black children experiencing significantly reduced access to transplant compared with white children. The reasons for these disparities remain poorly understood. Several recent studies provide new insights into the interplay of socioeconomic status, racial/ethnic disparities and access to pediatric kidney transplantation.
Recent findings
New evidence suggests that disparities are more pronounced in access to living vs. deceased donors. National allocation policies have mitigated racial differences in pediatric deceased donor kidney transplant (DDKT) access after waitlisting. However, disparities in access to DDKT are stark for minority emerging adults, who lose pediatric priority allocation. Although absence of health insurance poses an important barrier to transplant, even after adjustment for insurance status and neighborhood poverty, disparities persist. Differential access to care and unjust social structures are posited as important modifiable barriers to achieving equity in pediatric transplant access.
Summary
Future approaches to overcome disparities in pediatric kidney transplant access must focus on the continuum of the transplant process, including equitable health care access. Public health advocacy efforts to promote national policies that address disparate multilevel socioeconomic factors are essential.
Keywords: disparities, pediatric, transplant
INTRODUCTION
Racial disparities in access to kidney transplantation in the United States are well recognized among adults and the causes are multifactorial, including biologic, socioeconomic and cultural factors [1]. Fewer studies have examined disparities in pediatric end-stage renal disease (ESRD); however, findings parallel adult studies, with black (vs. white) children experiencing reduced access to deceased donor waitlisting [2] as well as lower rates of preemptive [3] and living donor transplants [4]. The root causes of disparities in pediatric kidney transplantation remain elusive [2,4–7]. This review highlights several articles published in 2012 that specifically address the interplay of race/ethnicity, socioeconomic status (SES) and biology and consider the impact of social structures and health care systems on access to kidney transplantation along the continuum of the transplant process.
THE CHANGING LANDSCAPE OF PEDIATRIC KIDNEY TRANSPLANTATION
Over the last decade, the demographics of pediatric ESRD and the distribution of living donor vs. deceased donor transplants have changed. The proportion of children with ESRD (<18 years) who are Hispanic has risen from 19.9% in 2000 to 26.7% in 2010 [8]. In addition, in September 2005, the United Network for Organ Sharing instituted the Share 35 allocation policy to provide priority allocation of organs from deceased donors younger than 35 years to pediatric kidney transplantation waiting list candidates younger than 18 years of age [9]. This policy was designed to promote the timely allocation of high-quality deceased donor organs to children. The policy was temporally associated with greater human leukocyte antigen (HLA) mismatching and a substantial shift in the distribution of donor source toward a greater proportion of deceased donor organs [10■■,11–13]. This review will consider racial and ethnic disparities in the context of the continuum of the transplant process, from referral to donor selection to transplantation and transplant survival. The role of national public health policies in reducing these disparities will also be addressed.
RACIAL DIFFERENCES IN THE STEPS TO PEDIATRIC KIDNEY TRANSPLANT
Transplantation is a process starting with recognition of renal disease progression and ending with long-term care of patients and allografts. There are many steps in between, including referral, evaluation, listing and organ receipt. Each step may pose distinct barriers to achieving the desired outcome of transplantation and must be considered singularly as a place where disparities may arise.
Recently, Patzer et al. [14■■] sought to better characterize along which steps in the pediatric kidney transplantation process racial disparities occur.
The authors examined racial/ethnic differences in overall time from ESRD start to deceased donor kidney transplant (DDKT) among incident ESRD patients younger than 21 years in the United States, 2000–2008. Two main substeps were examined, including access to the waiting list after incident ESRD, and access to DDKT among waitlisted patients. The authors also compared subjects 0–17 years with emerging adults 18–20 years.
In models adjusted for clinical, demographic and SES factors (including insurance and neighborhood poverty), black (vs. white) children (0–17 years) were 10% less likely to proceed from incident ESRD to DDKT, including lower rates of waitlisting and DDKT once waitlisted (Table 1) [14■■]. Hispanic (vs. white) children younger than 18 years experienced lower probability of proceeding from incident ESRD to DDKT, but had a similar rate of moving from waitlisting to DDKT. Importantly, Hispanic access to waitlisting after incident ESRD varied significantly by insurance status; uninsured Hispanics were 43% less likely to waitlist at any given time compared with uninsured whites.
Table 1.
Racial differences in the probability of accessing deceased donor kidney transplantation, from incident end-stage renal disease to waitlisting to transplant, among patients <21 years of age
| Hazard ratios (95% confidence intervals) |
||
|---|---|---|
| Transplant steps | Age 0-17 | Age 18-20 |
| Steps 1 and 2: Overall time from ESRD start to deceased donor transplantationa | ||
| Black versus white | ||
| Model 1: Crude | 0.89 (0.80-0.98) | 0.51 (0.42-0.62) |
| Model 2: clinical + demographic factors | 0.88 (0.79-0.98) | 0.55 (0.45-0.68) |
| Model 3: clinical + demographic + SES factors | 0.90 (0.80-1.00) | 0.60 (0.48-0.75) |
| Hispanic versus white | ||
| Model 1: Crude | 0.94 (0.85-1.03) | 0.45 (0.37-0.56) |
| Model 2: clinical + demographic factors | 0.91 (0.82-1.01) | 0.59 (0.47-0.75) |
| Model 3: clinical + demographic + SES factors | 0.91 (0.81-1.02) | 0.62 (0.48-0.79) |
| Step 1: time from ESRD start to waitlistinga | ||
| Black versus white | ||
| Model 1: Crude | 0.94 (0.87-1.02) | 0.72 (0.65-0.81) |
| Model 2: clinical + demographic factors | 0.92 (0.85-1.01) | 0.78 (0.68-0.84) |
| Model 3: clinical + demographic + SES factors | 0.92 (0.84-1.01) | 0.84 (0.74-0.96) |
| Hispanic versus white: (pooled estimates N/A)* | ||
| Step 2: time from waitlisting to deceased donor transplantationb | ||
| Black versus white | ||
| Model 1: Crude | 0.84 (0.76-0.93) | 0.53 (0.44-0.64) |
| Model 2: clinical + demographic factors | 0.92 (0.83-1.02) | 0.60 (0.50-0.81) |
| Model 3: clinical + demographic + SES factors | 0.89 (0.80-0.99) | 0.61 (0.49-0.76) |
| Hispanic versus white | ||
| Model 1: Crude | 0.79 (0.71-0.87) | 0.52 (0.42-0.64) |
| Model 2: clinical + demographic factors | 0.98 (0.88-1.09) | 0.65 (0.50-0.85) |
| Model 3: clinical + demographic + SES factors | 0.97 (0.86-1.08) | 0.66 (0.51-0.86) |
From Ref [14]. ESRD, end-stage renal disease.
Model 2 adjusted for age, sex, ESRD etiology, BMI, Share35 era, OPO region, blood type, ESA albumin, hemoglobin and PPRA. Model 3 adjusted for all factors in Model 2 plus zip code poverty and insurance.
Model 2 adjusted for age, sex, ESRD etiology, BMI, Share35 era, OPO region, blood type, ESA, albumin and hemoglobin. Model 3 adjusted for all factors in Model 2 plus zip code poverty and insurance.
Multivariable P-value for interaction between race and insurance P< 0.05 for both age groups.
Limited access to insurance posed a significant barrier to waitlisting for Hispanics but not for blacks. The reasons for this are unclear. Recent studies suggest that, among Hispanic children, language barriers and immigration status pose unique barriers to accessing insurance coverage, having a medical home and attaining subspecialty referrals [15–18]. These factors may preclude the coordinated medical care needed for waitlisting.
Another distinctive finding from this study is that disparities worsen for emerging adults. Among subjects 18–20 years of age, in multivariable models, blacks (vs. whites) were 40% less likely to proceed from incident ESRD to DDKT, including 16% less likely to waitlist and 39% less likely to move from the waiting list to DDKT (Table 1). Similarly, Hispanics (vs. whites) were significantly less likely to proceed from ESRD to DDKT and from waitlisting to DDKT. Again, insurance status significantly impacted the probability of Hispanic access to the waiting list. Emerging adults have previously been recognized as a group at heightened risk for allograft failure [19,20], but little is known about their kidney transplantation access in comparison with younger patients. In the United States, young adults are more likely to lack health insurance coverage and a usual source of medical care compared with older adults [21,22]. Although in Patzer's study there was a higher proportion of uninsured subjects 18–20 years old vs. 0–17 years, insurance status did not explain the observed disparity.
Patzer et al. note several differences in the medical treatment received across racial/ethnic groups. First, blacks and Hispanics had more anemia but less use of erythropoietin-stimulating agents preceding diagnosis of incident ESRD. In 2005, the CMS 2728 form was revised to capture several new data elements, including access to pre-ESRD nephrology care. Among the ESRD patients younger than 20 years from 2005 to 2008, only 53.2% Hispanics had pre-ESRD care vs. 63.3% blacks and 68.7% whites. Additionally, among DDKT waitlisted candidates, 21.9% of whites were waitlisted preemptively vs. only 10.9% of Hispanics and 12.8% of blacks. Preemptive kidney transplantation requires early recognition of kidney disease progression and thus necessitates early access to care. In a study of 111 children in Austria, those with at least 12 months of pre-ESRD nephrology care were significantly more likely to receive preemptive kidney transplantation (odds ratio 2.5; 95% confidence interval (CI): 1.06–5.91) vs. those with less than 1 year of care [23].
Further, the authors note racial disparities in access to living donor kidney transplantation (LDKT), such that blacks were 69% less likely [hazard ratio (HR) 0.31; 95% CI: 0.27–0.34] and Hispanics 53% less likely (HR 0.47; 95% CI: 0.43–0.52) to receive LDKT at any given time compared with whites. In adults, many barriers to living donation among minority patients have been cited, including donor factors, such as preexisting medical conditions, fear of surgery and financial concerns, and recipient factors, such as reluctance to ask potential donors, distrust of the medical community and lack of awareness about living donor benefits [24]. In addition, poverty has long been recognized as an adverse social determinant of health [25–27]. No studies have specifically examined barriers to living donation among family members of children with ESRD, though there are many stressors associated with poverty that might pose unique barriers to attaining a living donor source for a child in need of a transplant [26]. Although health care reform and the expansion of public insurance coverage for children have increased health care use and reduced out-of-pocket health care spending for low-income families [28], it has not eliminated household financial hardship. A recent study suggests that public insurance access does not significantly improve other deeply seeded problems of poverty, such as food insecurity and housing problems [29]. Low-income families may be particularly vulnerable to the financial burdens of living donation, as they are more likely to lack substantial savings [30].
Lastly, Patzer et al. showed that even after pediatric minority patients overcame the hurdle of placement on the waiting list, they remained at a significant disadvantage for obtaining a DDKT. This finding persisted in a substudy that excluded patients listed inactively. Neither clinical, demographic nor SES factors appeared to influence transplant access once patients were waitlisted. National data regarding transplant offers and refusals after waitlisting are limited. Thus, many questions persist regarding the context of reduced transplant access for minority children after waitlisting.
THE ROLE OF NATIONAL ALLOCATION POLICIES IN DISPARITIES
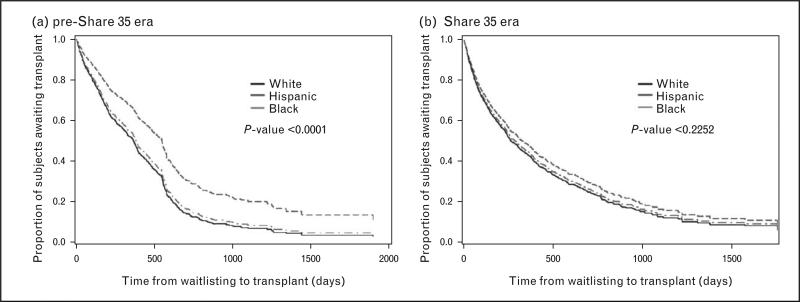
In 2012, Amaral et al. [10■■] examined the effects of the Share 35 policy on racial/ethnic disparities in kidney transplantation access. Access to DDKT improved for all children with ESRD, but improvements varied by race/ethnicity (Fig. 1). The probability of receiving a DDKT in the Share 35 (vs. pre-Share 35) era increased 81% for Hispanics (HR 1.81, 95% CI 1.48–2.21) vs. 45% for blacks and 37% for whites. In the Share 35 era (vs. pre-Share 35), Hispanics also received DDKT 201 days earlier after waitlisting (vs. 63 days earlier for whites and 90 days earlier for blacks).
FIGURE 1.
Comparison of racial differences in time to deceased donor kidney transplant in the pre-Share 35 vs. Share 35 eras, for children with ESRD. Adapted with permission [10■■].
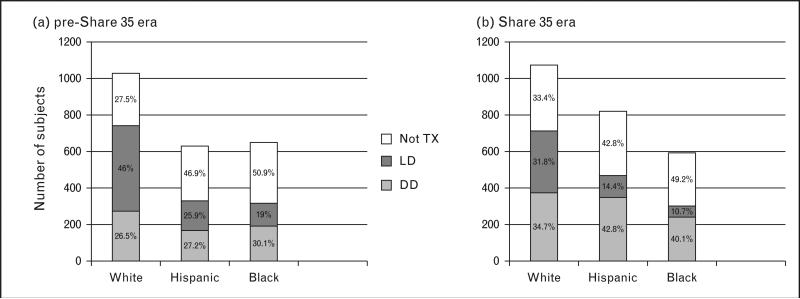
The authors also examined whether children were receiving equal quality donors. They noted that in the pre-Share 35 era, Hispanics and blacks received higher degrees of HLA mismatching; however, after Share 35, there were no longer significant racial/ethnic differences in HLA mismatching. This disparity reduction resulted primarily from a 46% increase in the proportion of whites receiving an HLA mismatch of five or six (39.7% pre-Share 35 vs. 57.8% Share 35). Additionally, there was a shift from LDKT to DDKT for all races after Share 35; however, the shift was greater for Hispanics and blacks, with a 25% reduction in living donor for whites vs. 48% reduction for Hispanics and 46% reduction for blacks (Fig. 2) [10■■]. Lastly, the overall proportion of white incident ESRD patients who were transplanted (living donor or deceased donor) in the Share 35 era (vs. pre-Share 35) decreased primarily because of declines in living donor rates that were not compensated by increases in deceased donor rates, whereas the proportion of incident ESRD patients transplanted in the Share 35 era (vs. pre-Share 35) increased for Hispanics and remained stable for blacks (Fig. 2). It is unknown why this shift in donor source occurred and why it varied by race. The authors speculate that by decreasing barriers to DDKT, patients and/or providers may have been less incentivized to attempt to overcome the barriers associated with obtaining living donors.
FIGURE 2.
Contrasts in the distribution of donor source and overall access to kidney transplant by race for pediatric patients in the pre-Share 35 vs. Share 35 eras. Adapted with permission [10■■].
The present study highlights that when racial disparities in transplant access are examined, one must consider the full context of organ availability and suitability – not just time to organ receipt but also quality of organs. In addition, an important goal of reducing disparities is to distribute opportunities for optimal health equitably, regardless of race. Although Share 35 shortened pediatric wait times across races, the policy may not have been as effective in providing high-quality donors to all pediatric candidates. Mitigating racial differences by redistributing a greater proportion of HLA mismatching among whites receiving DDKT and shifting from living donor to deceased donor for all races may have deleterious long-term outcomes on graft survival [31,32].
THE IMPACT OF THE SOCIAL ENVIRONMENT IN RACIAL DISPARITIES IN PEDIATRIC KIDNEY TRANSPLANT ACCESS
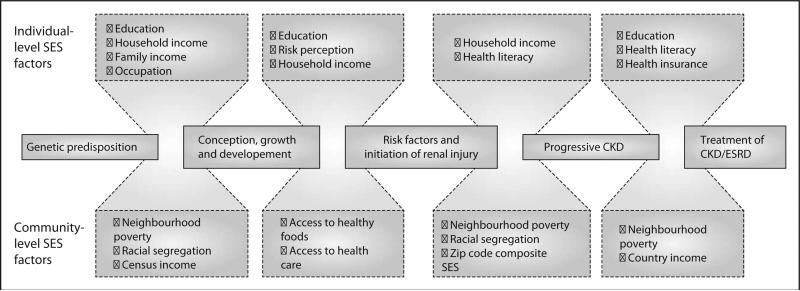
These recent studies [10■■,14■■] create a framework of contextual SES factors, which are hypothesized to inhibit opportunities for pediatric kidney transplantation among racial and ethnic minorities. In a recent bioethics piece, Moseley and Kershaw [33■] posit that the primary cause of disparities in pediatric kidney transplantation access between blacks and whites is ‘a social environment that limits the availability of suitable organs and makes adherence more difficult for (black families)’ compared with white (families). The authors delineate that health disparities are created when the resources available to achieve optimal health are unequally distributed. They emphasize that social structures, such as beliefs, attitudes and public policies, can substantially influence the distribution of ‘resources necessary to pursue a healthy lifestyle’ [33■]. The disparate distribution of these resources, including education, wealth, and employment, is the social determinant of health that influences the development of disease and subsequent access to healthcare. Patzer and McClellan [1] described a similar theoretical framework for racial/ethnic disparities in access to kidney transplantation for adults (Fig. 3).
FIGURE 3.
Conceptual model for health disparities in patients with kidney disease across the life span. CKD, chronic kidney disease; ESRD, end-stage kidney disease; SES, soicoeconomic status. Adapted with permission [1].
Moseley and Kershaw cite that a history of discrimination and racism has led to persistent societal bias in granting blacks access to desirable schools, jobs and neighborhoods [34]. Reduced access to these social benefits has resulted in a greater proportion of blacks living in poverty and experiencing reduced wealth, regardless of income [35]. The authors focus their examination of disparities on the lower rates of preemptive kidney transplantation for black vs. white children. Preemptive kidney transplantation, or transplantation prior to the need for dialysis initiation, is considered the optimal ESRD therapy, as it circumvents the increased morbidity and mortality associated with dialysis [36–38]. Moseley and Kershaw hypothesize five reasons for lower rates of preemptive kidney transplantation among blacks—all of which are created by circumstances of poverty and reduced access to optimal health care.
First, they cite the need for early recognition of advanced kidney disease to avoid dialysis. They suggest that black (vs. white) children present to nephrology care later in their disease course. Patzer et al. noted less pre-ESRD care among blacks and Hispanics vs. white children; however, whether minority children present later to nephrology care has not been definitively answered. Early access to care may be particularly important for blacks who appear to be at higher risk for reduced renal function as early as infancy. Among 152 US children with mean age of 1.5 years, blacks had lower estimated glomerular filtration rate (eGFR) than nonblacks [39]. Lower birth weight was significantly associated with lower eGFR among black (P = 0.012) but not nonblack children (P = 0.33). Factors that impact low birth weight include fetal exposures preceding conception and throughout pregnancy. A recent study suggested that SES (as measured by maternal educational attainment, income and marital status) was the single most important driving force of racial gaps in low birth weight [40]. It is unclear to what extent these early eGFR differences impact racial disparities in kidney transplantation access in childhood and throughout life, but this lends support to the hypothesis that the mechanisms by which SES influences disparities may begin early in life [1].
A second hypothesis for lower preemptive transplant among blacks vs. whites is family preference or distrust in the transplant process. Historical reports of black vs. white adults on dialysis reported such concerns [41]; however, a recent pediatric study showed no difference by race or ethnicity in the percentage of children listed for DDKT because of family choice [7]. Further studies to explore family beliefs about preemptive kidney transplantation and donor source are needed.
Third, the authors propose that black children are less likely to complete the pretransplant evaluation. Adult data support this finding [42,43], but studies are lacking in pediatrics. Several adult transplant centers have instituted interventions to consolidate the transplant evaluation or provide additional support and education to promote evaluation completion [44–46]. These approaches show promise toward overcoming racial and SES disparities in the transplant evaluation process.
A fourth conceived explanation for disparities in access to preemptive kidney transplantation includes lower rates of transplant referral from nephrologists. Furth et al. [47] reported that nephrologists were more likely to refer white vs. black children, even when patients had similar profiles of nonadherence. Moseley and Kershaw point out that the social, economic and health stressors associated with poverty may undermine the ability to comply with the medical regimen.
Lastly, Moseley and Kershaw suggest that blacks have fewer suitable living donors. Blacks have higher rates of obesity, diabetes and hypertension compared with whites, which may lead to lower eligibility rates for living donation [24,41]. Loss to follow-up of potential donors after initial contact is much greater among blacks vs. whites [48]. Because living donation incurs lost wages and necessitates social support, interested donors who live in poverty or are single care providers may feel unable to donate despite willingness. The issue of less suitable donors for minority children has been posited frequently as a potential cause of disparities in living donation; however, there is presently no literature to support this theory.
Moseley and Kershaw propose that one solution to address unfair social structures in the United States is to improve access to high-quality education across socioeconomic strata. A recent study from Western Europe lends some support to this approach. Schoenmaker et al. showed that even when universal health care coverage is provided, stark disparities in pediatric kidney transplantation access are evident: minority children in Belgium and the Netherlands stay on dialysis longer and are more likely to receive DDKT vs. LDKT [49,50]. The authors found significant differences in the educational attainment of caregivers and posited education, cultural and language barriers as contributing factors to renal disease complications and the ability to adhere to medical regimens.
Moseley and Kershaw invoke bioethicists to promote institutional and national public health policies that ensure equitable distribution of health care services among all patients, regardless of SES. Preliminary evidence from adult studies supports the efficacy of institutional policies that reduce barriers to completion of the transplant evaluation [44–46]. Nationally, Moseley and Kershaw cite inequity in the cessation of Medicare coverage for immunosuppressive medications 3 years after transplant because blacks are more likely to be Medicare-dependent. This perspective is particularly important in the present era of health care reform when the level of support for Medicaid and Medicare recipients is tenuous [51,52].
CONCLUSION
Racial and ethnic disparities persist along multiple steps of the pediatric kidney transplantation process. SES factors, including health insurance, neighborhood poverty and access to pre-ESRD nephrology care, contribute to barriers toward optimal health for pediatric minorities with ESRD. These barriers appear to increase for emerging adults (18–20 years), in which disparities are more pronounced than among children. Recent national allocation policy changes may have reduced disparities in kidney transplantation access for patients younger than 18 years; however, there may have been unintended consequences that may impact donor quality and future health outcomes. Additional research is needed to identify and target the root causes of disparities along the continuum of transplant access, including identifying the residual disparities in kidney transplantation access after waitlisting. Future policies that increase health care coverage and provide opportunities for better access to care may represent the most sustainable interventions to reduce disparities for children with ESRD, particularly for minorities who are more likely to have lower SES.
KEY POINTS.
Recent studies that examine racial/ethnic differences in pediatric kidney transplant access suggest that social structures and the contextual factors of socioeconomic status play an important role in disparities.
Future approaches to reduce disparities in pediatric kidney transplant access must delineate the root causes of disparities along the continuum of the transplant process, from the time of renal disease recognition through the long-term follow-up of patients and allografts.
Public health policies must address the disparate distribution of resources needed for children with renal disease to attain optimal long-term health, including not only equitable access to health care but also equitable access to education, employment and healthy social environments.
Acknowledgements
S.A. serves as an At Large Member of the Pediatric Transplantation Committee and Kidney Transplantation Committee, Organ Procurement and Transplantation Network/United Network for Organ Sharing. S.A. is supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases 1K23DK083529–01A2. R.E.P. is supported in part by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number ULl TR000454 and KL2TR0004SS as well as a grant from the National Institute on Minority Health and Health Disparities under award number 1R24MD008077-01.
Footnotes
Conflicts of interest There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 365–366).
- 1.Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8:533–541. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furth SL, Garg PP, Neu AM, et al. Racial differences in access to the kidney transplant waiting list for children and adolescents with end-stage renal disease. Pediatrics. 2000;106:756–761. doi: 10.1542/peds.106.4.756. [DOI] [PubMed] [Google Scholar]
- 3.Omoloja A, Mitsnefes M, Talley L, et al. Racial differences in graft survival: a report from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Clin J Am Soc Nephrol. 2007;2:524–528. doi: 10.2215/CJN.03100906. [DOI] [PubMed] [Google Scholar]
- 4.Omoloja A, Stolfi A, Mitsnefes M. Racial differences in pediatric renal transplantation 24-year single center experience. J Natl Med Assoc. 2006;98:154–157. [PMC free article] [PubMed] [Google Scholar]
- 5.Seikaly MG, Salhab N, Browne R. Patterns and time of initiation of dialysis in US children. Pediatr Nephrol. 2005;20:982–988. doi: 10.1007/s00467-004-1803-7. [DOI] [PubMed] [Google Scholar]
- 6.Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011. 63:1988–1997. doi: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen S, Martz K, Stablein D, Neu A. Wait list status of pediatric dialysis patients in North America. Pediatr Transplant. 2011;15:376–383. doi: 10.1111/j.1399-3046.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 8.US Renal Data System . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. [Google Scholar]
- 9. [20 January 2013];Organ Procurement and Transplantation Network: Policies. http://optn.trans plant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf.
- 10■■.Amaral S, Patzer RE, Kutner N, McClellan W. Racial disparities in access to pediatric kidney transplantation since Share 35. J Am Soc Nephrol. 2012;23:1069–1077. doi: 10.1681/ASN.2011121145. [The national Share 35 policy to preferentially allocate donors younger than 35 years to children younger than 18 years resulted in improved access to kidney transplantation for all pediatric ESRD patients. In addition, the disparity gap in transplant access was somewhat reduced following Share 35 implementation, with Hispanics experiencing the greatest improvement in transplant access.] [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S, Oak N, Siddique J, et al. Changes in pediatric renal transplantation after implementation of the revised deceased donor kidney allocation policy. Am J Transplant. 2009;9:1237–1242. doi: 10.1111/j.1600-6143.2009.02608.x. [DOI] [PubMed] [Google Scholar]
- 12.Axelrod DA, McCullough KP, Brewer ED, et al. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am J Transplant 2010. 10:987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 13.Abraham EC, Wilson AC, Goebel J. Current kidney allocation rules and their impact on a pediatric transplant center. Am J Transplant. 2009;9:404–408. doi: 10.1111/j.1600-6143.2008.02504.x. [DOI] [PubMed] [Google Scholar]
- 14■■.Patzer RE, Amaral S, Klein M, et al. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? Am J Transplant. 2012;12:369–378. doi: 10.1111/j.1600-6143.2011.03888.x. [Racial and ethnic disparities in waitlisting and transplant access exist even after accounting for demographic, clinical and socioeconomic differences among patients, but disparities are mitigated among Hispanics who have access to private health insurance. These racial/ethnic disparities worsen as ESRD patients enter young adulthood (18–20 years), and are unexplained by insurance coverage.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chando S, Tiro JA, Harris TR, et al. Effects of socioeconomic status and healthcare access on low levels of human papillomavirus vaccination among Spanish-speaking Hispanics in California. Am J Public Health. 2013;103:270–272. doi: 10.2105/AJPH.2012.300920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avila RM, Bramlett MD. Language and immigrant status effects on disparities in Hispanic children's health status and access to healthcare. Matern Child Health J. 2012 doi: 10.1007/s10995-012-0988-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Conrey EJ, Seidu D, Ryan NJ, Chapman DS. Access to patient-centered medical home among Ohio's children with special healthcare needs. J Child Healthcare. 2012 doi: 10.1177/1367493512456111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Fisher-Owens SA, Isong IA, Soobader MJ, et al. An examination of racial/ ethnic disparities in children's oral health in the United States. J Public Health Dent. 2012 doi: 10.1111/j.1752-7325.2012.00367.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Government Accountability Office [2 March, 2013];Report GA0-07-1117. End-stage renal disease: characteristics of kidney transplant recipients, frequency of transplant failures, and cost to Medicare: United States Government Accountability Office. 2007 http://www.gao.gov/assets/270/267345.pdf.
- 20.Foster BJ, Dahhou M, Zhang X, et al. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011;92:1237–1243. doi: 10.1097/TP.0b013e31823411d7. [DOI] [PubMed] [Google Scholar]
- 21. [20 January 2013];Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, January-March 2012. www.cdc.gov/nchs/data/nhis/ earlyrelease/insur201209.pdf.
- 22.Bloom BC, Young RA. Adults seeking medical care: do race and ethnicity matter? US Dept of Health and Human Services, CDC, Natl Ctr for Health Statistics; 2011. [Google Scholar]
- 23.Boehm M, Winkelmayer WC, Arbeiter K, et al. Late referral to paediatric renal failure service impairs access to preemptive kidney transplantation in children. Arch Dis Child. 2010;95:634–638. doi: 10.1136/adc.2009.174581. [DOI] [PubMed] [Google Scholar]
- 24.Shilling LM, Norman ML, Chavin KD, et al. Healthcare professionals’ perceptions of the barriers to living donor kidney transplantation among African Americans. J Natl Med Assoc. 2006;98:834–840. [PMC free article] [PubMed] [Google Scholar]
- 25.Moskowitz D, Vittinghoff E, Schmidt L. Reconsidering the effects of poverty and social support on health: a 5-year longitudinal test of the stress-buffering hypothesis. J Urban Health. 2013;90:175–184. doi: 10.1007/s11524-012-9757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattell V. Poor people, poor places, and poor health: the mediating role of social networks and social capital. Soc Sci Med. 2001;52:1501–1516. doi: 10.1016/s0277-9536(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 27.Gilman SE, Fitzmaurice GM, Bruce ML, et al. Economic inequalities in the effectiveness of a primary care intervention for depression and suicidal ideation. Epidemiology. 2013;24:14–22. doi: 10.1097/EDE.0b013e3182762403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney G. The impacts of the State Children's Health Insurance Program on children who enroll: findings from ten states. Health Serv Res. 2007;42:1520–1543. doi: 10.1111/j.1475-6773.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saloner B. Does expanding public insurance prevent material hardship for families with children? Med Care Res Rev. 2013 doi: 10.1177/1077558712470566. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Rank MR. Asset building across the life course. In: McKernan S, Sherraden M, editors. Asset building and low-income families. 1st ed. The Urban Institute Press; Washington, DC: 2008. pp. 67–88. [Google Scholar]
- 31.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2012. p. 43. Tables KI8.26-8.27. [Google Scholar]
- 32.Opelz G, Dohler B. Pediatric kidney transplantation: analysis of donor age, HLA match, and posttransplant non-Hodgkin lymphoma: a collaborative transplant study report. Transplantation. 2010;90:292–297. doi: 10.1097/TP.0b013e3181e46a22. [DOI] [PubMed] [Google Scholar]
- 33■.Moseley KL, Kershaw DB. African American and white disparities in pediatric kidney transplantation in the United States – unfortunate or unjust? Camb Q Healthc Ethics. 2012;21:353–365. doi: 10.1017/S0963180112000072. [Continued discrimination and bias toward racial minorities in the United States leads to disparities, such as the black to white disparity observed in access to preemptive kidney transplantation. Solutions to overcoming this disparity must address the unfair social structures through policies that remediate past injustices.] [DOI] [PubMed] [Google Scholar]
- 34.Byrd WM, Clayton LA. Race, medicine, and healthcare in the United States: a historical survey. J Natl Med Assoc. 2001;93:11S–34S. [PMC free article] [PubMed] [Google Scholar]
- 35.Kochhar R, Fry R, Taylor P. Pew Research Social & Demographic Trends. Pew Research Center; Washington, DC: 2011. Wealth gaps rise to record highs between whites, blacks, Hispanics: twenty-to-one. [Google Scholar]
- 36.Liem YS, Weimar W. Early living-donor kidney transplantation: a review of the associated survival benefit. Transplantation. 2009;87:317–318. doi: 10.1097/TP.0b013e3181952710. [DOI] [PubMed] [Google Scholar]
- 37.Butani L, Perez RV. Effect of pretransplant dialysis modality and duration on long-term outcomes of children receiving renal transplants. Transplantation. 2011;91:447–451. doi: 10.1097/TP.0b013e318204860b. [DOI] [PubMed] [Google Scholar]
- 38.Sinha R, Marks SD. Comparison of parameters of chronic kidney disease following paediatric preemptive versus nonpreemptive renal transplantation. Pediatr Transplant. 2010;14:583–588. doi: 10.1111/j.1399-3046.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 39.Cassidy-Bushrow AE, Wegienka G, Barone CJ, 2nd, et al. Race-specific relationship of birth weight and renal function among healthy young children. Pediatr Nephrol. 2012;27:1317–1323. doi: 10.1007/s00467-012-2136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lhila A, Long S. What is driving the black-white difference in low birthweight in the US? Health Econ. 2012;21:301–315. doi: 10.1002/hec.1715. [DOI] [PubMed] [Google Scholar]
- 41.Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why. Curr Opin Organ Transplant. 2011;16:243–249. doi: 10.1097/MOT.0b013e3283447b1c. [DOI] [PubMed] [Google Scholar]
- 42.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the southeastern United States. Am J Transplant. 2012;12:358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis. 2005;46:734–745. doi: 10.1053/j.ajkd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Patzer RE, Perryman JP, Pastan S, et al. Impact of a patient education program on disparities in kidney transplant evaluation. Clin J Am Soc Nephrol. 2012;7:648–655. doi: 10.2215/CJN.10071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan C, Leon JB, Sayre SS, et al. Impact of navigators on completion of steps in the kidney transplant process: a randomized, controlled trial. Clin J Am Soc Nephrol. 2012;7:1639–1645. doi: 10.2215/CJN.11731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Formica RN, Jr, Barrantes F, Asch WS, et al. A one-day centralized work-up for kidney transplant recipient candidates: a quality improvement report. Am J Kidney Dis. 2012;60:288–294. doi: 10.1053/j.ajkd.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Furth SL, Hwang W, Neu AM, et al. Effects of patient compliance, parental education and race on nephrologists’ recommendations for kidney transplantation in children. Am J Transplant. 2003;3:28–34. doi: 10.1034/j.1600-6143.2003.30106.x. [DOI] [PubMed] [Google Scholar]
- 48.Lunsford SL, Simpson KS, Chavin KD, et al. Racial disparities in living kidney donation: is there a lack of willing donors or an excess of medically unsuitable candidates? Transplantation. 2006;82:876–881. doi: 10.1097/01.tp.0000232693.69773.42. [DOI] [PubMed] [Google Scholar]
- 49.Schoenmaker NJ, Tromp WF, van der Lee JH, et al. Disparities in dialysis treatment and outcomes for Dutch and Belgian children with immigrant parents. Pediatr Nephrol. 2012;27:1369–1379. doi: 10.1007/s00467-012-2135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tromp WF, Schoenmaker NJ, van der Lee JH, et al. Important differences in management policies for children with end-stage renal disease in the Netherlands and Belgium: report from the RICH-Q study. Nephrol Dial Transplant. 2012;27:1984–1992. doi: 10.1093/ndt/gfr570. [DOI] [PubMed] [Google Scholar]
- 51.Carroll AE, Frakt AB. The health policy election. JAMA. 2012;308:1633–1634. doi: 10.1001/jama.2012.13667. [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum S. Medicaid and access to healthcare: a proposal for continued inaction? N Engl J Med. 2011;365:102–104. doi: 10.1056/NEJMp1106046. [DOI] [PubMed] [Google Scholar]