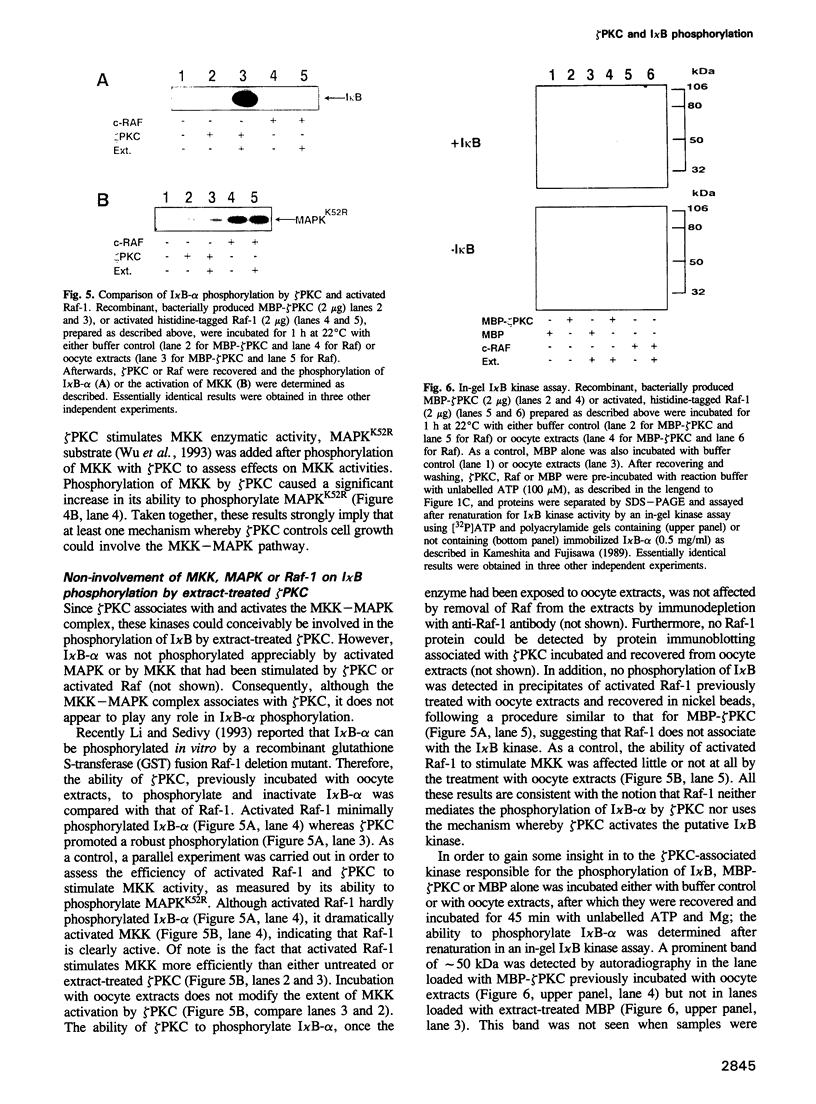
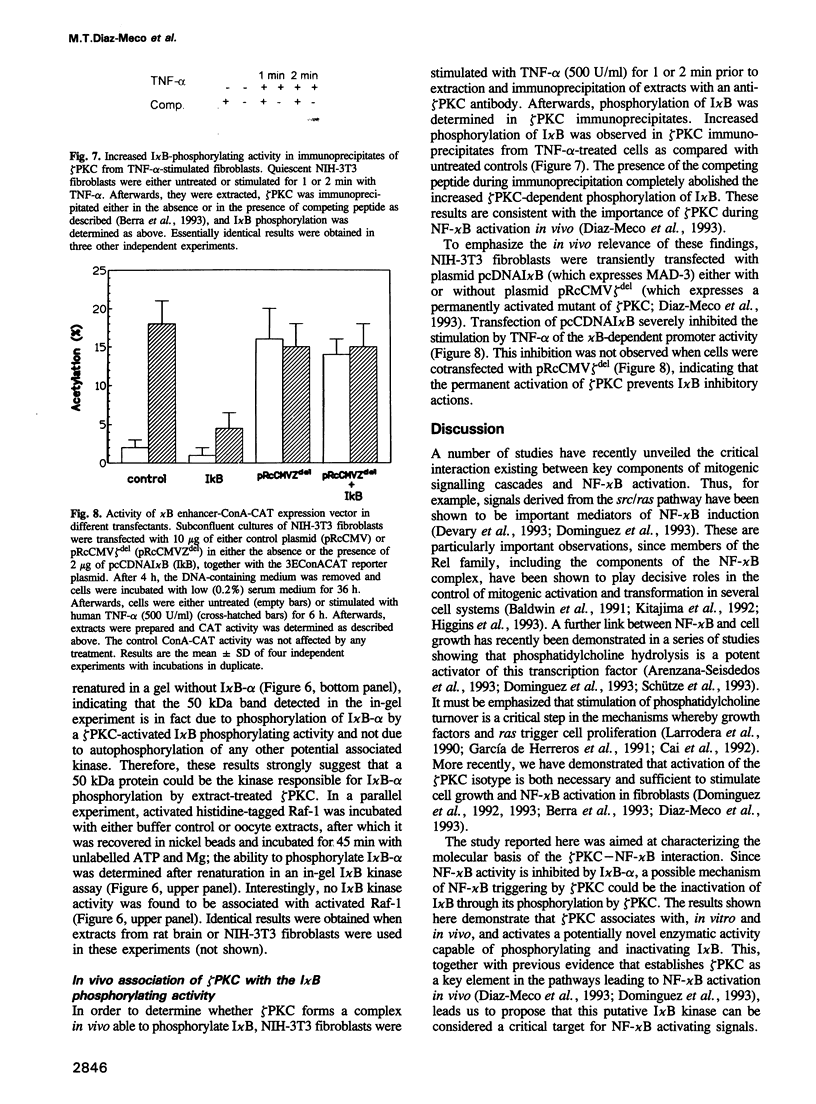
Abstract
The zeta isotype of protein kinase C (zeta PKC), a distinct PKC unable to bind phorbol esters, is required during NF-kappa B activation as well as in mitogenic signalling in Xenopus oocytes and mammalian cells. To investigate the mechanism(s) for control of cellular functions by zeta PKC, this enzyme was expressed in Escherichia coli as a fusion protein with maltose binding protein (MBP), to allow immobilization on amylose beads to study signalling proteins in cell extracts that might form complex(es) with zeta PKC. The following evidence for interaction with the NF-kappa B/I kappa B pathway was obtained. MBP-zeta PKC, but not MBP, bound and activated a potentially novel I kappa B kinase of approximately 50 kDa molecular weight able to regulate I kappa B-alpha function. Activation of the I kappa B kinase was dependent on zeta PKC enzymatic activity and ATP, suggesting that zeta PKC controls, directly or indirectly, the activity of a functionally significant I kappa B kinase. Importantly, zeta PKC immunoprecipitates from TNF-alpha-stimulated NIH-3T3 fibroblasts displayed a higher I kappa B phosphorylating activity than untreated controls, indicating the in vivo relevance of these findings. We also show here that zeta PKC associates with and activates MKK-MAPK in vitro, suggesting that one of the mechanisms whereby overexpression of zeta PKC leads to deregulation of cell growth may be accounted for at least in part by activation of the MKK-MAPK complex. However, neither MKK nor MAPK is responsible for the putative I kappa B phosphorylating activity. These data provide a decisive step towards understanding the functions of zeta PKC.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenzana-Seisdedos F., Fernandez B., Dominguez I., Jacqué J. M., Thomas D., Diaz-Meco M. T., Moscat J., Virelizier J. L. Phosphatidylcholine hydrolysis activates NF-kappa B and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J Virol. 1993 Nov;67(11):6596–6604. doi: 10.1128/jvi.67.11.6596-6604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Azizkhan J. C., Jensen D. E., Beg A. A., Coodly L. R. Induction of NF-kappa B DNA-binding activity during the G0-to-G1 transition in mouse fibroblasts. Mol Cell Biol. 1991 Oct;11(10):4943–4951. doi: 10.1128/mcb.11.10.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993 Aug 13;74(3):555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Brown K., Park S., Kanno T., Franzoso G., Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Tan T. H., Weske J. C., Derse D., Rapp U. R. Oncogene activation of HIV-LTR-driven expression via the NF-kappa B binding sites. Nucleic Acids Res. 1993 Nov 11;21(22):5229–5234. doi: 10.1093/nar/21.22.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Erhardt P., Szeberényi J., Diaz-Meco M. T., Johansen T., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine is stimulated by Ras proteins during mitogenic signal transduction. Mol Cell Biol. 1992 Dec;12(12):5329–5335. doi: 10.1128/mcb.12.12.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Erhardt P., Troppmair J., Diaz-Meco M. T., Sithanandam G., Rapp U. R., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine couples Ras to activation of Raf protein kinase during mitogenic signal transduction. Mol Cell Biol. 1993 Dec;13(12):7645–7651. doi: 10.1128/mcb.13.12.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Devary Y., Rosette C., DiDonato J. A., Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993 Sep 10;261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Berra E., Municio M. M., Sanz L., Lozano J., Dominguez I., Diaz-Golpe V., Lain de Lera M. T., Alcamí J., Payá C. V. A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993 Aug;13(8):4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I., Diaz-Meco M. T., Municio M. M., Berra E., García de Herreros A., Cornet M. E., Sanz L., Moscat J. Evidence for a role of protein kinase C zeta subspecies in maturation of Xenopus laevis oocytes. Mol Cell Biol. 1992 Sep;12(9):3776–3783. doi: 10.1128/mcb.12.9.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I., Sanz L., Arenzana-Seisdedos F., Diaz-Meco M. T., Virelizier J. L., Moscat J. Inhibition of protein kinase C zeta subspecies blocks the activation of an NF-kappa B-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993 Feb;13(2):1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco T. S., Baldwin A. S., Jr Kappa B site-dependent induction of gene expression by diverse inducers of nuclear factor kappa B requires Raf-1. J Biol Chem. 1993 Aug 25;268(24):17676–17679. [PubMed] [Google Scholar]
- García de Herreros A., Dominguez I., Diaz-Meco M. T., Graziani G., Cornett M. E., Guddal P. H., Johansen T., Moscat J. Requirement of phospholipase C-catalyzed hydrolysis of phosphatidylcholine for maturation of Xenopus laevis oocytes in response to insulin and ras p21. J Biol Chem. 1991 Apr 15;266(11):6825–6829. [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Higgins K. A., Perez J. R., Coleman T. A., Dorshkind K., McComas W. A., Sarmiento U. M., Rosen C. A., Narayanan R. Antisense inhibition of the p65 subunit of NF-kappa B blocks tumorigenicity and causes tumor regression. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kitajima I., Shinohara T., Bilakovics J., Brown D. A., Xu X., Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-kappa B. Science. 1992 Dec 11;258(5089):1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Force T. L., Rapp U. R., Bonventre J. V., Avruch J. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase-kinase. J Biol Chem. 1993 Jul 25;268(21):16009–16019. [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Larrodera P., Cornet M. E., Diaz-Meco M. T., Lopez-Barahona M., Diaz-Laviada I., Guddal P. H., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is an important step in PDGF-stimulated DNA synthesis. Cell. 1990 Jun 15;61(6):1113–1120. doi: 10.1016/0092-8674(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Li S., Sedivy J. M. Raf-1 protein kinase activates the NF-kappa B transcription factor by dissociating the cytoplasmic NF-kappa B-I kappa B complex. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9247–9251. doi: 10.1073/pnas.90.20.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meichle A., Schütze S., Hensel G., Brunsing D., Krönke M. Protein kinase C-independent activation of nuclear factor kappa B by tumor necrosis factor. J Biol Chem. 1990 May 15;265(14):8339–8343. [PubMed] [Google Scholar]
- Muslin A. J., MacNicol A. M., Williams L. T. Raf-1 protein kinase is important for progesterone-induced Xenopus oocyte maturation and acts downstream of mos. Mol Cell Biol. 1993 Jul;13(7):4197–4202. doi: 10.1128/mcb.13.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Paya C. V., Ten R. M., Bessia C., Alcami J., Hay R. T., Virelizier J. L. NF-kappa B-dependent induction of the NF-kappa B p50 subunit gene promoter underlies self-perpetuation of human immunodeficiency virus transcription in monocytic cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7826–7830. doi: 10.1073/pnas.89.16.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears C., Schaap D., Parker P. J. The regulatory domain of protein kinase C-epsilon restricts the catalytic-domain-specificity. Biochem J. 1991 May 15;276(Pt 1):257–260. doi: 10.1042/bj2760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993 Apr;13(4):2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shibuya E. K., Polverino A. J., Chang E., Wigler M., Ruderman J. V. Oncogenic ras triggers the activation of 42-kDa mitogen-activated protein kinase in extracts of quiescent Xenopus oocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. G., Roberts T. M., Li P. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2922–2926. doi: 10.1073/pnas.89.7.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Dent P., Lynch K. R., Weber M. J., Sturgill T. W. Identification and characterization of a new mammalian mitogen-activated protein kinase kinase, MKK2. Mol Cell Biol. 1993 Aug;13(8):4539–4548. doi: 10.1128/mcb.13.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]