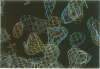Abstract
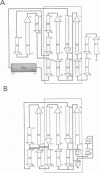
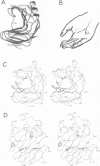
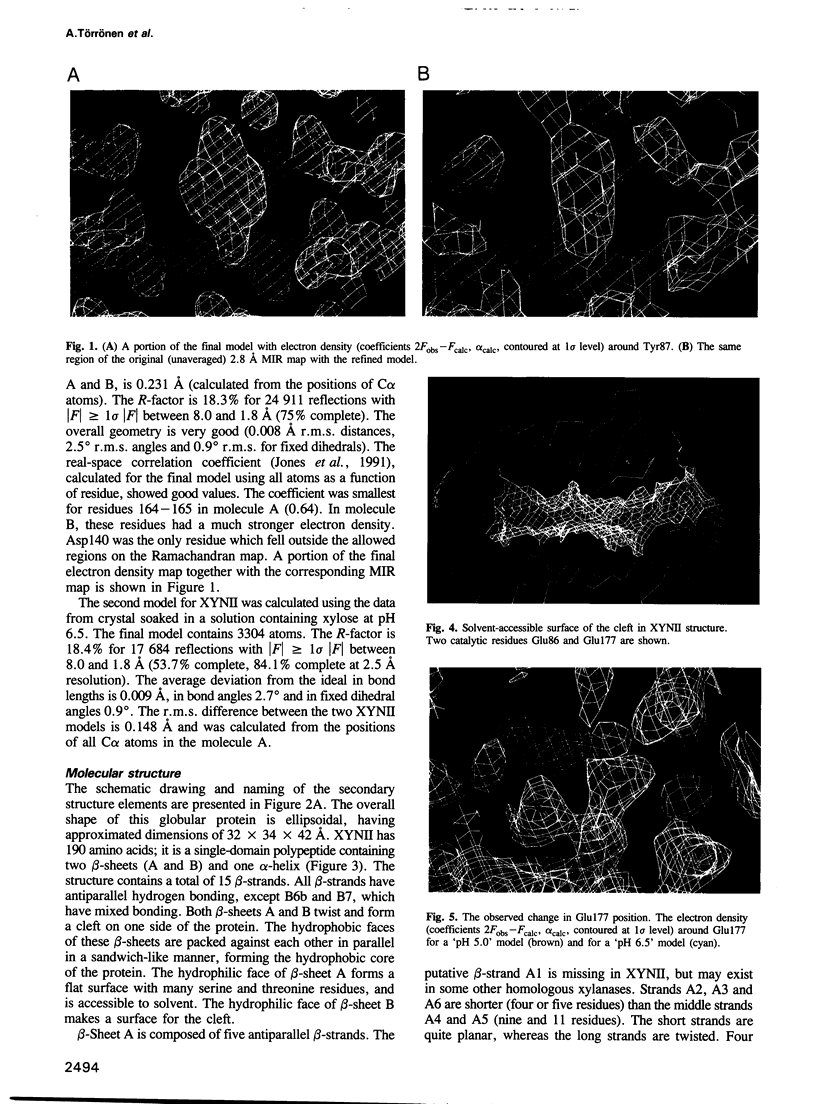
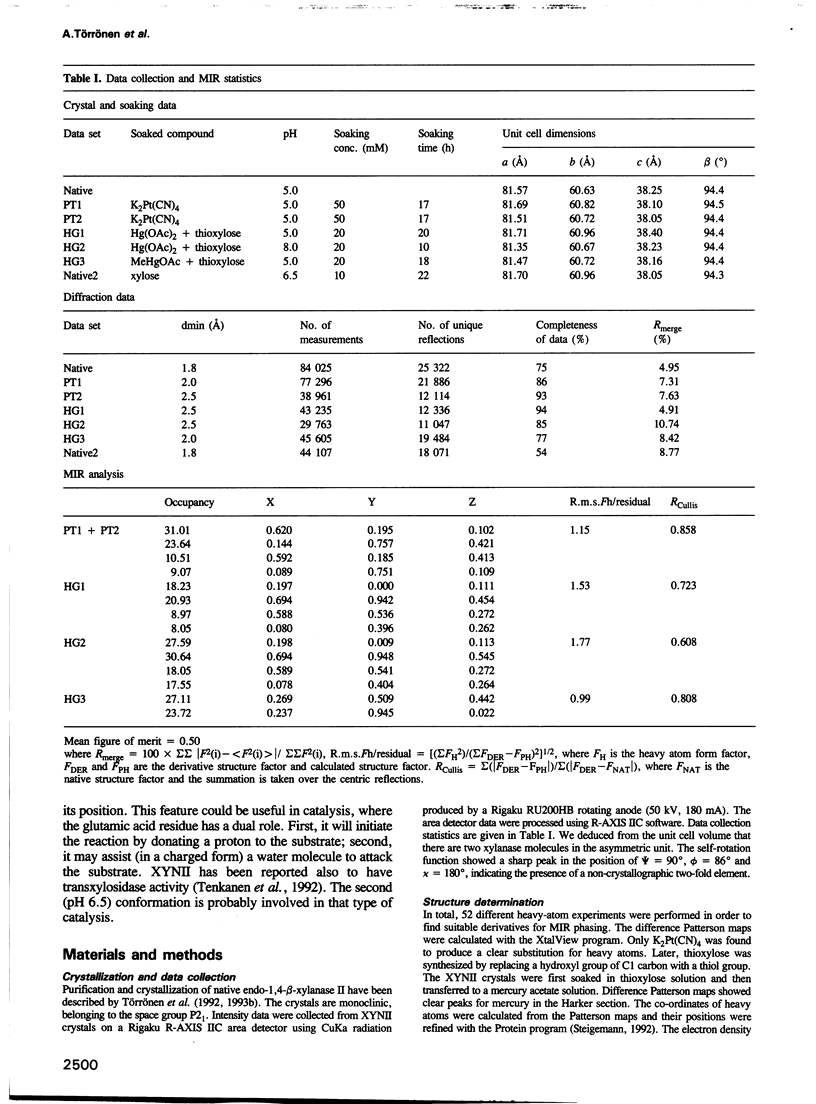
The three-dimensional structure of endo-1,4-beta-xylanase II (XYNII) from Trichoderma reesei has been determined by X-ray diffraction techniques and refined to a conventional R-factor of 18.3% at 1.8 A resolution. The 190 amino acid length protein was found to exist as a single domain where the main chain folds to form two mostly antiparallel beta-sheets, which are packed against each other in parallel. The beta-sheet structure is twisted, forming a large cleft on one side of the molecule. The structure of XYNII resembles that of Bacillus 1,3-1,4-beta-glucanase. The cleft is an obvious suggestion for an active site, which has putative binding sites for at least four xylose residues. The catalytic residues are apparently the two glutamic acid residues (Glu86 and Glu177) in the middle of the cleft. One structure was determined at pH 5.0, corresponding to the pH optimum of XYNII. The second structure was determined at pH 6.5, where enzyme activity is reduced considerably. A clear structural change was observed, especially in the position of the side chain of Glu177. The observed conformational change is probably important for the mechanism of catalysis in XYNII.
Full text
PDF
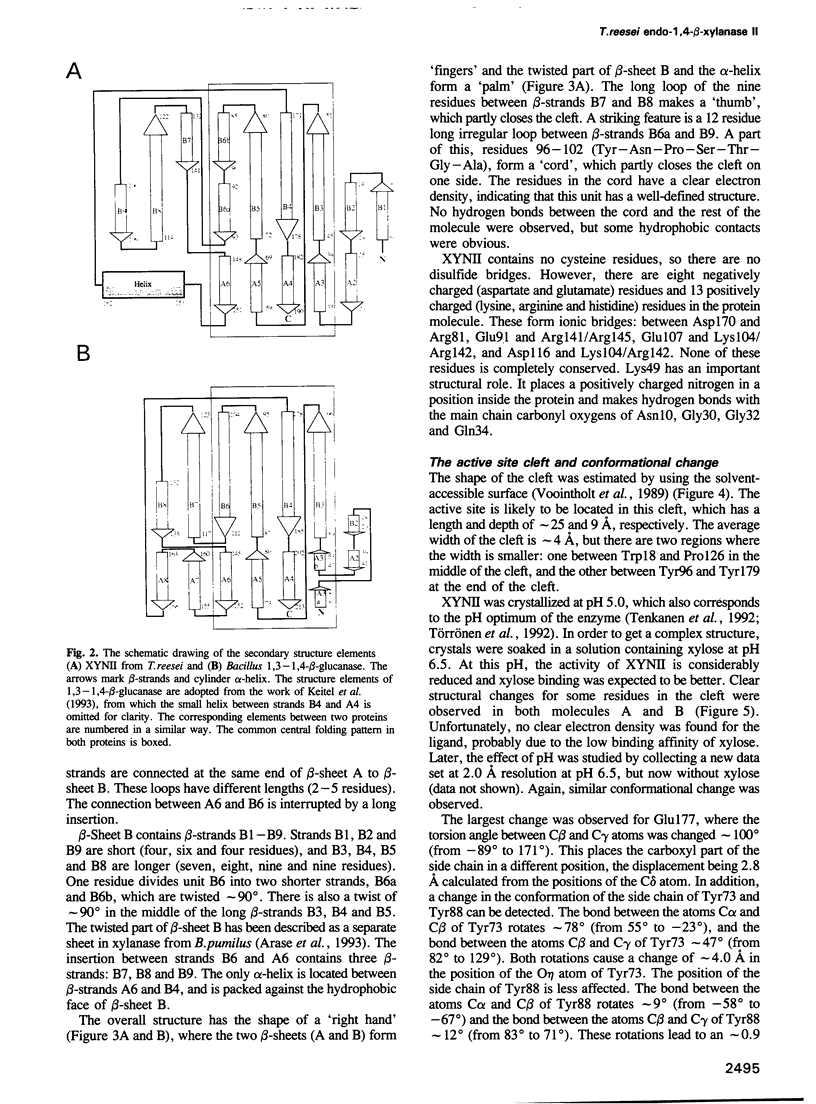
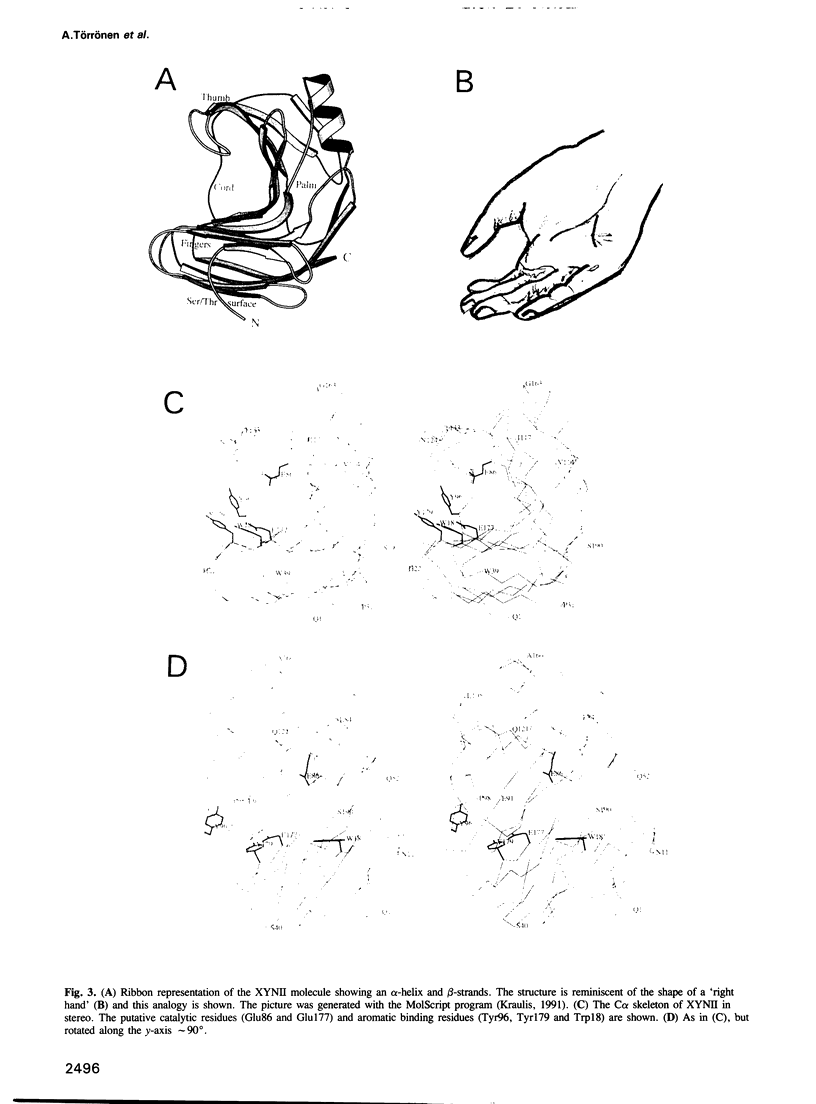
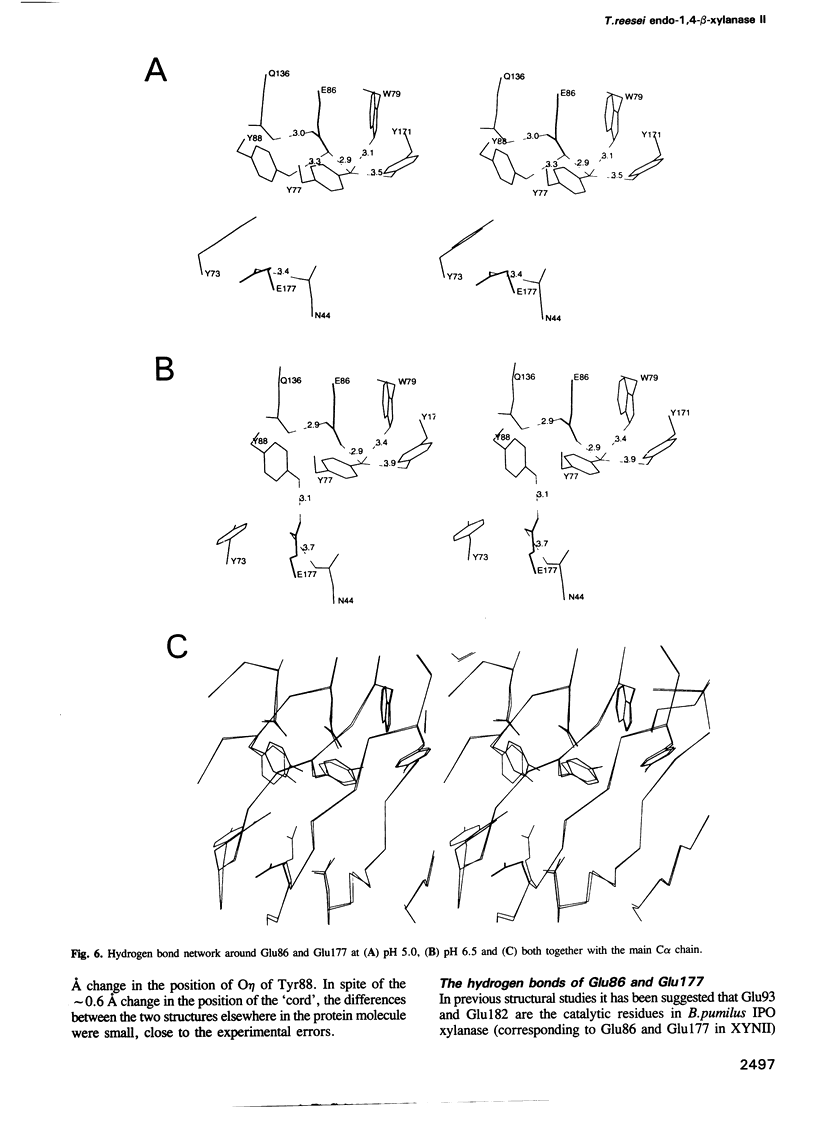
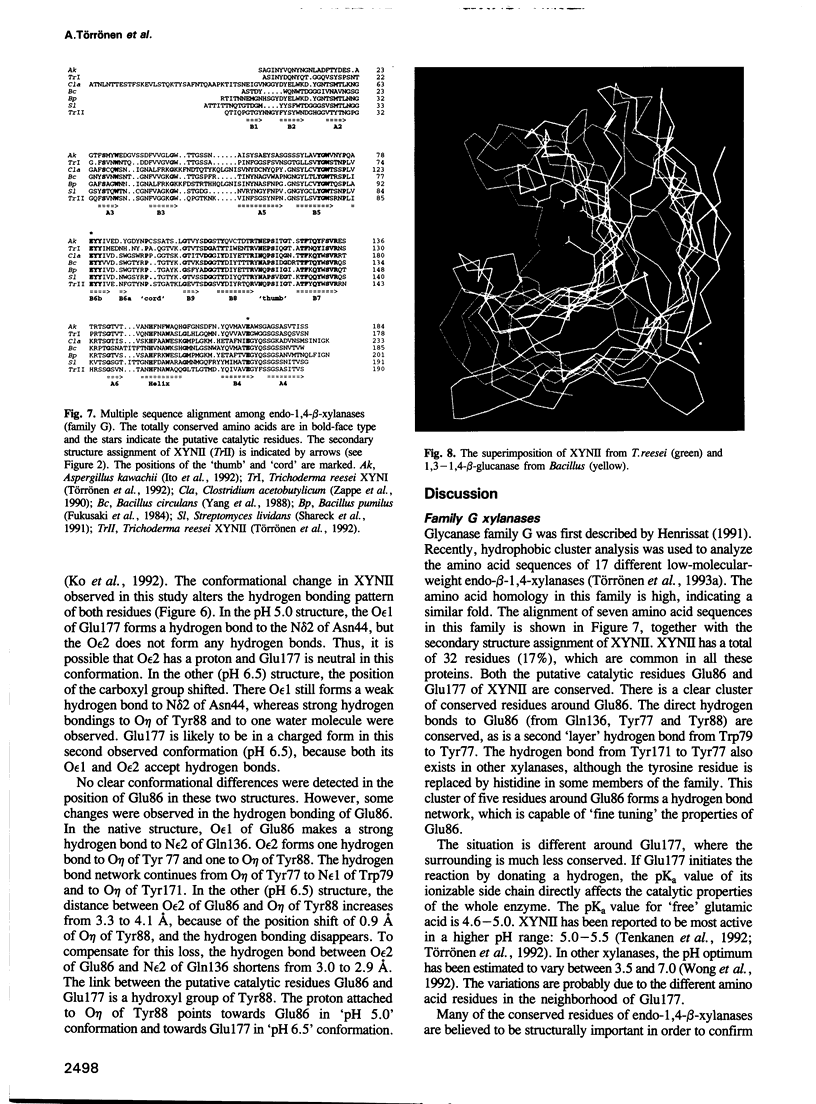


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arase A., Yomo T., Urabe I., Hata Y., Katsube Y., Okada H. Stabilization of xylanase by random mutagenesis. FEBS Lett. 1993 Jan 25;316(2):123–127. doi: 10.1016/0014-5793(93)81199-a. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Davies G. J., Dodson G. G., Hubbard R. E., Tolley S. P., Dauter Z., Wilson K. S., Hjort C., Mikkelsen J. M., Rasmussen G., Schülein M. Structure and function of endoglucanase V. Nature. 1993 Sep 23;365(6444):362–364. doi: 10.1038/365362a0. [DOI] [PubMed] [Google Scholar]
- Gebler J., Gilkes N. R., Claeyssens M., Wilson D. B., Béguin P., Wakarchuk W. W., Kilburn D. G., Miller R. C., Jr, Warren R. A., Withers S. G. Stereoselective hydrolysis catalyzed by related beta-1,4-glucanases and beta-1,4-xylanases. J Biol Chem. 1992 Jun 25;267(18):12559–12561. [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubev A. M., Kilimnik AYu, Neustroev K. N., Pickersgill R. W. Crystals of beta-xylanase from Aspergillus oryzae. J Mol Biol. 1993 Mar 20;230(2):661–663. doi: 10.1006/jmbi.1993.1177. [DOI] [PubMed] [Google Scholar]
- Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1991 Dec 1;280(Pt 2):309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Iwashita K., Iwano K. Cloning and sequencing of the xynC gene encoding acid xylanase of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992 Aug;56(8):1338–1340. doi: 10.1271/bbb.56.1338. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Keitel T., Simon O., Borriss R., Heinemann U. Molecular and active-site structure of a Bacillus 1,3-1,4-beta-glucanase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5287–5291. doi: 10.1073/pnas.90.11.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. J. Mechanism and stereoelectronic effects in the lysozyme reaction. CRC Crit Rev Biochem. 1987;22(4):283–315. doi: 10.3109/10409238709086959. [DOI] [PubMed] [Google Scholar]
- Ko E. P., Akatsuka H., Moriyama H., Shinmyo A., Hata Y., Katsube Y., Urabe I., Okada H. Site-directed mutagenesis at aspartate and glutamate residues of xylanase from Bacillus pumilus. Biochem J. 1992 Nov 15;288(Pt 1):117–121. doi: 10.1042/bj2880117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek C. P., Messner R., Gruber F., Mach R. L., Kubicek-Pranz E. M. The Trichoderma cellulase regulatory puzzle: from the interior life of a secretory fungus. Enzyme Microb Technol. 1993 Feb;15(2):90–99. doi: 10.1016/0141-0229(93)90030-6. [DOI] [PubMed] [Google Scholar]
- Moriyama H., Hata Y., Yamaguchi H., Sato M., Shinmyo A., Tanaka N., Okada H., Katsube Y. Crystallization and preliminary X-ray studies of Bacillus pumilus IPO xylanase. J Mol Biol. 1987 Jan 5;193(1):237–238. doi: 10.1016/0022-2836(87)90644-9. [DOI] [PubMed] [Google Scholar]
- Pickersgill R. W., Debeire P., Debeire-Gosselin M., Jenkins J. A. Crystallization and preliminary X-ray analysis of a thermophillic Bacillus xylanase. J Mol Biol. 1993 Mar 20;230(2):664–666. doi: 10.1006/jmbi.1993.1178. [DOI] [PubMed] [Google Scholar]
- Rose D. R., Birnbaum G. I., Tan L. U., Saddler J. N. Crystallization and preliminary X-ray diffraction study of a xylanase from Trichoderma harzianum. J Mol Biol. 1987 Apr 20;194(4):755–756. doi: 10.1016/0022-2836(87)90254-3. [DOI] [PubMed] [Google Scholar]
- Rouvinen J., Bergfors T., Teeri T., Knowles J. K., Jones T. A. Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990 Jul 27;249(4967):380–386. doi: 10.1126/science.2377893. [DOI] [PubMed] [Google Scholar]
- Shareck F., Roy C., Yaguchi M., Morosoli R., Kluepfel D. Sequences of three genes specifying xylanases in Streptomyces lividans. Gene. 1991 Oct 30;107(1):75–82. doi: 10.1016/0378-1119(91)90299-q. [DOI] [PubMed] [Google Scholar]
- Törrönen A., Kubicek C. P., Henrissat B. Amino acid sequence similarities between low molecular weight endo-1,4-beta-xylanases and family H cellulases revealed by clustering analysis. FEBS Lett. 1993 Apr 26;321(2-3):135–139. doi: 10.1016/0014-5793(93)80094-b. [DOI] [PubMed] [Google Scholar]
- Törrönen A., Mach R. L., Messner R., Gonzalez R., Kalkkinen N., Harkki A., Kubicek C. P. The two major xylanases from Trichoderma reesei: characterization of both enzymes and genes. Biotechnology (N Y) 1992 Nov;10(11):1461–1465. doi: 10.1038/nbt1192-1461. [DOI] [PubMed] [Google Scholar]
- Törrönen A., Rouvinen J., Ahlgren M., Harkki A., Visuri K. Crystallization and preliminary X-ray analysis of two major xylanases from Trichoderma reesei. J Mol Biol. 1993 Sep 20;233(2):313–316. doi: 10.1006/jmbi.1993.1509. [DOI] [PubMed] [Google Scholar]
- Viswamitra M. A., Bhanumoorthy P., Ramakumar S., Manjula M. V., Vithayathil P. J., Murthy S. K., Naren A. P. Crystallization and preliminary X-ray diffraction analysis of crystals of Thermoascus aurantiacus xylanase. J Mol Biol. 1993 Aug 5;232(3):987–988. doi: 10.1006/jmbi.1993.1444. [DOI] [PubMed] [Google Scholar]
- Voorintholt R., Kosters M. T., Vegter G., Vriend G., Hol W. G. A very fast program for visualizing protein surfaces, channels and cavities. J Mol Graph. 1989 Dec;7(4):243–245. doi: 10.1016/0263-7855(89)80010-4. [DOI] [PubMed] [Google Scholar]
- Yang R. C., MacKenzie C. R., Narang S. A. Nucleotide sequence of a Bacillus circulans xylanase gene. Nucleic Acids Res. 1988 Jul 25;16(14B):7187–7187. doi: 10.1093/nar/16.14.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe H., Jones W. A., Woods D. R. Nucleotide sequence of a Clostridium acetobutylicum P262 xylanase gene (xynB). Nucleic Acids Res. 1990 Apr 25;18(8):2179–2179. doi: 10.1093/nar/18.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]