Abstract
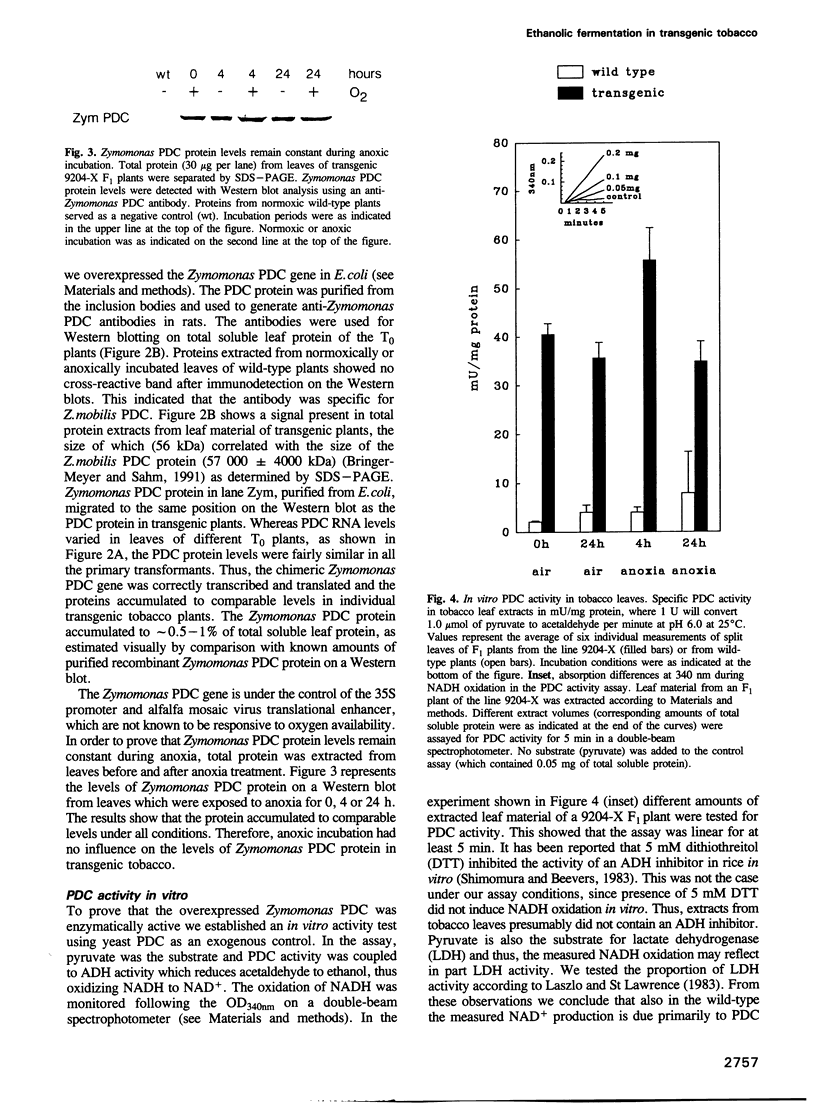
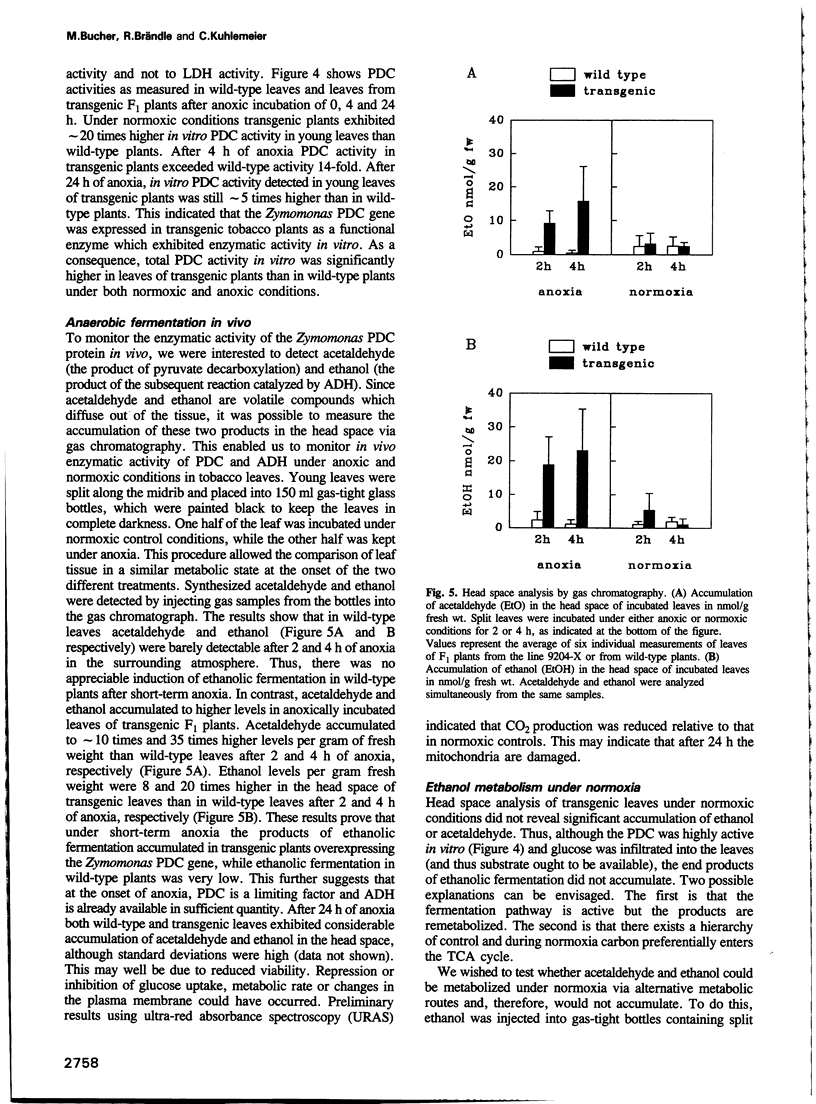
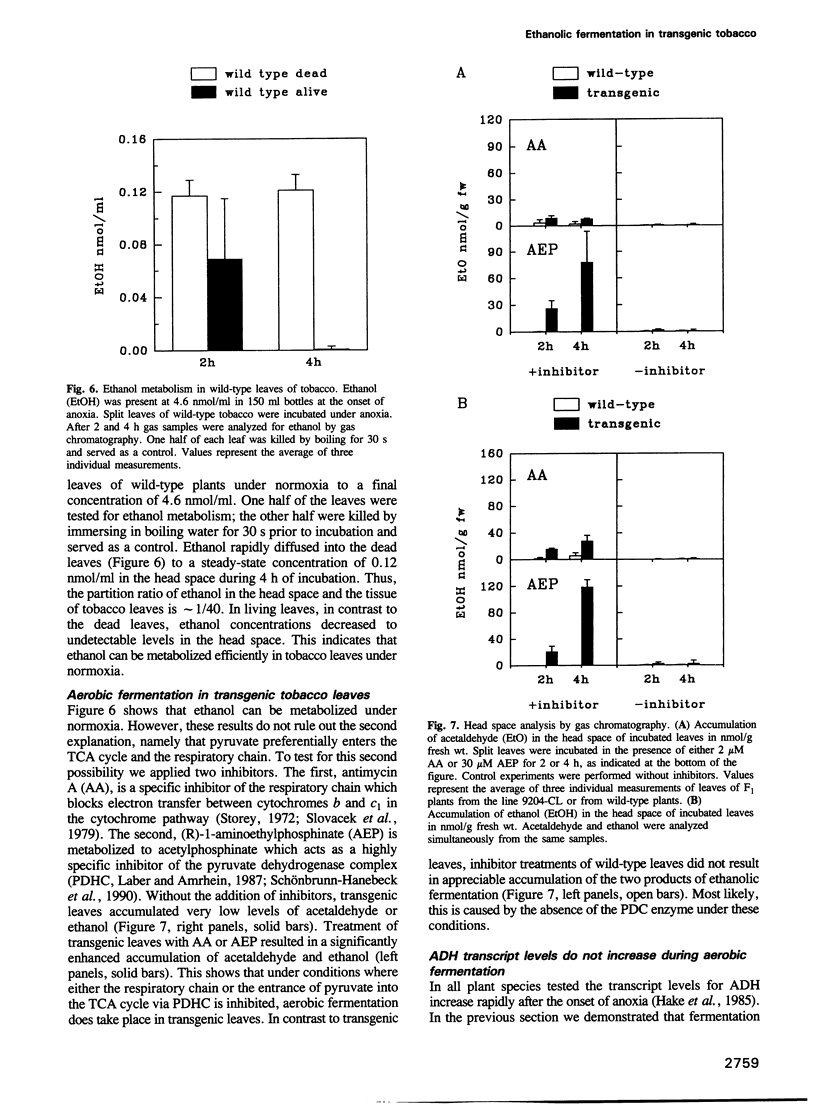
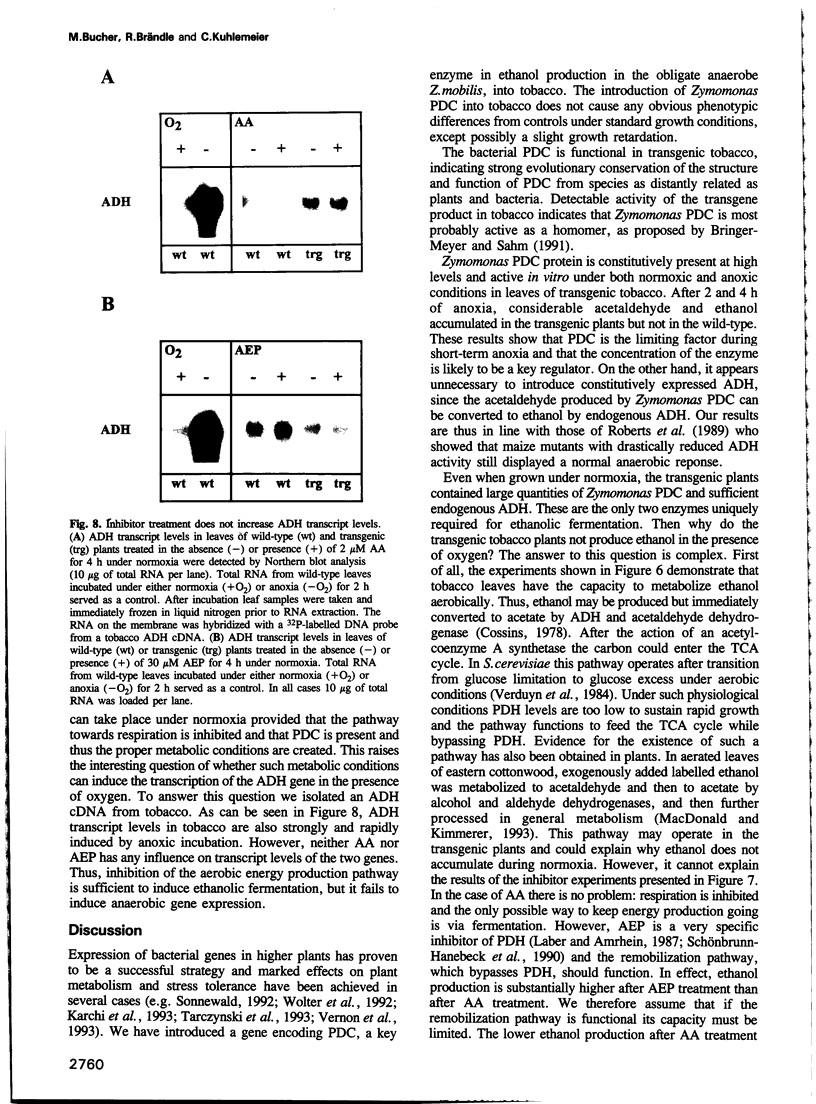
During oxygen limitation in higher plants, energy metabolism switches from respiration to fermentation. As part of this anaerobic response the expression of genes encoding pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) is strongly induced. In addition there is ample evidence for post-translational regulation. In order to understand this multi-level regulation of the anaerobic response, we provided tobacco with the constitutive capacity of ethanolic fermentation by expressing a PDC gene derived from the obligate anaerobe Zymomonas mobilis. The protein accumulated to high levels and was active in an in vitro assay. During the first 2-4 h of anoxia, acetaldehyde accumulated to 10- to 35-fold and ethanol to 8- to 20-fold higher levels than in wild-type. Under normoxic conditions no accumulation of acetaldehyde and ethanol could be measured. Instead, the two products may be immediately re-metabolized in tobacco leaf tissue. We show that aerobic fermentation takes place when the respiratory system is inhibited. Although these conditions enhance ethanolic fermentation under normoxia, they fail to increase ADH transcript levels. These results indicate that anaerobic transcription is triggered not by the metabolic consequences of oxygen limitation, but directly through an oxygen-sensing system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Appleby C. A., Tjepkema J. D., Trinick M. J. Hemoglobin in a nonleguminous plant, parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983 May 27;220(4600):951–953. doi: 10.1126/science.220.4600.951. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bucher M., Kuhlemeier C. Long-term anoxia tolerance. Multi-level regulation of gene expression in the amphibious plant Acorus calamus L. Plant Physiol. 1993 Oct;103(2):441–448. doi: 10.1104/pp.103.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
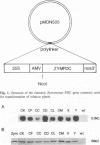
- Conway T., Osman Y. A., Konnan J. I., Hoffmann E. M., Ingram L. O. Promoter and nucleotide sequences of the Zymomonas mobilis pyruvate decarboxylase. J Bacteriol. 1987 Mar;169(3):949–954. doi: 10.1128/jb.169.3.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Ribbons D. W., Large P. J. The route of ethanol formation in Zymomonas mobilis. Biochem J. 1966 Mar;98(3):795–803. doi: 10.1042/bj0980795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Good A. G., Muench D. G. Long-Term Anaerobic Metabolism in Root Tissue (Metabolic Products of Pyruvate Metabolism). Plant Physiol. 1993 Apr;101(4):1163–1168. doi: 10.1104/pp.101.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake S., Kelley P. M., Taylor W. C., Freeling M. Coordinate induction of alcohol dehydrogenase 1, aldolase, and other anaerobic RNAs in maize. J Biol Chem. 1985 Apr 25;260(8):5050–5054. [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Kelley P. M. Maize pyruvate decarboxylase mRNA is induced anaerobically. Plant Mol Biol. 1989 Aug;13(2):213–222. doi: 10.1007/BF00016139. [DOI] [PubMed] [Google Scholar]
- Laber B., Amrhein N. Metabolism of 1-aminoethylphosphinate generates acetylphosphinate, a potent inhibitor of pyruvate dehydrogenase. Biochem J. 1987 Dec 1;248(2):351–358. doi: 10.1042/bj2480351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois A. F., Weinstein M., Ditta G. S., Helinski D. R. Autophosphorylation and phosphatase activities of the oxygen-sensing protein FixL of Rhizobium meliloti are coordinately regulated by oxygen. J Biol Chem. 1993 Feb 25;268(6):4370–4375. [PubMed] [Google Scholar]
- Lowry C. V., Zitomer R. S. Oxygen regulation of anaerobic and aerobic genes mediated by a common factor in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6129–6133. doi: 10.1073/pnas.81.19.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., Kimmerer T. W. Metabolism of Transpired Ethanol by Eastern Cottonwood (Populus deltoides Bartr.). Plant Physiol. 1993 May;102(1):173–179. doi: 10.1104/pp.102.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer K., Kim K. S., Kogan S., Guarente L. Functional dissection and sequence of yeast HAP1 activator. Cell. 1989 Jan 27;56(2):291–301. doi: 10.1016/0092-8674(89)90903-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Jardetzky O., Walbot V., Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Chang K., Webster C., Callis J., Walbot V. Dependence of Ethanolic Fermentation, Cytoplasmic pH Regulation, and Viability on the Activity of Alcohol Dehydrogenase in Hypoxic Maize Root Tips. Plant Physiol. 1989 Apr;89(4):1275–1278. doi: 10.1104/pp.89.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Kennedy R. A. Anaerobic Metabolism in Germinating Seeds of Echinochloa crus-galli (Barnyard Grass) : METABOLITE AND ENZYME STUDIES. Plant Physiol. 1981 Jul;68(1):165–168. doi: 10.1104/pp.68.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaff I., Heinisch J., Zimmermann F. K. Overproduction of glycolytic enzymes in yeast. Yeast. 1989 Jul-Aug;5(4):285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- Schönbrunn-Hanebeck E., Laber B., Amrhein N. Slow-binding inhibition of the Escherichia coli pyruvate dehydrogenase multienzyme complex by acetylphosphinate. Biochemistry. 1990 May 22;29(20):4880–4885. doi: 10.1021/bi00472a018. [DOI] [PubMed] [Google Scholar]
- Shimomura S., Beevers H. Alcohol dehydrogenase and an inactivator from rice seedlings. Plant Physiol. 1983 Apr;71(4):736–741. doi: 10.1104/pp.71.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek R. E., Crowther D., Hind G. Cytochrome function in the cyclic electron transport pathway of chloroplasts. Biochim Biophys Acta. 1979 Jul 10;547(1):138–148. doi: 10.1016/0005-2728(79)90102-6. [DOI] [PubMed] [Google Scholar]
- Sonnewald U. Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J. 1992 Jul;2(4):571–581. [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. 13. Redox state changes of cytochrome b 562 in mung bean seedling mitochondria treated with antimycin A. Biochim Biophys Acta. 1972 Apr 20;267(1):48–64. doi: 10.1016/0005-2728(72)90137-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tarczynski M. C., Jensen R. G., Bohnert H. J. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science. 1993 Jan 22;259(5094):508–510. doi: 10.1126/science.259.5094.508. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe G. C., McIntosh L. Coordinate regulation of cytochrome and alternative pathway respiration in tobacco. Plant Physiol. 1992 Dec;100(4):1846–1851. doi: 10.1104/pp.100.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F. P., Schmidt R., Heinz E. Chilling sensitivity of Arabidopsis thaliana with genetically engineered membrane lipids. EMBO J. 1992 Dec;11(13):4685–4692. doi: 10.1002/j.1460-2075.1992.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]