Abstract
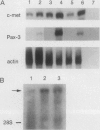
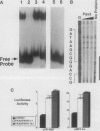
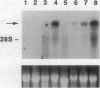
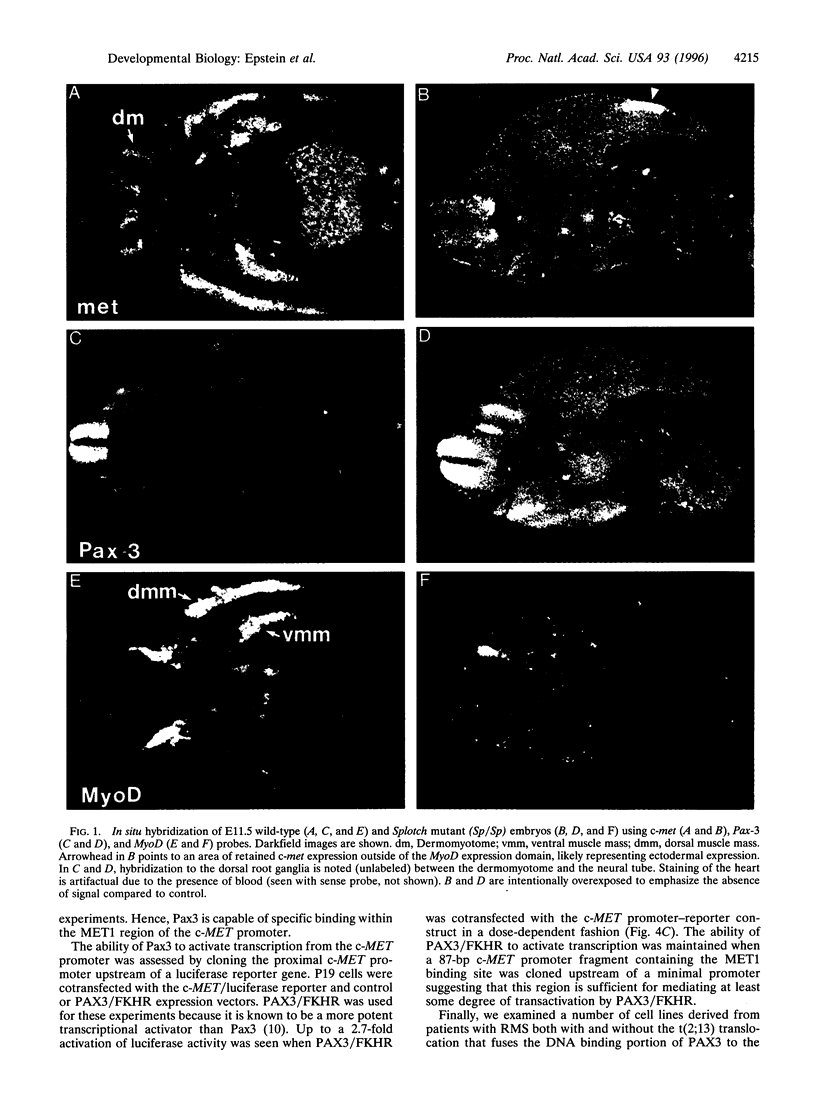
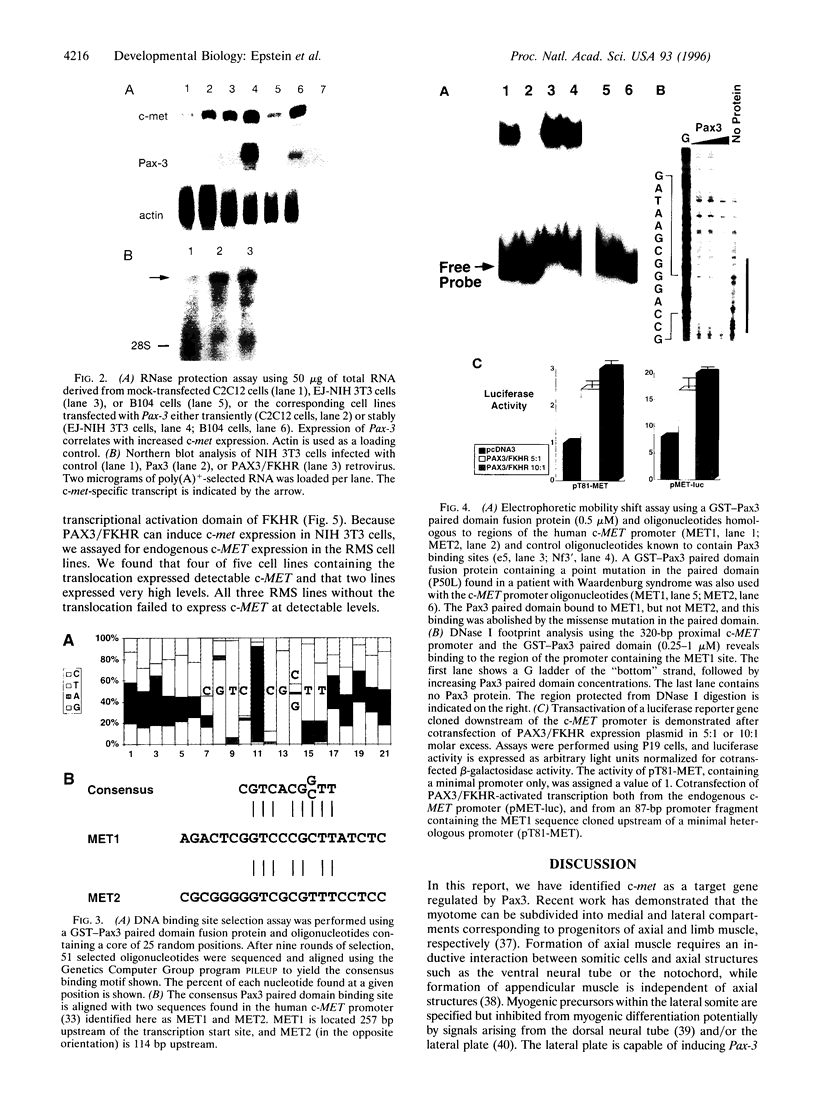
Pax3 is a transcription factor whose expression has been used as a marker of myogenic precursor cells arising in the lateral somite destined to migrate to and populate the limb musculature. Accruing evidence indicates that the embryologic origins of axial and appendicular muscles are distinct, and limb muscle abnormalities in both mice and humans harboring Pax3 mutations support this distinction. The mechanisms by which Pax3 affects limb muscle development are unknown. The tyrosine kinase receptor for hepatocyte growth factor/scatter factor encoded by the c-met protooncogene is also expressed in limb muscle progenitors and, like Pax-3, is required in the mouse for limb muscle development. Here, we show that c-met expression is markedly reduced in the lateral dermomyotome of Splotch embryos lacking Pax3. We show that Pax3 can stimulate c-met expression in cultured cells, and we identify a potential Pax3 binding site in the human c-MET promoter that may contribute to direct transcriptional regulation. In addition, we have found that several cell lines derived from patients with rhabdomyosarcomas caused by a t(2;13) chromosomal translocation activating PAX3 express c-MET, whereas those rhabdomyosarcoma cell lines examined without the translocation do not. These results are consistent with a model in which Pax3 modulates c-met expression in the lateral dermomyotome, a function that is required for the appropriate migration of these myogenic precursors to the limb where the ligand for c-met (hepatocyte growth factor/scatter factor) is expressed at high levels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. T., Hoth C. F., Amos J. A., da-Silva E. O., Milunsky A. An exonic mutation in the HuP2 paired domain gene causes Waardenburg's syndrome. Nature. 1992 Feb 13;355(6361):637–638. doi: 10.1038/355637a0. [DOI] [PubMed] [Google Scholar]
- Barr F. G., Galili N., Holick J., Biegel J. A., Rovera G., Emanuel B. S. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Feb;3(2):113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995 Aug 31;376(6543):768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bober E., Franz T., Arnold H. H., Gruss P., Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994 Mar;120(3):603–612. doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991 Feb 15;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Buffinger N., Stockdale F. E. Myogenic specification of somites is mediated by diffusible factors. Dev Biol. 1995 May;169(1):96–108. doi: 10.1006/dbio.1995.1130. [DOI] [PubMed] [Google Scholar]
- Chalepakis G., Gruss P. Identification of DNA recognition sequences for the Pax3 paired domain. Gene. 1995 Sep 11;162(2):267–270. doi: 10.1016/0378-1119(95)00345-7. [DOI] [PubMed] [Google Scholar]
- Cvekl A., Kashanchi F., Sax C. M., Brady J. N., Piatigorsky J. Transcriptional regulation of the mouse alpha A-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol Cell Biol. 1995 Feb;15(2):653–660. doi: 10.1128/mcb.15.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A., Sax C. M., Li X., McDermott J. B., Piatigorsky J. Pax-6 and lens-specific transcription of the chicken delta 1-crystallin gene. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4681–4685. doi: 10.1073/pnas.92.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Busslinger M. DNA-binding and transactivation properties of Pax-6: three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5). Mol Cell Biol. 1995 May;15(5):2858–2871. doi: 10.1128/mcb.15.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Schaffner G., Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993 Oct;7(10):2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Epstein D. J., Vogan K. J., Trasler D. G., Gros P. A mutation within intron 3 of the Pax-3 gene produces aberrantly spliced mRNA transcripts in the splotch (Sp) mouse mutant. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):532–536. doi: 10.1073/pnas.90.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. A., Glaser T., Cai J., Jepeal L., Walton D. S., Maas R. L. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994 Sep 1;8(17):2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- Epstein J. A., Lam P., Jepeal L., Maas R. L., Shapiro D. N. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem. 1995 May 19;270(20):11719–11722. doi: 10.1074/jbc.270.20.11719. [DOI] [PubMed] [Google Scholar]
- Epstein J., Cai J., Glaser T., Jepeal L., Maas R. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J Biol Chem. 1994 Mar 18;269(11):8355–8361. [PubMed] [Google Scholar]
- Franz T., Kothary R., Surani M. A., Halata Z., Grim M. The Splotch mutation interferes with muscle development in the limbs. Anat Embryol (Berl) 1993 Feb;187(2):153–160. doi: 10.1007/BF00171747. [DOI] [PubMed] [Google Scholar]
- Fredericks W. J., Galili N., Mukhopadhyay S., Rovera G., Bennicelli J., Barr F. G., Rauscher F. J., 3rd The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol. 1995 Mar;15(3):1522–1535. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili N., Davis R. J., Fredericks W. J., Mukhopadhyay S., Rauscher F. J., 3rd, Emanuel B. S., Rovera G., Barr F. G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Nov;5(3):230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- Gambarotta G., Pistoi S., Giordano S., Comoglio P. M., Santoro C. Structure and inducible regulation of the human MET promoter. J Biol Chem. 1994 Apr 29;269(17):12852–12857. [PubMed] [Google Scholar]
- Glaser T., Jepeal L., Edwards J. G., Young S. R., Favor J., Maas R. L. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994 Aug;7(4):463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Goulding M. D., Chalepakis G., Deutsch U., Erselius J. R., Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991 May;10(5):1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M., Lumsden A., Paquette A. J. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994 Apr;120(4):957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Helwig U., Imai K., Schmahl W., Thomas B. E., Varnum D. S., Nadeau J. H., Balling R. Interaction between undulated and Patch leads to an extreme form of spina bifida in double-mutant mice. Nat Genet. 1995 Sep;11(1):60–63. doi: 10.1038/ng0995-60. [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Wang S., Dörfler P., Adams B., Busslinger M. The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol Cell Biol. 1992 Jun;12(6):2662–2672. doi: 10.1128/mcb.12.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B., Kuratani S., Cooney A. J., Wawersik S., Tsai S. Y., Eichele G., Tsai M. J. Developmental regulation of the orphan receptor COUP-TF II gene in spinal motor neurons. Development. 1994 Jan;120(1):25–36. doi: 10.1242/dev.120.1.25. [DOI] [PubMed] [Google Scholar]
- Moase C. E., Trasler D. G. Delayed neural crest cell emigration from Sp and Spd mouse neural tube explants. Teratology. 1990 Aug;42(2):171–182. doi: 10.1002/tera.1420420208. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Ordahl C. P., Le Douarin N. M. Two myogenic lineages within the developing somite. Development. 1992 Feb;114(2):339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Kaul K., Braun M. J., Gonda M. A., Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquié O., Coltey M., Bréant C., Le Douarin N. M. Control of somite patterning by signals from the lateral plate. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3219–3223. doi: 10.1073/pnas.92.8.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt S. C. Expression of Pax-3- and neuroectoderm-inducing activities during differentiation of P19 embryonal carcinoma cells. Development. 1992 Nov;116(3):573–583. doi: 10.1242/dev.116.3.573. [DOI] [PubMed] [Google Scholar]
- Pöpperl H., Bienz M., Studer M., Chan S. K., Aparicio S., Brenner S., Mann R. S., Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995 Jun 30;81(7):1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Richardson J., Cvekl A., Wistow G. Pax-6 is essential for lens-specific expression of zeta-crystallin. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P. M., Teillet M. A., Ziller C., Le Douarin N. M. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992 Jul;115(3):657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- Shapiro D. N., Sublett J. E., Li B., Downing J. R., Naeve C. W. Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res. 1993 Nov 1;53(21):5108–5112. [PubMed] [Google Scholar]
- Sonnenberg E., Meyer D., Weidner K. M., Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993 Oct;123(1):223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T., Read A. P. PAX genes. Curr Opin Genet Dev. 1994 Jun;4(3):427–438. doi: 10.1016/0959-437x(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Sublett J. E., Jeon I. S., Shapiro D. N. The alveolar rhabdomyosarcoma PAX3/FKHR fusion protein is a transcriptional activator. Oncogene. 1995 Aug 3;11(3):545–552. [PubMed] [Google Scholar]
- Treisman J., Harris E., Desplan C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991 Apr;5(4):594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]
- Tsarfaty I., Rong S., Resau J. H., Rulong S., da Silva P. P., Vande Woude G. F. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994 Jan 7;263(5143):98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- Walther C., Guenet J. L., Simon D., Deutsch U., Jostes B., Goulding M. D., Plachov D., Balling R., Gruss P. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991 Oct;11(2):424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Williams B. A., Ordahl C. P. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994 Apr;120(4):785–796. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- Xu W., Rould M. A., Jun S., Desplan C., Pabo C. O. Crystal structure of a paired domain-DNA complex at 2.5 A resolution reveals structural basis for Pax developmental mutations. Cell. 1995 Feb 24;80(4):639–650. doi: 10.1016/0092-8674(95)90518-9. [DOI] [PubMed] [Google Scholar]
- Yang X. M., Park M. Expression of the met/hepatocyte growth factor/scatter factor receptor and its ligand during differentiation of murine P19 embryonal carcinoma cells. Dev Biol. 1993 Jun;157(2):308–320. doi: 10.1006/dbio.1993.1137. [DOI] [PubMed] [Google Scholar]
- Zannini M., Francis-Lang H., Plachov D., Di Lauro R. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol. 1992 Sep;12(9):4230–4241. doi: 10.1128/mcb.12.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]