Summary
Zolpidem has been reported as an “awakening drug” in some patients with disorders of consciousness (DOC). We here present the results of a prospective open-label study in chronic DOC patients. Sixty patients (35±15 years; 18 females; mean time since insult ± SD: 4±5.5 years; 31 with traumatic etiology) with a diagnosis of vegetative state/unresponsive wakefulness syndrome (n=28) or minimally conscious state (n=32) were behaviorally assessed using the Coma Recovery Scale-Revised (CRS-R) before and one hour after administration of 10 mg of zolpidem. At the group level, the diagnosis did not change after intake of zolpidem (p=0.10) and CRS-R total scores decreased (p=0.01). Twelve patients (20%) showed improved behaviors and/or CRS-R total scores after zolpidem administration but in only one patient was the diagnosis after zolpidem intake found to show a significant improvement (functional object use), which suggested a change of diagnosis. However, in this patient, a double-blind placebo-controlled trial was performed in order to better specify the effects of zolpidem, but the patient, on this trial, failed to show any clinical improvements.
The present open-label study therefore failed to show any clinically significant improvement (i.e., change of diagnosis) in any of the 60 studied chronic DOC patients.
Keywords: disorders of consciousness, minimally conscious state, treatment, vegetative state, zolpidem
Introduction
Zolpidem is often reported, particularly in the non-scientific literature (e.g., Interlandi, 2011), as a “miracle drug” that awakens patients with disorders of consciousness (DOC). As a consequence, families of patients with DOC are likely to invest much hope in this drug. However, the proportion of DOC patients in whom zolpidem produces such an effect is not well documented. Currently, patients with DOC are increasingly well defined, especially since the publication, in 2002, of diagnostic criteria for the minimally conscious state [MCS - presence of limited but clear signs of consciousness without effective communication (Giacino et al., 2002)] and the vegetative state, recently renamed unresponsive wakefulness syndrome [VS/UWS – wakefulness without signs of awareness (Laureys et al., 2010)]. However, there are no evidence-based recommendations regarding pharmacological treatment capable of improving the level of consciousness in DOC patients (Demertzi et al., 2008).
A number of studies, essentially case reports, have nevertheless reported zolpidem as a possible treatment, found to result in a transient recovery of consciousness in some DOC patients (Table I, over). Whereas zolpidem, an imidazopyridine, is generally used as a sedative-hypnotic drug, it seems that it occasionally produces, temporarily, a surprising paradoxical effect on the level of consciousness in some DOC patients, irrespective of whether they have a traumatic or a non-traumatic etiology (Gosseries et al., 2013). A wide range of behavioral improvements have been reported including the emergence of visual pursuit, command following, verbalizations, functional communication, motor improvements (e.g., ability to walk) and/or cognitive (e.g., reading, counting, writing) improvements (Brefel-Courbon et al., 2007; Clauss and Nel, 2006; Clauss et al., 2000; Cohen and Duong, 2008; Shames and Ring, 2008; Whyte and Myers, 2009) (Table I). Despite the existence of numerous case reports about zolpidem in DOC, only Whyte and Myers (2009) have investigated the incidence of responders among DOC patients (11). In their study, only one out of 15 patients evolved from VS/UWS to MCS (6.7% responder rate) and demonstrated behavioral improvements, namely visual pursuit and response to command.
Table I.
Pharmacological studies on zolpidem in post-comatose patients with disorders of consciousness.
| Type of study | n (etiology) | Diagnosis | Time since insult | Dose | Main results | MRI or CT of zolpidem responders |
|---|---|---|---|---|---|---|
| Williams et al., 2013 | ||||||
| EEG case study | 3 (TBI, stroke, TBI/hypoxic) | MCS | 5, 6, 9 y | 10 mg | Behavioral improvements: verbal fluency, functional use of objects, functional communication. EEG: ↓ of power and coherence at ∼6–10 Hz and ↑ in power at ∼15–30 Hz. Changes in brain metabolism (PET): ↑ in anterior forebrain with the largest ↑ in the lateral frontal cortex |
Unavailable |
| Du et al., 2013 | ||||||
| Cohort study using SPECT | 127 [brain contrecoup contusion, primary brainstem injury, space-occupying brain compression injury and secondary brainstem injury] | VS/UWS (N.B.: no behavioral standard) | Unavailable | 10 mg | ↑ cerebral state index, ↑ cerebral perfusion, ↓ burst suppression in contrecoup contusion and space-occupying compression group. No changes in primary and secondary brainstem injury groups | Unavailable |
| Machado et al., 2011 | ||||||
| Case study | 1 (stroke) | VS/UWS | 5 y | 10 mg | Signs of arousal (eye movements and yawns), heart rate ↑, EEG changes from subdelta band to the lower delta frequency range | Lesions in rostral part of the pons, mesencephalon and both thalami |
| Snyman et al., 2010 | ||||||
| Double-blind placebo-controlled randomized trial | 3 pediatrics (1 TBI) | VS/UWS | 2, 14, 13 y | 0.14-0.2 mg/kg | No change of RLAS scores, ↑ CNCS scores (reduced responsiveness) | (No responder) |
| Whyte & Myers, 2009 | ||||||
| Multi-centric, double-blind, randomized study | 15 (8 TBI) | VS/UWS, MCS | 3 m–23 y | 10 mg | 1 responder to treatment (VS/UWS to MCS), ↑ CRS-R score, visual pursuit, response to command | Bilateral temporal lobe contusion, intraventricular hemorrhage |
| Cohen & Duong, 2008 | ||||||
| Case study | 1 (anoxic) | MCS | 8 m | 5–20 mg | ↑ lucidity, ↑ interactions, ↑ verbal and social responses | Unavailable |
| Shames & Ring, 2008 | ||||||
| Case study | 1 (anoxic) | MCS | 18 m | 10 mg | ↑ lucidity, ↑ RLAS score, ↑ verbal communication, reading, counting, verbal production, self-feeding | Mild ventricular dilatation |
| Singh et al., 2008 | ||||||
| Double-blind case study | 1 (TBI) | MCS | 4 y | 10 mg | No effect on tests with instructions which ↑ in complexity | (No responder) |
| Lo et al., 2008 | ||||||
| Double-blind case study | 2 (anoxic) | VS/UWS | ±1 m | 10 mg | No arousal improvement, no recovery of consciousness, no ↑ GCS score | (No responder) |
| Brefel-Courbon et al., 2007 | ||||||
| Double-blind, placebo-controlled randomized case study | 1 (anoxic) | MCS | 2 y | 20 mg | ↑ arousal, ↑ motor and neuropsychological performance (functional communication, eating, walking, reading and repeating words) | Unavailable |
| Clauss & Nel, 2006 | ||||||
| Case studies | 3 (2 TBI) | VS/UWS | 3–5 y | 10 mg | ↑ rousal, ↑ GCS and RLAS scores | Unavailable |
| Clauss et al., 2000 | ||||||
| Case study | 1 (TBI) | ‘Semi-comatose’ | 3 y | 10 mg | Verbal response after 15 min, talking, answering simple questions, spontaneous interaction, counting, writing | Hemorrhage in left lentiform nucleus, thalamus and cerebellar peduncles. Intra-ventricular blood in occipital horn of lateral ventricles, lesion in brainstem |
Abbreviations: n=number of patients; TBI=traumatic brain injury; VS/UWS=vegetative state/unresponsive wakefulness syndrome; MCS=minimally conscious state; ↑=increase; ↓=decrease; y=year(s); m=month(s); EEG=electroencephalogram; SPECT=single photon emission computed tomography; CRS-R=Coma Recovery Scale–Revised; RLAS=Rancho Los Amigos Scale; GCS=Glasgow Coma Scale; CNCS=Coma/Near Coma Scale; PET=positron emission tomography
Abbreviations: n=number of patients; TBI=traumatic brain injury; VS/UWS=vegetative state/unresponsive wakefulness syndrome; MCS=minimally conscious state; ↑=increase; ↓=decrease; y=year(s); m=month(s); EEG=electroencephalogram; SPECT=single photon emission computed tomography; CRS-R=Coma Recovery Scale–Revised; RLAS=Rancho Los Amigos Scale; GCS=Glasgow Coma Scale; CNCS=Coma/Near Coma Scale
The numerous case reports present in the literature may result in overestimation of the rate of patients in whom zolpidem works as a “waking up pill”. On the other hand, only one study has assessed the responder rate in a small sample. The aim of the present prospective, uncontrolled, unblinded study was thus to estimate the frequency of beneficial clinical changes after zolpidem intake and to characterize these changes in a larger cohort of chronic DOC patients.
Materials and methods
We prospectively enrolled patients with chronic (>4 weeks post injury) VS/UWS or MCS. The patients underwent repeated (≥5) standardized behavioral assessments using the Coma Recovery Scale-Revised [CRSR, (4)], administered by trained examiners over a one-week period. The CRS-R was also administered before and one hour after (considering the approximate moment of the peak plasma concentration) a single dose of zolpidem (10 mg administered orally or via a feeding tube). If the clinical diagnosis, according to the CRS-R results, improved in the open-label zolpidem trial, a double-blind placebo-controlled trial was performed in order to confirm the improvement. Informed consent was obtained from each participant’s legally authorized representative. Statistical analyses were performed on diagnosis and CRS-R total scores using non-parametric statistics (Wilcoxon’s signed rank test, SPSS 16.0, 2007, SPSS Inc.). Data were corrected for multiple comparisons (Bonferroni’s correction, p<0.05, one-tailed).
Results
Sixty patients (18 females, mean age±SD: 36±14 years) with a diagnosis of VS/UWS (n=28, 47%) or MCS (n=32, 53%) were assessed between four weeks and 26 years post-injury (mean time since injury±SD: 4±5.5 years). The etiology was traumatic in 31 (52%) and non-traumatic in 29 (48%) patients [i.e., anoxic encephalopathy (n=18), hemorrhagic or ischemic stroke (n=6), metabolic encephalopathy (n=2) and mixed etiology (n= 3)].
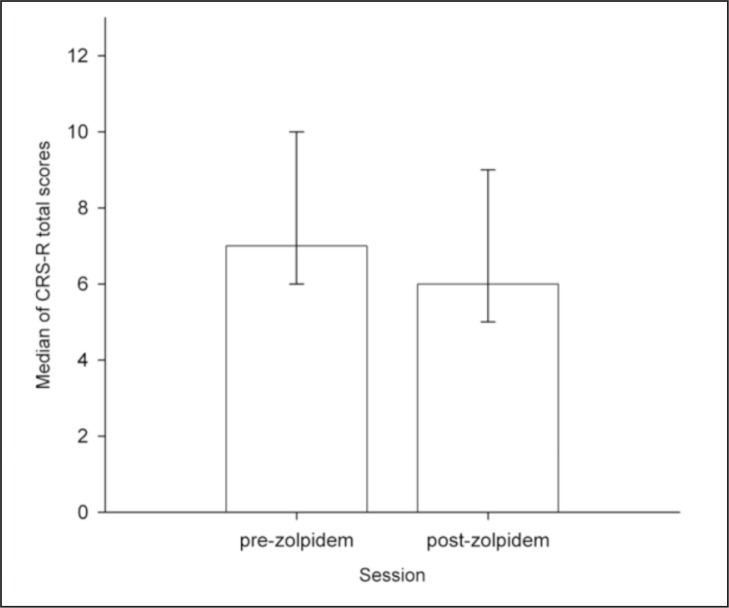
At the group level, the diagnosis did not change after zolpidem intake (z=−1.7, p=0.10, r=0.15) and CRS-R total scores decreased [pre-zolpidem median=7, post-zolpidem median=6, interquartile range (IQR)=1, z=−2.7, p=0.01, r=0.24, Fig. 1], and the CRS-R subscores showed no significant change pre- versus post-zolpidem. However, at the individual level, 12 patients showed improved behaviors and/or increased CRS-R total scores after zolpidem (Table II). Of these 12 patients, four (2 traumatic brain injury, 1 anoxia and 1 metabolic etiology, with different patterns of regional brain damage) showed improvements not observed in any other CRS-R assessments performed outside the zolpidem trial (e.g., reproducible command following, object recognition, vocalization; Table II). However, in only one of these patients did the improvement suggest a change of diagnosis. Patient n. 45, a 30-year-old man who suffered a traumatic brain injury nine years before the study, was diagnosed as MCS on the basis of all the pre-zolpidem CRS-R assessments. He presented conscious behaviors such as command following, object recognition and visual pursuit. However, he showed functional use of objects only on the post-zolpidem CRS-R assessment, which thus suggests that he emerged from MCS. A double-blind placebo-controlled study was consequently performed, which, however, failed to confirm the clinical improvement; i.e., there was no increase in any of his CRS-R subscores and, therefore, no change of diagnosis.
Figure 1.
Significant decrease of CRS-R total scores (ranging from 0 to 23) after zolpidem intake (interquartile range represented by errors bars) in the entire sample (n=60).
Table II.
Data regarding 12 out of 60 DOC patients who showed improved behaviors and/or increased CRS-R total scores after zolpidem administration.
| Patient | Age | Etiology | Time since injury | Diagnosis (CRS-R total score) | Improvements compared with pre-zolpidem baseline | Improvements compared with with one-week baseline assessments | ||
|---|---|---|---|---|---|---|---|---|
| All CRS-R | Pre-zolpidem | Post-zolpidem | ||||||
| 45*† | 30 | TBI | 9 y | MCS (14) | MCS (9) | EMCS (17) | • Reproducible command following | • Functional use of objects |
| • Object localization | ||||||||
| • Functional use of objects | ||||||||
| 52* | 48 | Anoxia | 1 y | MCS (10) | MCS (7) | MCS (12) | • Reproducible command following | • Reproducible command following |
| • Object recognition | • Object recognition | |||||||
| 4* | 21 | TBI | 5 y | MCS (10) | MCS (8) | MCS (14) | • Systematic command following | • Intentional communication |
| • Automatic motor reaction | ||||||||
| • Vocalization | ||||||||
| • Intentional communication | ||||||||
| 15* | 53 | Metabolic | 1 y | MCS (15) | MCS (15) | MCS (16) | • Object localization | • Object localization |
| • Automatic motor reaction | ||||||||
| 19 | 20 | TBI | 3.5 y | MCS (10) | MCS (10) | MCS (10) | • Auditory startle | - |
| 59 | 24 | TBI | 3 y | MCS (10) | MCS (9) | MCS (11) | • Reproducible command following | - |
| 9 | 23 | TBI | 1 y | MCS (10) | MCS (11) | MCS (10) | • Vocalization | - |
| 58 | 24 | TBI | 9 m | MCS (10) | VS/UWS (8) | MCS (11) | • Automatic motor reaction | - |
| 46 | 67 | Stroke | 4 y | MCS (4) | VS/UWS (4) | VS/UWS (5) | • Eye opening without stimulation | - |
| 50 | 22 | TBI | 6 m | VS/UWS (8) | VS/UWS (6) | VS/UWS (7) | • Auditory startle | - |
| 21 | 19 | TBI | 9 m | MCS (11) | MCS (11) | MCS (11) | • Vocalization | - |
| 53 | 49 | Anoxia | 8 y | VS/UWS (6) | VS/UWS (4) | VS/UWS (3) | • Eye opening without stimulation | - |
Patients (n=4) showing improvements post-zolpidem compared to all CRS-R assessments.
In this patient, a placebo-controlled double-blind zolpidem trial failed to show any clinical improvement.
Abbreviations: TBI=traumatic brain injury; VS/UWS=vegetative state/unresponsive wakefulness syndrome; MCS=minimally conscious state; EMCS=emergence from minimally conscious state; CRS-R=Coma Recovery Scale-Revised; y=year(s); m=month(s)
Discussion
The aim of this prospective uncontrolled study was to estimate the frequency of beneficial clinical changes after zolpidem intake and to characterize these changes in a large population of chronic DOC patients.
Overall, zolpidem intake did not induce a beneficial effect in chronic DOC patients. On the contrary, zolpidem led to decreased CRS-R total scores. At the individual level, however, 12 patients showed an increase of CRS-R total scores compared to the pre-zolpidem baseline CRS-R assessment. Some might argue that these slight improvements could have been the result of spontaneous fluctuations of vigilance and responsiveness, which are well known to occur in patients with DOC. Therefore, we also compared the post-zolpidem status to the best clinical status during the one week of repeated CRS-R assessments. Only four patients (6.7% of the present cohort) showed some functional improvements. Although this responder rate is identical to the 6.7% obtained by Whyte and Myers (2009), it is important to note that, contrary to our study, the clinical improvements observed in one of Whyte and Myers’ patients resulted in a change of diagnosis. Indeed, the positive changes observed in our patients did not, ultimately, result in a change of diagnosis. This result also conflicts with previous reports of changes of diagnosis after zolpidem intake in some patients (e.g., Brefel-Courbon et al., 2007; Clauss and Nel, 2006; Clauss et al., 2000; Shames and Ring, 2008); indeed, although one of our patients did show a significant improvement, the follow-up double-blind placebo-controlled trial failed to confirm the zolpidem effect. Instead of having an all-or-none effect, zolpidem seems to have a gradual effect, not necessarily sufficient to justify a change of diagnosis. Given the current results, it is important to temper the enthusiasm in the media about zolpidem as a “miracle awakening pill”. Extraordinary cases with regained speech or other impressive effects exist and are reported in the literature, but they seem to be exceptions rather than the rule. Families of patients have to be informed that this treatment possibility exists but, as clinicians, we should properly inform patients’ relatives about the low responder rate, and the sometimes limited clinical improvements observed.
In conclusion, no change in outcome was observed after zolpidem administration even in DOC patients who transiently showed some increased alertness. Only a small minority of our patients showed partial beneficial effects after zolpidem (4/60; 6.7% of the present cohort), and the observed clinical changes did not lead to a change in diagnosis in any of the cases included in this study. However, these partial beneficial effects, although not significant, suggest that the effect in DOC patients does not seem to be bimodal, but rather more subtle and possibly gradual in some patients. Therefore, in order to more precisely characterize and understand the effects of zolpidem, we suggest that, in the future, the positive behavioral changes observed should be classified as small (i.e., increase in arousal), medium (i.e., improved responsiveness but same diagnosis) or significant (i.e., change of diagnosis). Recent case-report studies have used neuroimaging and electroencephalography techniques to document the effect of zolpidem (Machado et al., 2013; Snyman et al., 2010; Williams et al., 2013). Further studies should focus on large patient series aiming to identify the characteristics of zolpidem responders using clinical, functional neuroimaging and electrophysiological techniques in order to better understand the precise underlying mechanisms of action of the effect of zolpidem in patients with DOC (Schiff, 2010).
Acknowledgments
This research was funded by the Belgian National Funds for Scientific Research (FNRS), the European Commission, Fonds Léon Fredericq, the James McDonnell Foundation, the Mind Science Foundation, the French Speaking Community Concerted Research Action (ARC 06/11-340), “Fondazione Europea di Ricerca Biomedica” and the University and University Hospital of Liège.
References
- Brefel-Courbon C, Payoux P, Ory F, et al. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann Neurol. 2007;62:102–105. doi: 10.1002/ana.21110. [DOI] [PubMed] [Google Scholar]
- Clauss R, Nel W. Drug induced arousal from the permanent vegetative state. NeuroRehabilitation. 2006;21:23–28. [PubMed] [Google Scholar]
- Clauss RP, Güldenpfennig WM, Nel HW, Sathekge MM, Venkannagari RR. Extraordinary arousal from semi-comatose state on zolpidem. A case report. S Afr Med J. 2000;90:68–72. [PubMed] [Google Scholar]
- Cohen SI, Duong TT. Increased arousal in a patient with anoxic brain injury after administration of zolpidem. Am J Phys Med Rehabil. 2008;87:229–231. doi: 10.1097/PHM.0b013e318161971b. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Vanhaudenhuyse A, Bruno MA, et al. Is there anybody in there? Detecting awareness in disorders of consciousness. Expert Rev Neurother. 2008;8:1719–1730. doi: 10.1586/14737175.8.11.1719. [DOI] [PubMed] [Google Scholar]
- Du B, Shan A, Zhang Y, Zhong X, Chen D, Cai K. Zolpidem arouses patients in vegetative state after brain injury: quantitative evaluation and indications. Am J Med Sci. 2013 Mar 4; doi: 10.1097/MAJ.0b013e318287c79c. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–253. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Gosseries O, Charland-Verville V, Thonnard M, Bodart O, Laureys S, Demertzi A. Amantadine, apomorphine and zolpidem in the treatment of disorders of consciousness. Curr Pharm Des. 2013 Sep 11; [Epub ahead of print] [PubMed] [Google Scholar]
- Interlandi J. Waking Chris. The New York Times, The New York Times Company; New York: 2011. p. 42. [Google Scholar]
- Laureys S, Celesia GG, Cohadon F, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YL, Tan EK, Ratnagopal P, Chan LL, Tan TE. Zolpidem and its effects on hypoxic encephalopathy. Ann Neurol. 2008;64:477–478. doi: 10.1002/ana.21183. [DOI] [PubMed] [Google Scholar]
- Machado C, Estévez M, Rodríguez R, et al. Zolpidem arousing effect in persistent vegetative state patients: autonomic, EEG and behavioral assessment. Curr Pharm Des. 2013 Sep 10; [Epub ahead of print] [PubMed] [Google Scholar]
- Machado C, Estévez M, Pérez-Nellar J, et al. Autonomic, EEG, and behavioral arousal signs in a PVS case after Zolpidem intake. Can J Neurol Sci. 2011;38:341–344. doi: 10.1017/s0317167100011562. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames JL, Ring H. Transient reversal of anoxic brain injury-related minimally conscious state after zolpidem administration: a case report. Arch Phys Med Rehabil. 2008;89:386–388. doi: 10.1016/j.apmr.2007.08.137. [DOI] [PubMed] [Google Scholar]
- Snyman N, Egan JR, London K, et al. Zolpidem for persistent vegetative state - a placebo-controlled trial in pediatrics. Neuropediatrics. 2010;41:223–227. doi: 10.1055/s-0030-1269893. [DOI] [PubMed] [Google Scholar]
- Singh R, McDonald C, Dawson K, et al. Zolpidem in a minimally conscious state. Brain Inj. 2008;22:103–106. doi: 10.1080/02699050701829704. [DOI] [PubMed] [Google Scholar]
- Williams ST, Conte MM, Goldfine AM, et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severely brain-injured subjects. eLife. 2013 Nov 19;2(0):e01157. doi: 10.7554/eLife.01157. doi: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J, Myers R. Incidence of clinically significant responses to zolpidem among patients with disorders of consciousness: a preliminary placebo controlled trial. Am J Phys Med Rehabil. 2009;88:410–418. doi: 10.1097/PHM.0b013e3181a0e3a0. [DOI] [PubMed] [Google Scholar]