Abstract
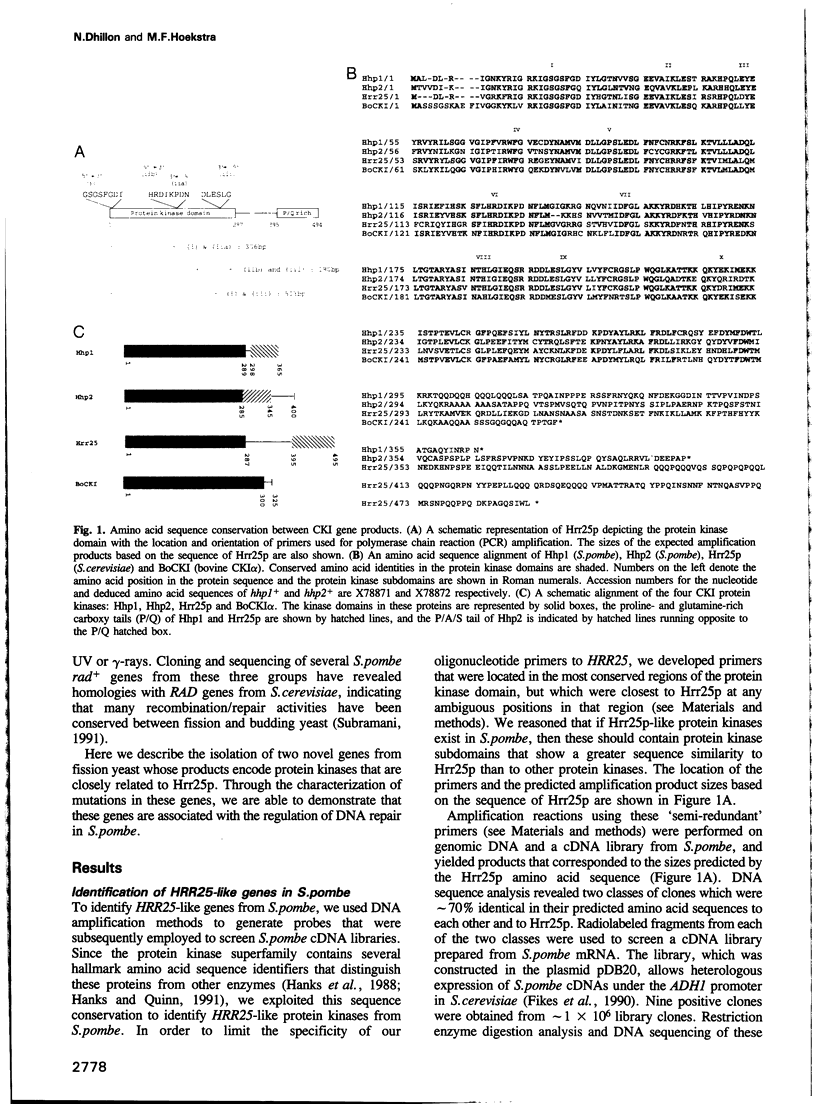
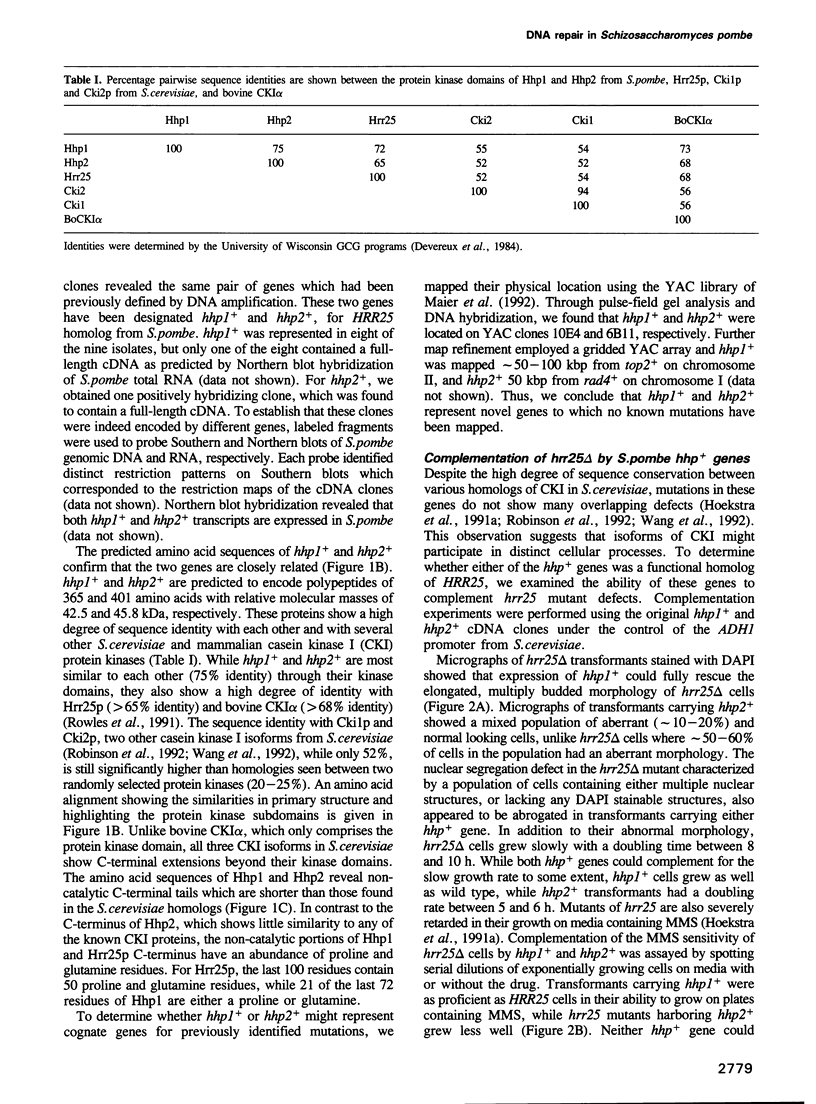
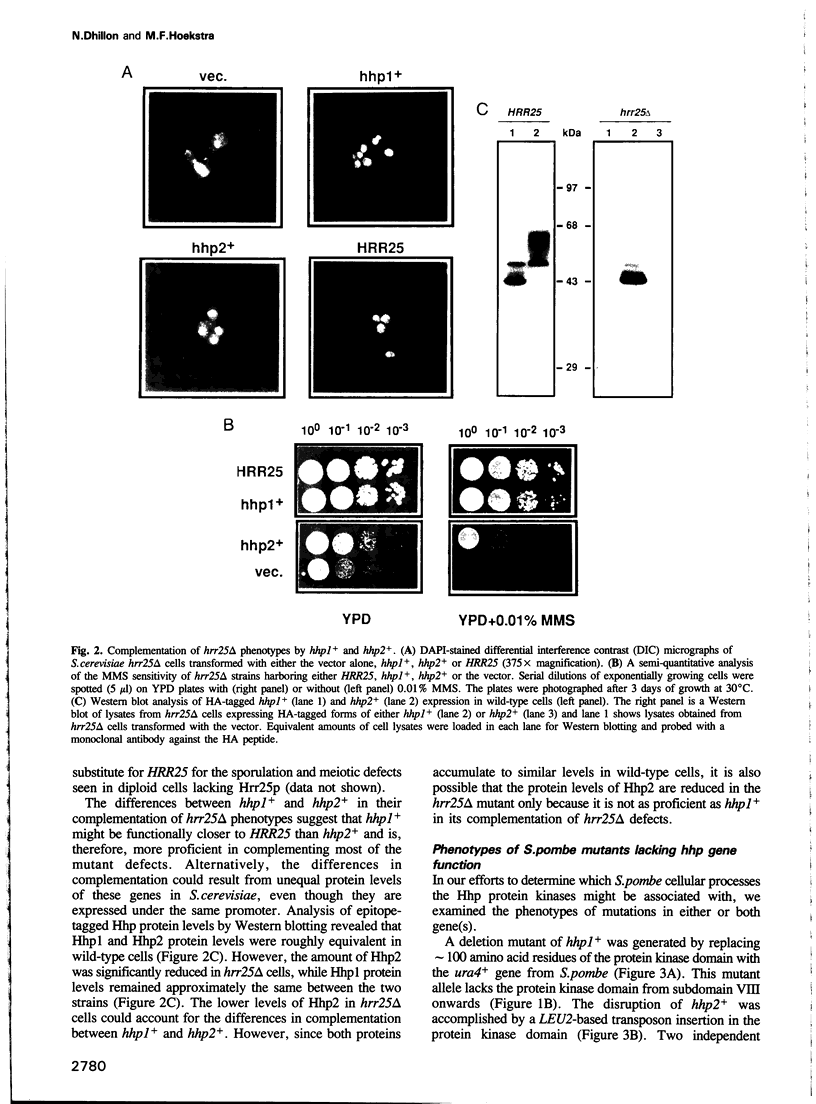
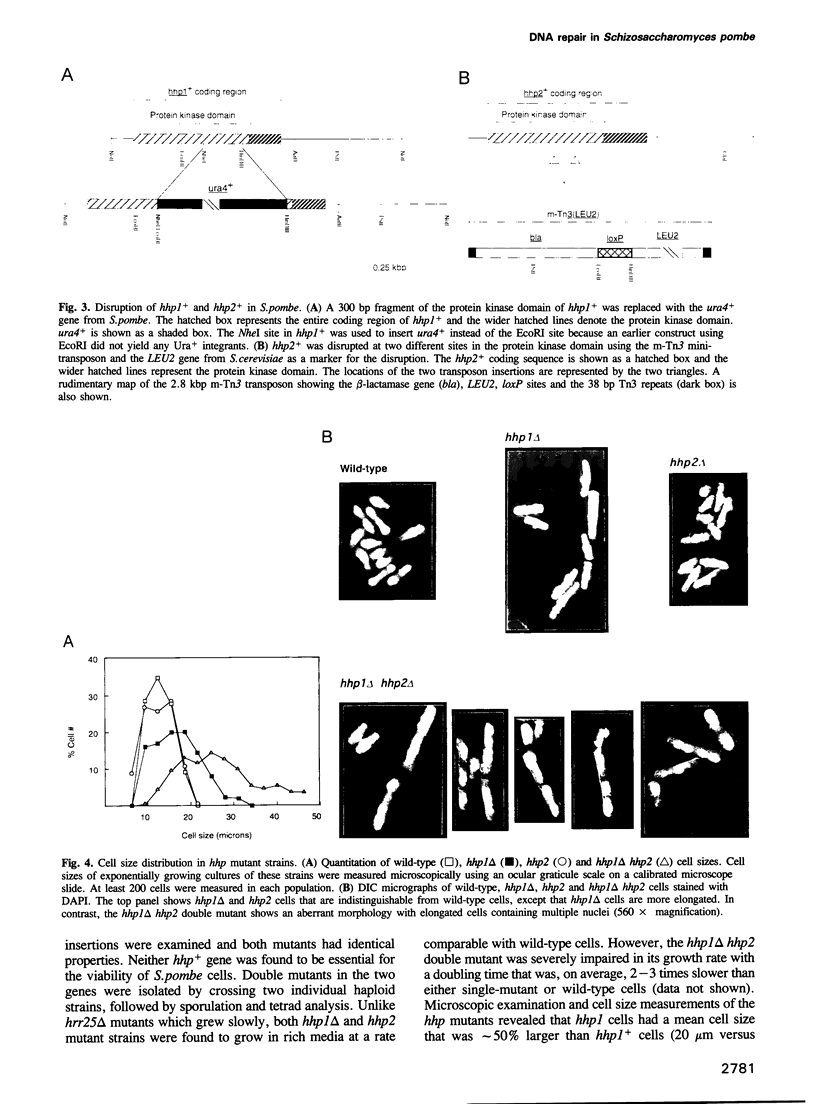
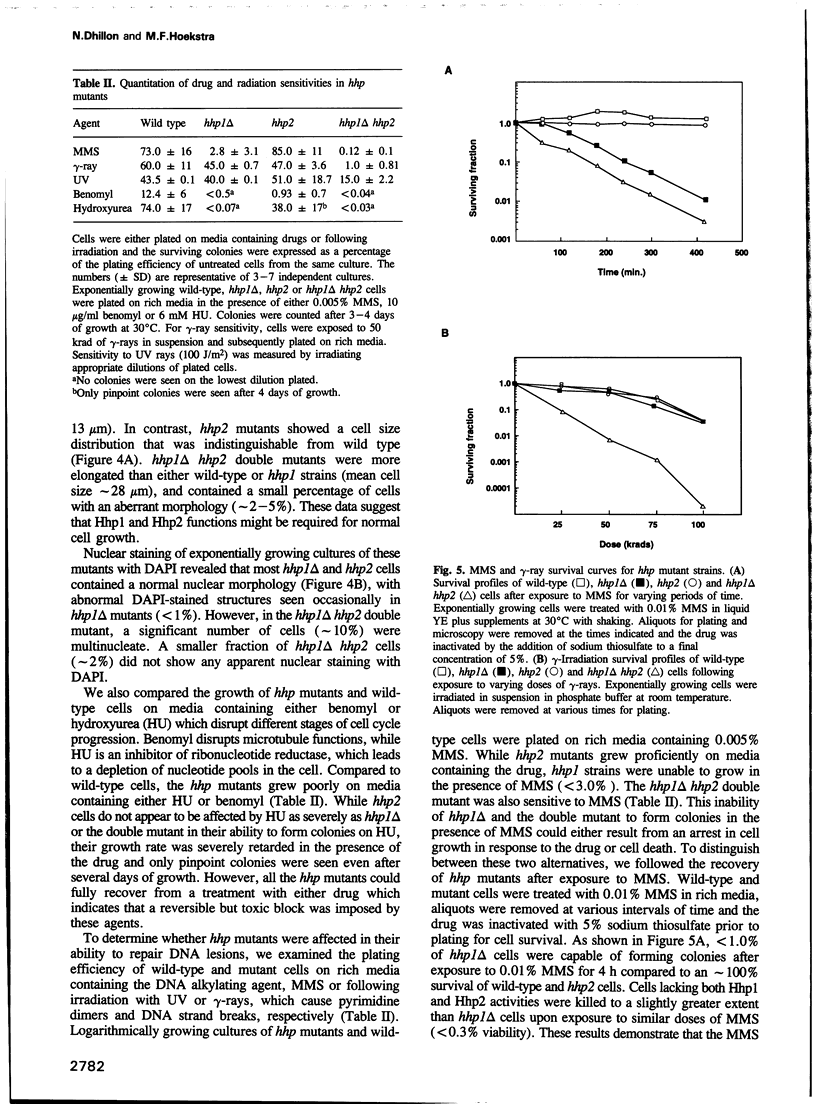
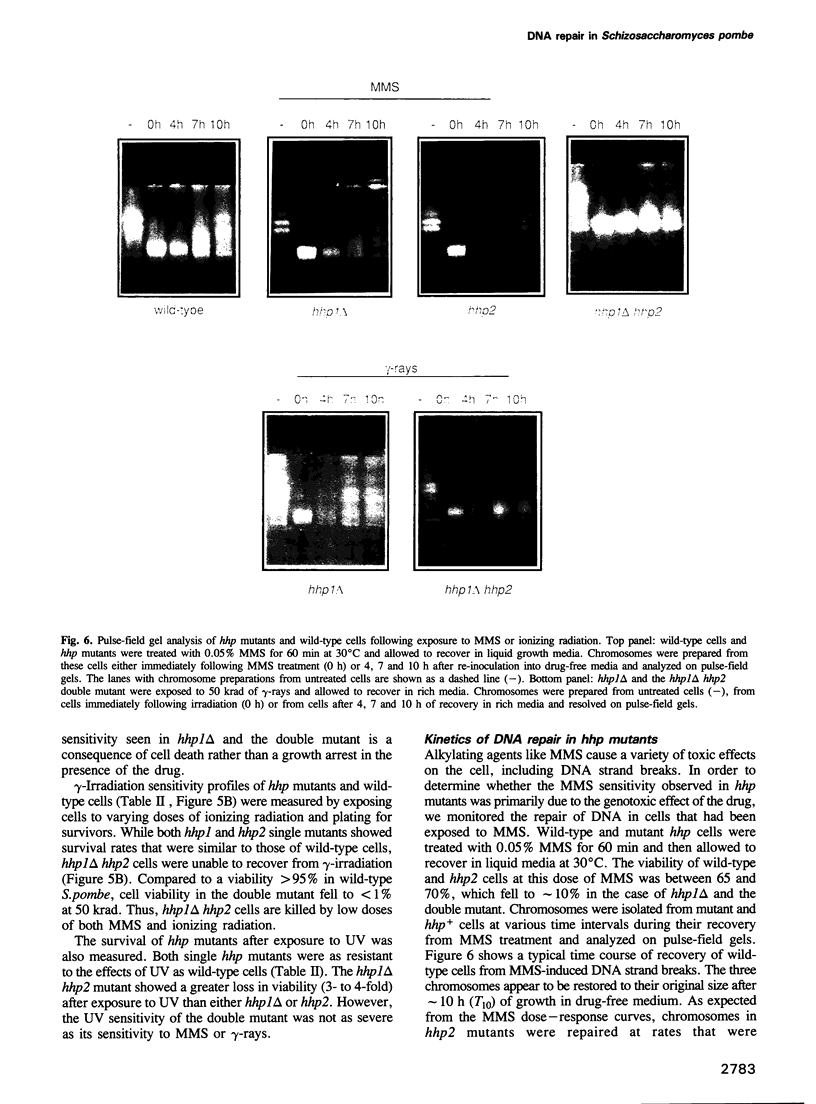
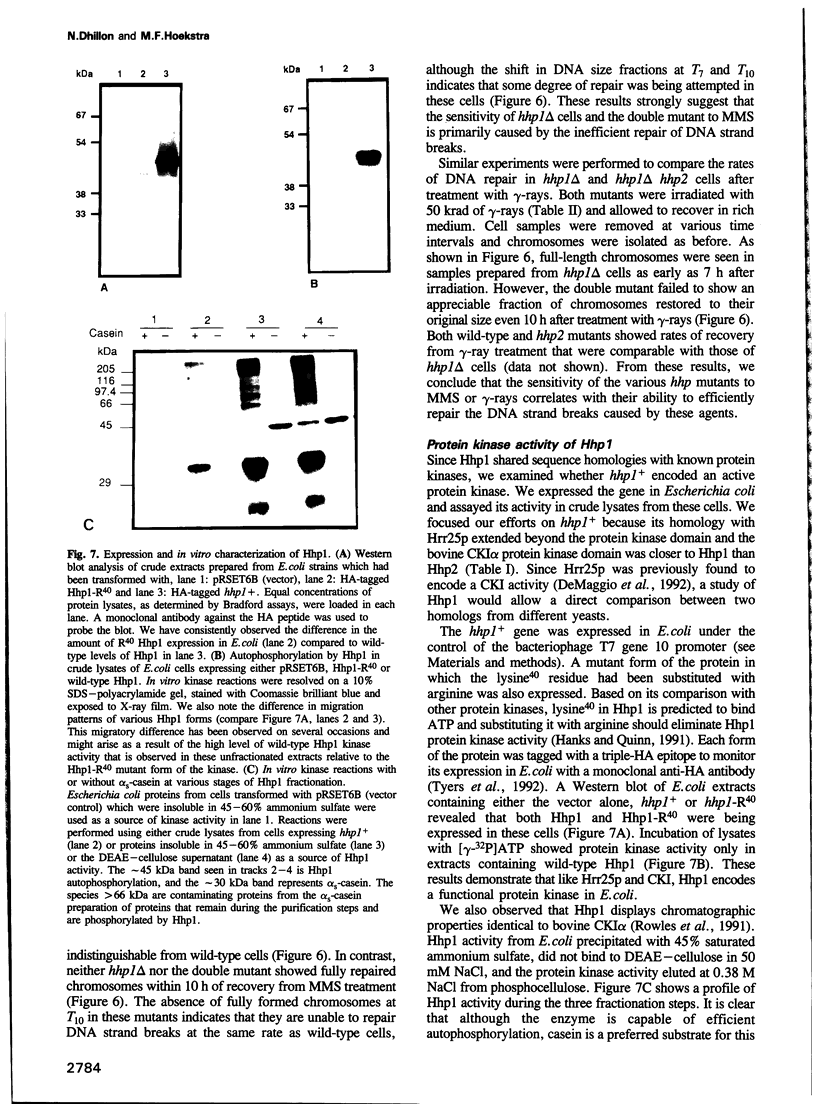
We have identified two novel genes designated hhp1+ and hhp2+ in the fission yeast Schizosaccharomyces pombe. The hhp1+ and hhp2+ genes encode two closely related protein kinases that share significant sequence identities with Hrr25p from Saccharomyces cerevisiae. Characterization of strains harboring single and double mutations in the hhp+ genes reveals DNA repair defects in these cells. Schizosaccharomyces pombe strains lacking either or both Hhp activities reveal differences in their ability to withstand DNA lesions caused by either methyl methanesulfonate (MMS) or gamma-rays which correlate with their ability to repair DNA strand breaks caused by these agents. We suggest that Hhp1 and Hhp2 are involved in the regulation of distinct and overlapping DNA repair pathways in S. pombe.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankmann M., Prakash L., Prakash S. Yeast RAD14 and human xeroderma pigmentosum group A DNA-repair genes encode homologous proteins. Nature. 1992 Feb 6;355(6360):555–558. doi: 10.1038/355555a0. [DOI] [PubMed] [Google Scholar]
- Chen J., Derfler B., Samson L. Saccharomyces cerevisiae 3-methyladenine DNA glycosylase has homology to the AlkA glycosylase of E. coli and is induced in response to DNA alkylation damage. EMBO J. 1990 Dec;9(13):4569–4575. doi: 10.1002/j.1460-2075.1990.tb07910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaggio A. J., Lindberg R. A., Hunter T., Hoekstra M. F. The budding yeast HRR25 gene product is a casein kinase I isoform. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7008–7012. doi: 10.1073/pnas.89.15.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Carr A. M., Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 1992 Nov;6(11):2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Fan J. B., Chikashige Y., Smith C. L., Niwa O., Yanagida M., Cantor C. R. Construction of a Not I restriction map of the fission yeast Schizosaccharomyces pombe genome. Nucleic Acids Res. 1989 Apr 11;17(7):2801–2818. doi: 10.1093/nar/17.7.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Becker D. M., Winston F., Guarente L. Striking conservation of TFIID in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Nature. 1990 Jul 19;346(6281):291–294. doi: 10.1038/346291a0. [DOI] [PubMed] [Google Scholar]
- Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:1–863. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A. Mutagenesis and deoxyribonucleotide pool imbalance. Mutat Res. 1988 Jul-Aug;200(1-2):133–147. doi: 10.1016/0027-5107(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Legerski R., Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992 Sep 3;359(6390):70–73. doi: 10.1038/359070a0. [DOI] [PubMed] [Google Scholar]
- Lieberman H. B., Riley R., Martel M. Isolation and initial characterization of a Schizosaccharomyces pombe mutant exhibiting temperature-dependent radiation sensitivity due to a mutation in a previously unidentified rad locus. Mol Gen Genet. 1989 Sep;218(3):554–558. doi: 10.1007/BF00332423. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Maier E., Hoheisel J. D., McCarthy L., Mott R., Grigoriev A. V., Monaco A. P., Larin Z., Lehrach H. Complete coverage of the Schizosaccharomyces pombe genome in yeast artificial chromosomes. Nat Genet. 1992 Jul;1(4):273–277. doi: 10.1038/ng0792-273. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Murray J. M., Doe C. L., Schenk P., Carr A. M., Lehmann A. R., Watts F. Z. Cloning and characterisation of the S. pombe rad15 gene, a homologue to the S. cerevisiae RAD3 and human ERCC2 genes. Nucleic Acids Res. 1992 Jun 11;20(11):2673–2678. doi: 10.1093/nar/20.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975 Aug 14;256(5518):547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Phipps J., Nasim A., Miller D. R. Recovery, repair, and mutagenesis in Schizosaccharomyces pombe. Adv Genet. 1985;23:1–72. doi: 10.1016/s0065-2660(08)60511-8. [DOI] [PubMed] [Google Scholar]
- Robinson L. C., Hubbard E. J., Graves P. R., DePaoli-Roach A. A., Roach P. J., Kung C., Haas D. W., Hagedorn C. H., Goebl M., Culbertson M. R. Yeast casein kinase I homologues: an essential gene pair. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):28–32. doi: 10.1073/pnas.89.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles J., Slaughter C., Moomaw C., Hsu J., Cobb M. H. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley R., Subramani S., Young P. G. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992 Apr;11(4):1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel C., Kirchhoff S., Schmidt H. The protein sequence and some intron positions are conserved between the switching gene swi10 of Schizosaccharomyces pombe and the human excision repair gene ERCC1. Nucleic Acids Res. 1992 Dec 11;20(23):6347–6353. doi: 10.1093/nar/20.23.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Radiation resistance in Schizosaccharomyces pombe. Mol Microbiol. 1991 Oct;5(10):2311–2314. doi: 10.1111/j.1365-2958.1991.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Nash R., Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992 May;11(5):1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. C., Vancura A., Desai A., Carmel G., Kuret J. Cytoplasmic forms of fission yeast casein kinase-1 associate primarily with the particulate fraction of the cell. J Biol Chem. 1994 Apr 22;269(16):12014–12023. [PubMed] [Google Scholar]
- Wang P. C., Vancura A., Mitcheson T. G., Kuret J. Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol Biol Cell. 1992 Mar;3(3):275–286. doi: 10.1091/mbc.3.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Derfler B., Chen J., Samson L. Primary sequence and biological functions of a Saccharomyces cerevisiae O6-methylguanine/O4-methylthymine DNA repair methyltransferase gene. EMBO J. 1991 Aug;10(8):2179–2186. doi: 10.1002/j.1460-2075.1991.tb07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Khodairy F., Carr A. M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992 Apr;11(4):1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]