Abstract
Neuroscience research has thoroughly studied how nonliteral language is processed during metaphor comprehension. However, it is not clear how the brain actually creates nonliteral language. Therefore, the present study for the first time investigates the neural correlates of metaphor production. Participants completed sentences by generating novel metaphors or literal synonyms during functional imaging. Responses were spoken aloud in the scanner, recorded, and subsequently rated for their creative quality. We found that metaphor production was associated with focal activity in predominantly left-hemispheric brain regions, specifically the left angular gyrus, the left middle and superior frontal gyri—corresponding to the left dorsomedial prefrontal (DMPFC) cortex—and the posterior cingulate cortex. Moreover, brain activation in the left anterior DMPFC and the right middle temporal gyrus was found to linearly increase with the creative quality of metaphor responses. These findings are related to neuroscientific evidence on metaphor comprehension, creative idea generation and episodic future thought, suggesting that creating metaphors involves the flexible adaptation of semantic memory to imagine and construct novel figures of speech. Furthermore, the left DMPFC may exert executive control to maintain strategic search and selection, thus facilitating creativity of thought.
Keywords: Language production, Metaphor, Mental simulation, Creativity, fMRI
Highlights
-
•
Figurative language production was studied for the first time with fMRI.
-
•
Verbal responses were recorded and analyzed for quality.
-
•
Generation of novel metaphors relies on activity in left AG and the PCC.
-
•
Activity in left DMPFC increases linearly as a function of metaphor creativity.
Introduction
From eminent poetry to everyday prose, metaphor is a familiar form of figurative language. Such nonliteral expressions are widely used to express symbolism in the arts (Kennedy, 2008) and convey imagery in everyday conversations (Carter, 2004). Psycholinguistic (Gibbs, 1994; Kintsch, 2000; Lackoff and Johnson, 1980) and neuroscientific (Mashal et al., 2007; Rapp et al., 2004) research has thoroughly investigated the cognitive processes and neural correlates of metaphor comprehension. Yet little is known about how new metaphors are produced. Recent behavioral research has begun to shed light on the cognitive abilities underlying metaphor production (Beaty and Silvia, 2013; Chiappe and Chiappe, 2007; Silvia and Beaty, 2012), and suggests an important role of controlled attention and strategic semantic search processes. Nevertheless, an investigation of how the brain produces new metaphors remains elusive. In the present study, we explored this question by taking a first look at the neural correlates of figurative language production.
Metaphor comprehension and production
Metaphor comprehension involves forming an abstract connection between two concepts in semantic memory. Such a link, or attributive category, is established by extracting and relating similar properties of different concepts in memory (Glucksberg, 2001, 2003). For example, the metaphor music is medicine involves identifying the conceptual category “something that is healing”, abstracting the properties of music and medicine that are related, and inhibiting the properties that are unrelated. This model has also been used to conceptualize metaphor production. Recently, Beaty and Silvia (2013) examined the cognitive processes involved in producing conventional (i.e., familiar) and creative (i.e., novel) metaphors. The ability to produce creative metaphors was more strongly associated with fluid intelligence and verbal fluency, pointing to the involvement of executive functions; in contrast, the ability to produce conventional metaphors was associated with general vocabulary knowledge. The processes involved in verbal fluency tasks mirror some of the theoretical functions of metaphor comprehension; for example, verbal fluency requires the generation and maintenance of a semantic cue (e.g., searching memory for synonyms for “good”), which closely resemble the demands of an attributive category (searching memory for “something that is healing”). Taken together, metaphor comprehension and production thus seem to involve some of the same underlying cognitive processes.
Neuroscientific research on metaphor has, so far, largely focused on metaphor comprehension. Such studies typically contrast brain activation during passive processing of literal with nonliteral statements (e.g., Rapp et al., 2004). Recently, a number of meta-analyses have tried to summarize findings across fMRI studies on figurative language processing (Bohrn et al., 2012; Rapp et al., 2012; Vartanian, 2012; Yang, 2012). These meta-analyses report consistent patterns of activation in frontal, temporal and parietal regions located predominately in the left hemisphere. The processing of nonliteral sentences was commonly related to activations in the left inferior frontal gyrus (IFG), left middle and superior temporal gyri (MTG and STG), and left inferior parietal cortex (IPC), and parahippocampal gyri.
These brain regions are believed to play discriminable roles for the comprehension of nonliteral language. Metaphors are usually not correct in a literal sense and thus can only be understood when the nonliteral meaning is extracted. Traditional views on metaphor processing assume that the literal meaning has to be processed and discarded in the first place, paving the way for a subsequent recognition of the nonliteral meaning (e.g., Clark and Lucy, 1975). According to the “parallel hypothesis” both meanings are processed concurrently (McElree and Nordlie, 1999). In this context, the left IFG (BA45/47) is thought to be relevant for the selection of the appropriate meaning and the suppression of inappropriate or irrelevant meanings (Badre and Wagner, 2007; Glucksberg et al., 2001; Rapp et al., 2012). Metaphor processing was also consistently related to activations in the left MTG and STG. The MTG and STG are at the core of a richly interconnected language network reaching to frontal and parietal structures and thus are conceived to play a general role in language comprehension (Turken and Dronkers, 2011) that may be especially taxed during the probably more complex processing of figurative language. Finally, the left IPC, and more specifically the left angular gyrus (AG), are thought to play an important role for metaphor processing through its function to integrate individual conceptual representations into a coherent meaning (e.g., Bambini et al., 2011; Binder et al., 2009).
While language processing is traditionally known to be dominant in the left hemisphere, a number of studies examining figurative language processing deficits in patients with unilateral brain damage suggested an important role of the right hemisphere for comprehending figurative language (Schmidt et al., 2010; Thoma and Daum, 2006). In this context, it was suggested that the specific neuroanatomic structure of right-hemispheric language areas results in a coarser semantic coding of information that may facilitate coactivation between remote semantic concepts (Jung-Beeman, 2005). Findings from fMRI studies, however, have been inconsistent (e.g., Rapp et al., 2007) and meta-analytic evidence does not support a strong specific role of the right hemisphere in metaphor processing (Bohrn et al., 2012; Rapp et al., 2012).
A more consistent involvement of the right hemisphere has been observed in studies comparing the processing of novel versus conventional metaphors (Mashal et al., 2009; Rutter et al., 2012; Subramaniam et al., 2012). Unfamiliar metaphoric expressions appear to recruit different frontal brain regions, including the bilateral IFG and left middle frontal gyrus, as well as temporal regions of the right hemisphere (Bambini et al., 2011; Mashal et al., 2008, 2009; Rutter et al., 2012; Yang, 2012). This is in line with the “graded salience hypothesis” (Giora, 1997), which assumes that the right hemisphere is particularly involved in the processing of novel, non-salient figurative language. In contrast, in familiar metaphors, the metaphoric meaning is salient and hence does not depend as much on right hemispheric processing.
Metaphor and creative idea generation
The study of metaphor production offers a new approach to the longstanding problem of how people come up with new ideas. Previous neuroimaging studies have used a range of approaches to investigate the brain regions involved in different types of creative cognition, such as insight problem solving, creative idea generation (i.e., divergent thinking), story generation, and visual problem solving (e.g., Aziz-Zadeh et al., 2012; Bowden et al., 2005; Fink et al., 2009; Goel and Vartanian, 2005; Howard-Jones et al., 2005; for reviews, see Arden et al., 2010; Dietrich and Kanso, 2010; Fink and Benedek, in press). Studies focusing on divergent thinking usually ask participants to generate novel responses to open-ended problems. For example, Fink et al. (2009) compared performance on tasks with greater creative demands (i.e., generating novel uses for objects) with tasks involving lower creative demands (i.e., generating typical characteristics of objects). Generating novel ideas was associated with increased activation in the left angular gyrus and decreased activation in the right temporoparietal junction (see also Abraham et al., 2012).
Furthermore, Benedek et al. (2013) assessed the novelty of verbal responses to an alternate uses task during functional imaging. Generating novel uses—responses participants identified as unfamiliar to them prior to scanning—was related to stronger activation in the left inferior parietal cortex as compared to generating previously known uses—responses participants had retrieved from memory. The left inferior parietal cortex plays an important role in semantic integration (Binder et al., 2009) and mental simulation (Hassabis and Maguire, 2007). This region is thought to contribute to the brain's ability to flexibly recombine stored information in memory into novel mental representations (e.g., episodic future thinking; Cabeza et al., 2008; Schacter et al., 2007, 2012). Finally, there is evidence that the generation of more creative ideas is related to activation of left prefrontal brain regions (Benedek et al., 2013; Fink et al., 2012), possibly subserving executive processes needed to inhibit dominant response tendencies. Taken together, several related literature provide converging evidence on how the brain integrates knowledge to produce novel ideas; however, the extent to which such processes contribute to the production of figurative language remains unknown.
The present research
The present study used fMRI to examine the neural correlates of figurative language production. We presented participants with brief phrases relating objects to characteristics (e.g., the lamp is [glaring]), and asked them to complete the phrases with metaphors or literal expressions. Responses were spoken aloud in the scanner, recorded, and later coded for accuracy and creative quality. The present research had two goals: (1) to provide a first look at the neural correlates of metaphor production, and (2) to determine what brain regions are related to the creativity of responses. Based on the available evidence on metaphor processing and creative idea generation, metaphor generation should be associated with focal activity in the left hemisphere, especially the left inferior parietal cortex (IPC). Moreover, based on the evidence on metaphor novelty and creativity, we expected the creative quality of metaphor responses to be associated with activation in the left prefrontal cortex (PFC) and potentially with an additional recruitment of the right hemisphere.
Material and methods
Participants
The original sample consisted of 32 adults. Four participants were excluded, two for excessive head movements (> 1.5 mm without online motion correction), one for noncompliance, and one for aborting the scanner session early. After exclusions, the final sample consisted of 28 healthy adults (18 females; mean age: 26.2 years, age range: 19–49). The participants were drawn from a larger pool recruited via newspaper advertisement. All participants were right-handed native-German speakers, with normal or corrected-to-normal vision and no reported history of CNS-affecting drugs or neurological disease. Participants gave written informed consent and were paid for participation. The study was approved by the local ethics committee.
Experimental task and procedure
Participants worked on a metaphor production task and a control task that required production of literal responses (i.e., synonyms). Both tasks presented short phrases relating a noun to an adjective in parentheses, e.g., “The lamp is (glaring)”. In the metaphor production task, participants were asked to produce a creative (i.e., novel and appropriate) metaphor that conveys the meaning of the adjective, and thus may replace it in the phrase (e.g., “a supernova”). In the literal control task, participants were asked to produce a synonym that conveys the meaning of the adjective as closely as possible, and thus may replace it in the phrase (e.g., “bright”).
The sentences were presented in white letters at the middle of a black screen. In both tasks, participants had 10 s to think of a response. If they produced a response in less than 10 s, they were encouraged to come up with an even more creative metaphor, or a more adequate synonym, respectively. After 10 s, the stimulus turned green for 5 s, indicating that participants should now vocalize their response (see Fig. 1). The temporal separation of idea generation and response periods is commonly employed in neuroscientific studies on creative idea generation to avoid artifacts related to overt responses (Fink and Benedek, in press). Participants were told to respond only with the new continuation of the sentence, not to repeat the entire sentence (e.g., “a supernova,” not “The lamp is a supernova.”). If they were unable to come up with a response, they were asked to respond with “don't know”. The responses were recorded by means of an MRI-compatible microphone and transcribed for further analyses.
Fig. 1.
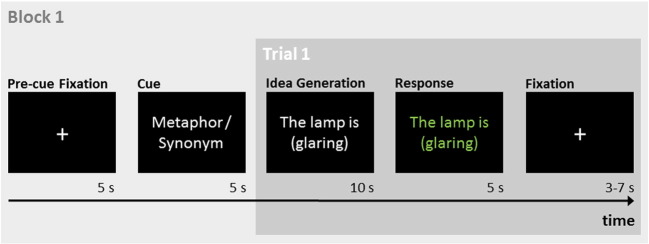
Schematic sequence of first trial within a task block. After an initial fixation period a cue indicated whether participants should generate metaphors or synonyms (literal control task) in this block. In each trial, participants had 10 s to complete the sentence by generating a metaphor, or a synonym. Responses were given in the subsequent response period (5 s) indicated by the stimulus word changing its color to green. Trials were separated by jittered fixation periods.
Participants performed a total of 48 trials using 48 different stimulus phrases (see Appendix). Some of the phrases were adapted from previous behavioral studies on metaphor production (Beaty and Silvia, 2013; Chiappe and Chiappe, 2007) and others were devised by the authors. For each participant, half of the phrases were randomly assigned to either task (i.e., metaphor and literal). To maximize the power of the task contrast, trials were grouped to eight task blocks (four metaphors, four synonyms) in an ABBAABBA/BAABBAAB fashion, with each block containing six trials of one task.
Fig. 1 depicts the experimental paradigm. A block started with a fixation period (5 s), followed by a cue (5 s) indicating the task to be performed in that block (metaphor or synonym). After the cue, six trials were presented separated in time by jittered (3–7 s) fixation null periods. Additional 10-s fixation periods were presented at the beginning and end of the session.
Before the scanner session, participants received thorough task instructions explaining the difference between metaphoric and literal responses followed by eight exercise trials. Participants then performed the tasks in a single fMRI run, a T1-scan, and another unrelated task. After the scanner session, participants rated the difficulty of the metaphor and synonym task on a 5-point rating scale from 1 (very easy) to 5 (very difficult).
Imaging procedure
Whole brain imaging was performed on a 3T Siemens Skyra MRI system (Siemens Medical Systems, Erlangen, Germany) using a 32-channel head coil. BOLD-sensitive T2*-weighted functional images were acquired using a single shot gradient-echo EPI pulse sequence (TR = 2400 ms, TE = 30 ms, flip angle = 90°, 35 axial slices, 3.5 × 3.5 × 3.5 mm, distance factor 20%, FoV = 240 × 240 mm, interleaved slice ordering) and corrected online for head motion. The first two volumes were discarded to allow for T1 equilibration effects. Head motion was restricted using firm padding that surrounded the head. Visual stimuli were presented using the software Presentation (Neurobehavioral Systems, Albany, CA) onto a screen and viewed through a mirror attached to the head coil. Verbal responses were recorded by means of a MRI-compatible noise canceling microphone (FOMRI-III; Optoacoustics, Mazor, Israel) also attached to the head coil.
Analysis of response behavior
All responses were transcribed to a spreadsheet and pooled for each item and task across participants, resulting in 48 item-specific response lists for both tasks. Responses were examined for validity by two raters who attained consensual agreement on the accuracy of responses. The raters marked responses as invalid when participants responded “don't know” or when they gave a literal response in the metaphor task, or vice versa.
Metaphor responses were also scored for creative quality using the subjective scoring method (Benedek et al., 2013; Christensen et al., 1957; Silvia et al., 2008). Three raters scored responses independently using a three-point scale (1 = not at all creative, 3 = very creative). The raters were trained to score responses based on criteria of remoteness, novelty, and cleverness (Christensen et al., 1957). Remoteness reflected the conceptual distance of the response from the topic; novelty reflected originality; and cleverness reflected whether a response was witty, funny, or interesting. The three criteria were factored into a single, holistic score and applied to each response (Beaty and Silvia, 2013; Silvia and Beaty, 2012).
Functional imaging analysis
Functional MRI data analysis was performed using SPM 8 software (Wellcome Department of Imaging Neuroscience, London, UK). For each participant, approximately 450 functional images were obtained. Preprocessing steps included slice time acquisition correction, motion correction, spatial normalization to an averaged EPI template in standard Montreal Neurological Institute (MNI) space, and smoothing with a 10-mm full-width at half-maximum Gaussian kernel.
Effects were estimated with a subject-specific fixed effects model including the conditions CUE (i.e., task cue), METAPHOR (i.e., generating metaphor responses), LITERAL (i.e., generating synonym responses), and SPEECH (i.e., vocalization of responses). Generation periods (metaphor or literal) that did not result in valid responses were modeled as separate regressors of no interest, as were motion parameters. Linear contrasts were used to obtain subject-specific estimates for each effect. These estimates were entered into a second-level analysis treating subjects as a random effect with a one-sample t-test against a contrast value of zero at each voxel.
The brain activation specific for metaphor production was examined with the contrast of METAPHOR > LITERAL and LITERAL > METAPHOR, respectively. Moreover, we performed a parametric analysis to examine the brain regions sensitive to the creativity of metaphor responses. To this end, we added a regressor to the first-level model coding the average creativity rating (averaged across raters) of each valid metaphor response. Voxel-based results are reported when they are significant at a level of p < .05, corrected for multiple comparisons by means of family-wise error (FWE) correction. Finally, the direction (activation or deactivation) and amplitude of the signal change over time was explored for all significant task effects using MarsBaR 0.43 (Brett et al., 2002).
Results
Behavioral results
On average, participants were able to produce valid responses in 87% of metaphor trials and 90% of the literal control trials, thus showing no significant performance differences between tasks, t(27) = 1.50, p = .15. Moreover, self-reported task difficulty did not differ between tasks (mean difficulty rating: 2.18, and 1.96 for metaphor and literal, respectively; t(27) = 1.24, p = .23). The metaphor creativity scores from the three raters were averaged to form a composite for analysis (mean rating = 1.63, SD = 0.15).
Neural correlates of metaphor production
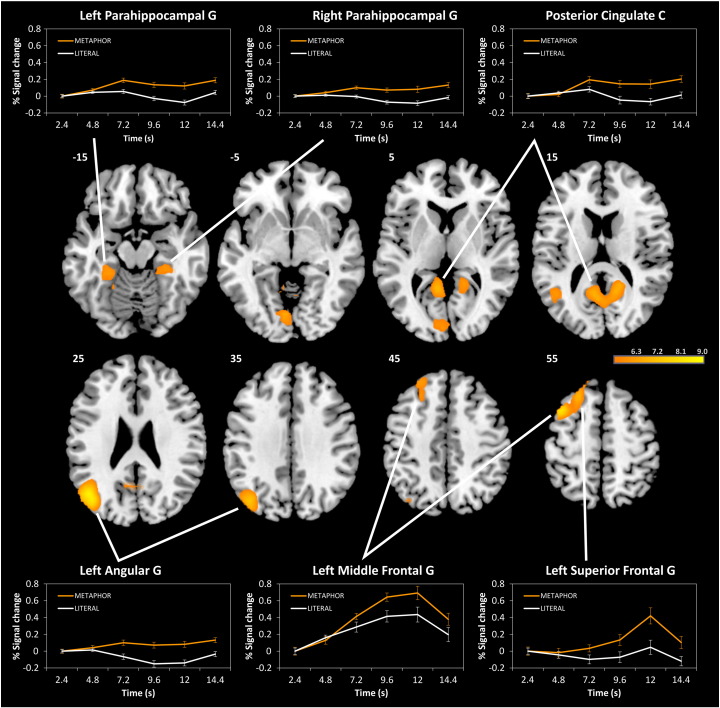
The whole-brain contrast of the tasks (METAPHOR > LITERAL; p < .05, FWE corrected, k > 20) revealed that metaphor production was associated with stronger brain activation than the control condition in seven clusters (see Fig. 2 and Table 1). The strongest effect was observed in the left inferior parietal cortex, peaking in the left angular gyrus (AG) and extending to posterior parts of the middle temporal gyrus (MTG) and adjacent occipital regions. Signal change analyses showed that activation in the left AG increased during metaphor production but decreased during the literal control task (see Fig. 2).
Fig. 2.
Whole brain analysis (T-maps) of the task contrast METAPHOR > LITERAL. Significant activation clusters (p < .05, FWE corrected, k > 20) are shown at different axial slices (z = − 15, − 5, 5, 15, 25, 35, 45, and 55). Additional, signal change is plotted over time (TR 1 to TR 6 after onset of idea generation period, corresponding to 2.4 to 14.4 s, respectively) for significant activation clusters. G = gyrus, C = cortex.
Table 1.
Whole-brain task effects (METAPHOR vs. LITERAL).
| Brain area | BA | MNI coordinates (x, y, z) | k | Peak T | ||
|---|---|---|---|---|---|---|
| METAPHOR > LITERAL | ||||||
| L angular G, L MTG | 39 | − 47 | − 67 | 24 | 224 | 8.99 |
| L MFG (DMPFC) | 6/8 | − 40 | 11 | 59 | 122 | 8.37 |
| L SFG (DMPFC) | 8 | − 26 | 42 | 48 | l.m. | 6.62 |
| PCC, precuneus | 23/30 | 13 | − 56 | 17 | 289 | 7.33 |
| L lingual G | 18 | − 8 | − 81 | − 5 | 103 | 7.11 |
| L Parahipp. G, fusiform G | 37 | − 33 | − 35 | − 19 | 88 | 6.84 |
| R posterior cerebellum | 20 | − 77 | − 33 | 49 | 6.83 | |
| R Parahipp. G, fusiform G | 37 | 27 | − 32 | − 22 | 35 | 6.62 |
| LITERAL > METAPHOR | ||||||
| – | ||||||
Notes: MTG = Middle temporal gyrus, MFG = Middle frontal gyrus, SFG = Superior frontal gyrus, DMPFC = Dorsomedial prefrontal cortex, PCC = Posterior cingulate cortex, Parahipp. = Parahippocampal, G = gyrus; l.m. = local maximum. Results are corrected for multiple comparisons (p < .05, FWE-corrected, k > 20).
Metaphor production was also related to stronger activation in a left-hemispheric cluster comprising the dorsal–medial middle frontal gyrus (MFG) and dorsal superior frontal gyrus (SFG) which has been labeled dorsomedial prefrontal cortex (DMPFC; cf. Binder et al., 2009). To analyze signal change in the left DMPFC separately for the MFG and the SFG subregions, we generated ROIs at their local peaks (see Table 1) with a sphere of 5 mm. In the left MFG, brain activation strongly increased from the beginning during both tasks; however, this activation was stronger during metaphor production than during the control task. In the SFG, a significant activation increase was only observed during metaphor production and especially towards the end of the task. Metaphor production was also related to significantly stronger bilateral activation of the posterior cingulate cortex (PCC) and the adjacent ventral precuneus. Finally, metaphor production was related to stronger activations in bilateral parahippocampal and fusiform gyri, as well as in the left lingual gyrus and the right posterior cerebellum. The reversed contrast (LITERAL > METAPHOR) did not reveal further significant effects.
Neural correlates of metaphor creativity
A parametric analysis was used to analyze the brain activation related to the creative quality of metaphor responses (p < .05, FWE corrected). This analysis revealed that brain activity linearly increased with creativity ratings in the central dorsomedial part of the left SFG, corresponding to the anterior DMPFC (peak coordinates x, y, z = − 15, 42, 52; k = 3, Tmax = 6.28), as well as in the right anterior middle temporal gyrus (MTG; peak coordinates x, y, z = 48, 0, − 22; k = 8, Tmax = 5.88). Notably, the activation cluster in the DMPFC overlapped with the significant DMPFC cluster from the task contrast (METAPHOR > LITERAL). Metaphor creativity was not associated with any significant decreases in brain activation.
Task-general effects
For reasons of comparison with other studies of idea generation, we also report the task-general activation pattern related to both tasks (metaphor production and synonym production; METAPHOR & LITERAL > 0; p < .05, FWE corrected). The tasks were associated with brain activation in extended brain areas, most prominently in the left inferior frontal gyrus (IFG), left middle temporal gyrus (MTG), bilaterally in the insula, the precentral gyrus, the lingual gyrus, and the posterior cerebellum, and with deactivations (METAPHOR & LITERAL < 0) in the right temporoparietal junction (TPJ) and, to a weaker extent, in the left inferior parietal cortex (IPC) and the anterior cingulate (AC).
Discussion
The present study investigated the neural correlates of metaphor generation. Participants generated novel metaphors or literal responses in the scanner, and functional imaging was used to explore the brain regions unique to producing metaphors. We found that metaphor production was associated with increased activation in predominantly left-hemispheric brain regions, specifically the left angular gyrus (AG), the left dorsomedial prefrontal cortex (DMPFC), and the posterior cingulate cortex (PCC). Moreover, brain activation in the left DMPFC and the right middle temporal gyrus (MTG) increased as a function of the creative quality of responses. These results are discussed in the context of the literatures on metaphor processing and creative cognition.
Neural correlates of metaphor production
As expected, metaphor production was related to a left-lateralized activation pattern, including activation of the left AG. The left AG is part of the inferior parietal cortex (IPC) and has been consistently implicated in metaphor processing (Rapp et al., 2012) as well as creative idea generation (Fink et al., 2009). In a recent meta-analysis of 120 imaging studies, the left AG was identified as the most consistently activated region during tasks involving semantic processing (Binder et al., 2009). Due to its involvement in a variety of semantic processes, the left AG has been conceived as a supramodal association area, one that plays a key role in strategic knowledge retrieval and complex information integration. Further overlap with regions involved in metaphor processing was observed in the parahippocampal gyri. The parahippocampal gyri are considered part of the medial temporal lobe (MTL), a system that is essential for declarative memory (Squire et al., 2004). Together, these regions appear to be relevant for nonliteral language processing in general—both comprehension and production—by activating and relating shared semantic information between remotely associated concepts.
Meta-analyses on metaphor processing also consistently report brain activation in left IFG and left MTG (e.g., Bohrn et al., 2012; Rapp et al., 2012; Vartanian, 2012; Yang, 2012). Although these brain regions were found to be activated during both metaphoric and literal response generation, no significant task differences were observed in this study. The left IFG is conceived to be relevant for the evaluation and selection of meaning (Badre and Wagner, 2007; Turken and Dronkers, 2011). A central difference between metaphor comprehension and metaphor production tasks is that the former requires the extraction of the relevant semantic property conveyed by the metaphor, whereas in the latter, the relevant semantic feature is explicitly cued, and it requires finding a metaphor that serves as a vehicle for it. Therefore, since metaphor production does not primarily require the extraction of meaning behind a given metaphor but rather its generation, this may provide one explanation for the absence of activation differences in the left IFG.
While our results suggest that metaphor comprehension and production share some common neural substrates, we found several brain regions that appear to be unique to production. The most notable regions include the left DMPFC, the PCC, and the left lingual gyrus. The DMPFC encompasses the dorsal SFG extending to the posterior-medial part of the left MFG, roughly corresponding to BA 8 (Binder et al., 2009). Lesion studies have shown that damage to this region causes transcortical motor aphasia (Alexander and Benson, 1993; Freedman et al., 1984). Patients suffering from this condition can normally repeat words and name objects, but they are unable to generate responses from a larger set of possibilities (Robinson et al., 1998). Therefore, it was suggested that the DMPFC is specifically relevant for “self-guided, goal-directed retrieval of semantic information” and needed to “invent nonformulaic responses” (p. 2777; Binder et al., 2009).
This observation may provide a key insight into the nature of figurative language production. In this study, the DMPFC showed increased activation in both the main contrast of interest (i.e., METAPHOR > LITERAL) and as a function of response quality (i.e., creativity ratings). The metaphor production task was open-ended, and thus the range of possible responses was large. The range of responses to a given prompt appeared to be limited solely by the verbal ability and creative potential of the participants, which is a central characteristic of creative idea generation tasks. The DMPFC may therefore play an important role in the generation—or response invention (cf., Binder et al., 2009)—of new and meaningful figurative language, by supporting the effective goal-maintenance required for controlled semantic retrieval and the selection (and inhibition) of responses from a larger set of possibilities.
We also found that metaphor production was related to stronger activation in the left PCC. The PCC has been implicated in episodic memory retrieval (e.g., Vincent et al., 2006) and visuospatial mental imagery (e.g., Hassabis and Maguire, 2007). It is also worth noting that the AG showed the strongest effect in this study but only a minor effect in a recent meta-analysis on metaphor processing (Rapp et al., 2012). This suggests that the left AG could be even more relevant for metaphor production than for metaphor comprehension. The PCC and the left AG have both been conceived as central components of the semantic memory system (Binder et al., 2009). On the other hand, these regions have also been tied to the brain's default mode network (e.g., Raichle et al., 2001; see also, Seghier et al., 2010). It was proposed that task-unrelated and self-directed thoughts—trademarks of default mode activity—are essentially semantic cognitions because they involve the activation and manipulation of acquired knowledge (Binder et al., 2009). Furthermore, the activation of default mode network regions are also observed during forms of mental simulation involving spatial navigation or taking the perspective of others (Buckner and Carroll, 2007). Recent research on episodic memory has shown that the PCC and the lateral parietal cortex—together with medial–frontal and temporal regions—show comparable activation when participants are asked to recall an event from their past or imagine an event in the future (Addis et al., 2007; Schacter et al., 2007, 2012; Szpunar, 2010). These findings helped to inform the constructive hypothesis of episodic memory (cf., Hassabis and Maguire, 2007; Schacter et al., 2007), and led to the notion that retrieving information from past experiences is essential for constructing novel representations of the future. Similarly, theories of creative cognition are grounded in the assumption that novel ideas result from the recombination of relevant memory elements (Koestler, 1964; Mednick, 1962). When conceiving metaphor generation as a creative idea generation task (Beaty and Silvia, 2013; Silvia and Beaty, 2012), it becomes obvious that this task relies on the retrieval of acquired knowledge from memory which needs to be integrated to form a novel figures of speech.
Similar reasoning has been applied in another recent study on the neural basis of creative idea generation (Benedek et al., 2013). This study found that creating novel uses for objects elicited strong activation in the left inferior parietal cortex—a region tied to mental simulation and “mental time travel” (e.g., Nyberg et al., 2010; Schacter et al., 2007). Moreover, activation of the left AG was also found to be stronger during divergent thinking tasks that involve higher creative task demands (Fink et al., 2009). Taken together, metaphor production and mental simulation may both rely on common generative processes, drawing on stored knowledge to imagine and construct novel mental representations.
Neural correlates of metaphor creativity
Our analysis took a fine-grained approach to examining the role of novelty in metaphor production. We examined brain activation related to creative quality at the level of single ideas. Parametric analyses showed that activation linearly increased with creative quality in the left anterior DMPFC (dorsomedial part of SFG) and the right MTG. The cluster in the DMPFC overlaps with the DMPFC cluster observed the general task contrast (METAPHOR > LITERAL), suggesting that activity in this area is associated with creativity-related demands at both task and idea levels. The parametric effect in the left DMPFC supports our hypothesis that creativity of metaphors should be associated with activation of the left prefrontal cortex (PFC). Similarly, Fink et al. (2012) found greater activation in the same SFG region within the DMPFC after stimulating creativity by confronting people with common ideas generated by other people. In yet another related study, cognitive stimulation during creative idea generation also led to higher relative brain activation in the left medial superior frontal gyrus (Fink et al., 2010). Finally, Benedek et al. (2013) observed parametric effects of idea creativity in the alternate uses task located in the orbital part of the left inferior frontal gyrus. Taken together, converging evidence suggests that the left PFC might play a key role for the creativity aspect of novel ideas.
The present study may also be seen to provide parallels to behavioral studies of creative cognition. For example, the ability to generate creative ideas has been linked to higher-order cognitive abilities such as fluid intelligence (Beaty and Silvia, 2013; Jauk et al., 2013, in press), and executive processes such as pre-potent response inhibition (Benedek et al., 2012a). Such abilities are thought to play a key role in providing top-down control of attention and cognition during creative idea generation, by maintaining the task goal, exerting cognitive inhibition, and deploying strategic semantic search processes (Beaty and Silvia, 2012; Benedek and Neubauer, 2013; Benedek et al., 2012b; Gilhooly et al., 2007; Nusbaum and Silvia, 2011). The DMPFC has previously been implicated in goal-maintenance and uncued semantic retrieval during tasks that involve the flexible, non-formulaic use of language (Binder et al., 2009). Activation of the DMPFC during metaphor generation could thus reflect executive mechanisms needed to inhibit dominant responses or meanings (e.g., inhibiting literal in favor of nonliteral interpretations; Glucksberg et al., 2001; Thoma and Daum, 2006) and maintain the semantic search process en route to an original figurative response. Interestingly, signal change analysis indicated that activation in the anterior DMPFC increased only at the very end of the task. This delayed effect may correspond to an influence of executive processes at a later stage in the production process, whereby a larger number of competing responses are inhibited once a more adequate response is found. Alternatively, the DMPFC may also play an evaluative role, such as determining whether an idea fits the goal of the task (i.e., discernment; Silvia, 2008). Future research should further examine the DMPFC's role in creative thought.
The creative quality of metaphors was also related to greater activation of the right anterior MTG. Several studies reported that the right hemisphere plays a role for the processing of novel metaphors or non-salient meaning in language (e.g., Bambini et al., 2011; Bottini et al., 1994; Giora et al., 2000; Mashal et al., 2008; Pobric et al., 2008; Rapp et al., 2012; Yang, 2012). This finding is in line with the hypothesis that the generation of novel, creative metaphors may likewise lead to an additional recruitment of right-hemisphere regions. Specifically, it was proposed that right hemisphere regions are involved in coarse semantic processing (Jung-Beeman, 2005) and related to processing of non-salient semantic meanings as stated by the graded salience hypothesis (Giora, 1997).
The process of idea generation
The generation of metaphors and synonyms can be generally considered as divergent thinking tasks (i.e., idea generation tasks), since these tasks have various possible solutions that differ in quality (Guilford, 1967). The present results replicated the finding that divergent thinking is generally associated with strong activation in the left inferior frontal gyrus (IFG) and with deactivation in the right temporoparietal junction (TPJ; Abraham et al., 2012; Benedek et al., 2013; Fink et al., 2009). The IFG is known to be involved in general semantic processing and has been especially associated with verbal fluency (Binder et al., 2009; Costafreda et al., 2006). The sustained deactivation of the right TPJ is thought to indicate focused attention which helps to prevent reorienting to distracting bottom-up stimuli during divergent thought (Berkowitz and Ansari, 2010; Corbetta and Shulman, 2002; Corbetta et al., 2008). This is in line with consistent reports of increased EEG alpha activity (i.e., alpha synchronization) over the right parietal cortex during different types of divergent thinking tasks (Benedek et al., 2011; Fink and Benedek, 2013, in press).
Strengths, limitations, and future directions
The present study was strengthened by our ability to capture verbal responses in the scanner. This allowed us to monitor the accuracy of task performance, a methodological approach not possible in studies employing silent response generation. Moreover, recording verbal responses enabled a unique look at the creative quality of each idea, and a further examination of how quality related to brain activation. Our design was somewhat limited, however, by allowing only very brief periods of time for responses to be developed. Past research has found a strong correlation between time-on-task and creative quality of novel metaphors (Silvia and Beaty, 2012). The present design was constrained by the need to use a brief task that affords associating brain activity with well-defined cognitive processes. Nevertheless, this did not seem to compromise the results, as we were still able to capture an adequate range of differences in task performance and isolate regions specific to metaphor quality.
Our study suggests interesting parallels between the neural correlates of metaphor production and comprehension, but the basis for making inferences regarding such processes is limited. It certainly would have been interesting to directly contrast metaphor production with comprehension in the scanner; however, this was complicated by the fact that the generation of metaphors usually takes substantially longer than comprehension of metaphors. Future research should attempt to equate metaphor production and comprehension tasks, to allow for a direct comparison within the same experimental paradigm. Finally, one might assume that generating metaphors is more difficult than generating synonyms which might bias the contrast of these two tasks. However, pilot tests suggested that generating semantically accurate synonyms can also be quite difficult. This was confirmed by analyses of behavioral performance in the scanner showing that the tasks did neither differ in self-rated difficulty nor in the number of valid responses. We conclude that task difficulty does not have a major effect on our findings.
Conclusion
The present study examined the neural correlates of figurative language production. Our findings suggest that the generation of novel metaphors particularly relies on the left AG and the PCC, supporting the flexible integration of knowledge for the construction of novel semantic representations. Furthermore, the left DMPFC, which was activated during both metaphor production and as a function of metaphor creativity, is assumed to exert executive control to facilitate strategic retrieval processes and inhibit dominant or literal concepts. Taken together, this study provides a first investigation of the neural correlates of figurative language production, and points to an important role of left prefrontal and lateral parietal brain regions for the generation of new metaphors.
Acknowledgments
This research was supported by a grant from the Austrian Science Fund (FWF): P23914. The authors are grateful to Michael Achtner, Philip Brandner, Alexandra Lipfert, Jürgen Pretsch, and Martin Wammerl for their help in this study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
Supplementary material.
References
- Abraham A., Pieritz K., Thybush K., Rutter B., Kröger S., Schweckendiek J., Stark R., Windmann S., Hermann C. Creativity and the brain: uncovering the neural signature of conceptual expansion. Neuropsychologia. 2012;50:1906–1917. doi: 10.1016/j.neuropsychologia.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Addis D.R., Wong A.T., Schacter D.L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuroimage. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M.P., Benson D.F. The aphasias and related disturbances. In: Joynt R.J., editor. Clinical Neurology. J.B. Lipincott; Philadelphia (PA): 1993. pp. 1–58. [Google Scholar]
- Arden R., Chavez R.S., Grazioplene R., Jung R.E. Neuroimaging creativity: a psychometric review. Behav. Brain Res. 2010;214:143–156. doi: 10.1016/j.bbr.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L., Liew S.L., Dandekar F. Exploring the neural correlates of visual creativity. Soc. Cogn. Affect Neurosci. 2012;8:475–480. doi: 10.1093/scan/nss021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. Left ventrolateral cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bambini V., Gentili C., Ricciardi E., Bertinetto P.M., Pietrini P. Decomposing metaphor processing at the cognitive and neural level through functional magnetic resonance imaging. Brain Res. Bull. 2011;86:203–216. doi: 10.1016/j.brainresbull.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Beaty R.E., Silvia P.J. Why do ideas get more creative across time? An executive interpretation of the serial order effect in divergent thinking tasks. Psychol. Aesthet. Creat. 2012;6:309–319. [Google Scholar]
- Beaty R.E., Silvia P.J. Metaphorically speaking: cognitive abilities and the production of figurative speech. Mem. Cognit. 2013;41:255–267. doi: 10.3758/s13421-012-0258-5. [DOI] [PubMed] [Google Scholar]
- Benedek M., Neubauer A.C. Revisiting Mednick's model on creativity-related differences in associative hierarchies. Evidence for a common path to uncommon thought. J. Creat. Behav. 2013;47:273–289. doi: 10.1002/jocb.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Bergner S., Könen T., Fink A., Neubauer A.C. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia. 2011;49:3505–3511. doi: 10.1016/j.neuropsychologia.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Franz F., Heene M., Neubauer A.C. Differential effects of cognitive inhibition and intelligence on creativity. Personal. Individ. Differ. 2012;53:480–485. doi: 10.1016/j.paid.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Könen T., Neubauer A.C. Associative abilities underlying creativity. Psychol. Aesthet. Creat. 2012;6:273–281. [Google Scholar]
- Benedek M., Mühlmann C., Jauk E., Neubauer A.C. Assessment of divergent thinking by means of the subjective top-scoring method: Effects of the number of top-ideas and time-on-task on reliability and validity. Psychol. Aesthet. Creat. 2013;7:341–349. doi: 10.1037/a0033644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Jauk E., Fink A., Koschutnig K., Reishofer G., Ebner F., Neubauer A.C. To create or to recall? Neural mechanisms underlying the generation of creative new ideas, NeuroImage. 2013;88:125–133. doi: 10.1016/j.neuroimage.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A.L., Ansari D. Expertise-related deactivation of the right temporoparietal junction during musical improvisation. Neuroimage. 2010;49:712–719. doi: 10.1016/j.neuroimage.2009.08.042. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system. A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrn I.C., Altman U., Jacobs A.M. Looking at the brains behind figurative language—a quantitative meta-analysis of neuroimaging studies on metaphor, idiom, and irony processing. Neuropsychologia. 2012;50:2669–2683. doi: 10.1016/j.neuropsychologia.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Bottini G., Corcoran R., Sterzi R., Paulesu E., Schenone P., Scarpa P., Frackowiak R.S.J., Frith D. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Bowden E.M., Jung-Beeman M., Fleck J., Kounios J. New approaches to demystifying insight. Trends Cogn. Sci. 2005;9:322–328. doi: 10.1016/j.tics.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan. 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Buckner R.L., Carroll D.C. Self-projection and the brain. Trends Cogn. Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. Routledge; New York: 2004. Language and creativity: the art of common talk. [Google Scholar]
- Chiappe D.L., Chiappe P. The role of working memory in metaphor production and comprehension. J. Mem. Lang. 2007;56:172–188. [Google Scholar]
- Christensen P.R., Guilford J.P., Wilson R.C. Relations of creative responses to working time and instructions. J. Exp. Psychol. 1957;53:82–88. doi: 10.1037/h0045461. [DOI] [PubMed] [Google Scholar]
- Clark H.H., Lucy P. Understanding what is meant from what is said: a study in conversationally conveyed requests. J. Verb Learn. Verbal Behav. 1975;14:56–72. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;5:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S.G., Fu C.H.Y., Lee L., Everitt B., Brammer M.J., David A.S. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum. Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol. Bull. 2010;136:822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Fink A., Benedek M. The creative brain: brain correlates underlying the generation of original ideas. In: Vartanian O., Bristol A.S., Kaufman J.C., editors. Neuroscience of creativity. MIT Press; Cambridge: 2013. pp. 207–232. [Google Scholar]
- Fink A., Benedek M. EEG alpha power and creative ideation. Neurosci. Biobehav. 2013 doi: 10.1016/j.neubiorev.2012.12.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A., Grabner R.H., Benedek M., Reishofer G., Hauswirth V., Fally M., Neuper C., Ebner F., Neubauer A.C. The creative brain: investigation of brain activity during creative problem solving by means of EEG and fMRI. Hum. Brain Mapp. 2009;30:734–748. doi: 10.1002/hbm.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A., Grabner R.H., Gebauer D., Reishofer G., Koschutnig K., Ebner F. Enhancing creativity by means of cognitive stimulation: evidence from an fMRI study. NeuroImage. 2010;52:1687–1695. doi: 10.1016/j.neuroimage.2010.05.072. [DOI] [PubMed] [Google Scholar]
- Fink A., Koschutnig K., Benedek M., Reishofer G., Ischebeck A., Weiss E.M., Ebner F. Stimulating creativity via the exposure to other people's ideas. Hum. Brain Mapp. 2012;33:2603–2610. doi: 10.1002/hbm.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Alexander M.P., Naeser M.A. Anatomic basis of transcortical motor aphasia. Neurology. 1984;40:409–417. doi: 10.1212/wnl.34.4.409. [DOI] [PubMed] [Google Scholar]
- Gibbs R.W., Jr. Cambridge University Press; New York (NY): 1994. The Poetics of Mind: Figurative Thought, Language, and Understanding. [Google Scholar]
- Gilhooly K.J., Fioratou E., Anthony S.H., Wynn V. Divergent thinking: strategies and executive involvement in generating novel uses for familiar objects. Brit. J. Psychol. 2007;98:611–625. doi: 10.1111/j.2044-8295.2007.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Giora R. Understanding figurative and literal language: the graded salience hypothesis. Cogn. Linguist. 1997;7:183–206. [Google Scholar]
- Giora R., Zaidel E., Soroker N., Batori G., Kasher A. Differential effects of right- and left-hemisphere damage on understanding sarcasm and metaphor. Metaphor. Symb. 2000;15:63–83. [Google Scholar]
- Glucksberg S. Oxford University Press; New York (NY): 2001. Understanding Figurative Language: From Metaphors to Idioms. [Google Scholar]
- Glucksberg S. The psycholinguistics of metaphor. Trends Cogn. Sci. 2003;7:92–96. doi: 10.1016/s1364-6613(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Glucksberg S., Newsome M.R., Goldvarg Y. Inhibition of the literal: filtering metaphor-irrelevant information during metaphor comprehension. Metaphor. Symb. 2001;16:277–289. [Google Scholar]
- Goel V., Vartanian O. Dissociating the roles of the right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shit problems. Cereb. Cortex. 2005;15:1170–1177. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Guilford J.P. McGraw-Hill; New York (NY): 1967. The Nature of Human Intelligence. [Google Scholar]
- Hassabis D., Maguire E.A. Deconstructing episodic memory with construction. Trends Cogn. Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Howard-Jones P.A., Blakemore S.J., Samuel E.A., Summers I.R., Claxton G. Semantic divergence and creative story generation: an fMRI investigation. Cogn. Brain Res. 2005;25:240–250. doi: 10.1016/j.cogbrainres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Jauk E., Benedek M., Dunst B., Neubauer A.C. The relationship between intelligence and creativity: new support for the threshold hypothesis by means of empirical breakpoint detection. Intelligence. 2013;41:212–221. doi: 10.1016/j.intell.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauk E., Benedek M., Neubauer A.C. The road to creative achievement: a latent variable model of ability and personality predictors. Eur. J. Personal. 2013 doi: 10.1002/per.1941. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn. Sci. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kennedy J.M. Metaphors in art. In: Gibbs R.W., editor. Cambridge Handbook of Metaphor and Thought. Cambridge University Press; Cambridge (MA): 2008. pp. 447–461. [Google Scholar]
- Kintsch W. Metaphor comprehension: a computational theory. Psychon. Bull. Rev. 2000;7:257–266. doi: 10.3758/bf03212981. [DOI] [PubMed] [Google Scholar]
- Koestler A. Macmillan; New York (NY): 1964. The Act of Creation. [Google Scholar]
- Lackoff G., Johnson M. University of Chicago Press; Chicago (IL): 1980. Metaphors We Live by. [Google Scholar]
- Mashal N., Faust M., Hendler T., Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Lang. 2007;100:115–126. doi: 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mashal N., Faust M., Hendler T., Jung-Beeman M. Hemispheric differences in processing the literal interpretation of idioms: converging evidence from behavioral and fMRI studies. Cortex. 2008;44:848–860. doi: 10.1016/j.cortex.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Mashal N., Faust M., Hendler T., Jung-Beeman M. An fMRI study of processing novel metaphoric sentence. Laterality. 2009;14:30–54. doi: 10.1080/13576500802049433. [DOI] [PubMed] [Google Scholar]
- McElree B., Nordlie J. Literal and figurative interpretations are computed in equal time. Psychon. Bull. Rev. 1999;6:486–494. doi: 10.3758/bf03210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S.A. The associative basis of the creative process. Psychol. Rev. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- Nusbaum E.C., Silvia P.J. Are intelligence and creativity really so different? Fluid intelligence, executive processes, and strategy use in divergent thinking. Intelligence. 2011;39:36–45. [Google Scholar]
- Nyberg L., Kim A.S.N., Habib R., Levine B., Tulving E. Consciousness of subjective time in the brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22356–22359. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G., Mashal N., Faust M., Lavidor M. The role of the right cerebral hemisphere in processing novel metaphoric expressions: a transcranial magnetic stimulation study. J. Cogn. Neurosci. 2008;20:170–181. doi: 10.1162/jocn.2008.20005. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., McLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp A.M., Leube D.T., Erb M., Grodd W., Kircher T.T. Neural correlates of metaphor processing. Cogn. Brain Res. 2004;20:395–402. doi: 10.1016/j.cogbrainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Rapp A.M., Leube D.T., Erb M., Grodd W., Kircher T.T. Laterality in metaphor processing: lack of evidence from functional magnetic resonance imaging for the right hemisphere theory. Brain Lang. 2007;100:142–149. doi: 10.1016/j.bandl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rapp A.M., Mutschler D.E., Erb M. Where in the brain is nonliteral language? A coordinate-based meta-analysis of functional magnetic resonance imaging studies. Neuroimage. 2012;63:600–610. doi: 10.1016/j.neuroimage.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Robinson G., Blair J., Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Rutter B., Kröger S., Stark R., Schweckendiek J., Windmann S., Hermann C., Abraham A. Can clouds dance? Neural correlates of passive conceptual expansion using a metaphor processing task: implications for creative cognition. Brain Cogn. 2012;78:114–122. doi: 10.1016/j.bandc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R., Buckner R.L. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R., Hassabis D., Martin V.C., Spreng R.N., Szpunar K.K. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G.L., Kranjec A., Cardillo E.R., Chatterjee A. Beyond laterality: a critical assessment of research on the neural basis of metaphor. J. Int. Neuropsychol. Soc. 2010;16:1–5. doi: 10.1017/S1355617709990543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Fagan E., Price C.J. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J. Neurosci. 2010;30:16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia P.J. Discernment and creativity: how well can people identify their most creative ideas? Psychol. Aesthet. Creat. 2008;2:139–146. [Google Scholar]
- Silvia P.J., Beaty R.E. Making creative metaphors: the importance of fluid intelligence for creative thought. Intelligence. 2012;40:343–351. [Google Scholar]
- Silvia P.J., Winterstein B.P., Willse J.T., Barona C.M., Cram J.T., Hess K.I., Martinez J.L., Richard C.A. Assessing creativity with divergent thinking tasks: exploring the reliability and validity of new subjective scoring methods. Psychol. Aesthet. Creat. 2008;2:68–85. [Google Scholar]
- Squire L.R., Stark C.E.L., Clark R.E. The medial temproal lobe. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Subramaniam K., Faust M., Beeman M., Mashal N. The repetition paradigm: enhancement of novel metaphors and suppression of conventional metaphors in the left inferior parietal lobe. Neuropsychologia. 2012;50:2705–2719. doi: 10.1016/j.neuropsychologia.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Szpunar K.K. Episodic future thought: an emerging concept. Perspect. Psychol. Sci. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Thoma P., Daum I. Neurocognitive mechanisms of figurative language processing—evidence from clinical dysfunctions. Neurosci. Biobehav. Rev. 2006;30:1182–1205. doi: 10.1016/j.neubiorev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Turken A.U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian O. Dissociable neural systems for analogy and metaphor: implications for the neuroscience of creativity. Brit. J. Psychol. 2012;103:302–316. doi: 10.1111/j.2044-8295.2011.02073.x. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Snyder A.Z., Fox M.D., Shannon B.J., Andrews J.R., Raichle M.E., Buckner R.L. Coherent spontaneous activity identifies a hippocampal–parietal memory network. J. Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Yang J. The role of the right hemisphere in metaphor comprehension: a meta-analysis of functional magnetic resonance imaging studies. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.