Abstract
Background
γ-aminobutyric acidA (GABAA) receptors mediate the actions of several intravenous general anesthetics. However, the contribution of α3-containing GABAA receptors to the action of these drugs is unknown.
Methods
We compared anesthetic endpoints (hypnosis, immobility, hypothermia) in response to various intravenous anesthetics in mice lacking the α3 subunit of the GABAA receptor (α3KO) and wild type mice. Furthermore, we generated and analyzed conditional mutant mice expressing the GABAA receptor α3 subunit exclusively in noradrenergic neurons.
Results
α3KO mice displayed decreased hypnotic and hypothermic responses to etomidate and midazolam, but an increased response to pentobarbital. While the hypnotic response to ketamine was unaltered, the hypothermic response was increased. In contrast, the hypnotic but not the hypothermic response to medetomidine was increased. The combination of ketamine/xylazine displayed increased hypnotic, immobilizing, and hypothermic effects in α3KO mice. Mice expressing the α3 subunit exclusively in noradrenergic neurons were generated to assess whether the lack of α3 subunits on noradrenergic neurons may be responsible for this effect. In these mice, the increases of the hypnotic and immobilizing actions induced by ketamine/xylazine were largely absent, while the increase in the hypothermic action was still present.
Conclusion
α3-containing GABAA receptors bidirectionally regulate essential anesthetic actions: they mediate anesthetic actions of etomidate and midazolam, known to selectively act at GABAA receptors, and they negatively constrain anesthetic actions of compounds with targets partly or exclusively distinct from GABAA receptors such as medetomidine, ketamine and pentobarbital. Furthermore, our results indicate that α3-containing GABAA receptors on noradrenergic neurons may contribute to this constraint.
Introduction
GABAA receptors are chloride channels, which mediate fast synaptic inhibition and tonic inhibition in the central nervous system. They are composed of five subunits drawn from a repertoire of 8 different subunit families comprising at least 19 different genes (α1-6, β1-3, γ1-3, δ, ε, θ, π, ρ1-3).1-4 Depending on the exact subunit composition GABAA receptors possess different physiological and pharmacological properties.1,2 Many general anesthetics potentiate the activity of GABAA receptors and the role of several GABAA receptors subtypes in mediating anesthetic effects has been described.3,4 We have previously shown that β3(N265M) knock-in mice display dramatically reduced hypnotic responses to etomidate and pentobarbital and cannot be immobilized by these compounds, demonstrating that a subpopulation of GABAA receptors is essential for the anesthetic actions of these anesthetic agents.5 Most frequently, the GABAA receptors are classified by the α subunit that is present. Based on studies in global knockout mice, the α1 subunit6, but not the α57 and α6 subunits8, contribute to the hypnotic actions of intravenous general anesthetics. However, the significance of α3 subunit containing GABAA receptors for anesthetic effects remained to be clarified.
Performing stereotaxic surgeries we made the unexpected and surprising observation that a combination of ketamine, a N-methyl-D-aspartate receptor antagonist and xylazine, an α2-adrenergic agonist, induces remarkably prolonged anesthesia in α3KO mice compared to wild type mice. The α3 subunit of the GABAA receptor is present in 10-15% of all GABAA receptors in the central nervous system and GABAA receptors containing the α3 subunit are expressed widely in the brain1,2. However, in monoaminergic neurons the α3 subunit appears to be the major α subunit expressed. High expression levels in noradrenergic neurons9-11 suggest that α3 subunit-containing GABAA receptors might be important modulators of the noradrenergic system.
The noradrenergic system (cell groups A1-A10) which includes the locus coeruleus (A6) whose neurons project widely throughout the neuraxis and other noradrenergic nuclei (e.g. A1 and A2 which project mainly to the hypothalamus) plays an important role in the regulation of sleep and arousal as well as thermoregulation12-14. Activity of the noradrenaline-containing locus coeruleus neurons depends on the behavioral state (e.g. sleeping or waking) of the organism and this system is likely involved in switching between these different states.15 Moreover, α2-adrenoceptor agonists such as dexmedetomidine have been shown to exert their sedative/hypnotic effects through endogenous sleep pathways,16 at least in part through inhibition of the locus coeruleus.17
In order to obtain insights into the role of α3-containing GABAA receptors in general anesthetic actions, we investigated (1) in α3KO mice whether α3-containing GABAA receptors mediate the action of intravenous general anesthetics known to act at least in part via GABAA receptors such as etomidate, midazolam, and pentobarbital, (2) whether α3-containing GABAA receptors play a role in the actions of the N-methyl-D-aspartate receptor antagonist ketamine and of the α2-adrenergic agonist medetomidine, either alone or in combination, using α3KO mice and (3) whether α3-containing GABAA receptors on noradrenergic neurons are responsible for the increased sensitivity to ketamine/xylazine. To this end, using conditional cre-loxP-mediated recombination limited to noradrenergic neurons, we generated α3KO mice in which the α3 subunit was reintroduced exclusively in noradrenergic neurons.
Materials and Methods
Animals
Male mice on the C57BL/6J background between 8-18 weeks of age at beginning of experiments were used. Biochemical and morphological studies were performed in Zurich with approval of the cantonal veterinary office of Zurich (Zurich, Switzerland) and performed in accordance with international guidelines on animal use and care (European Community Council Directive 86 / 609 / EEC). All behavioral experiments were conducted in Belmont in accordance with the NIH guide for the Care and Use of Laboratory animals, and were approved by the McLean Hospital Institutional Animal Care and Use Committee, Belmont, MA. Mice lacking the α3 subunit of the GABAA receptor (α3KO mice) have been described previously.18 For behavioral testing mice were group-housed (3-4 per cage) and kept under a reversed 12/12h light-dark cycle (lights on from 2100- 0900h) for at least two weeks before beginning of experimental testing and throughout the study.
Generation of global rescue mice
The generation of mice lacking the α3 subunit of the GABAA receptor (α3KO mice) has been described in detail.18 The absence of α3 messenger RNA was demonstrated by reverse transcription polymerase chain reaction, and the absence of the α3 protein by Western blot and immunohistochemistry.18 As the nomenclature of exons in the Gabra3 gene has changed over time, exon 4 in Yee et al.18 is identical to exon 5 as described here. These mice carry an artificial exon flanked by loxP sites in the Gabra3 gene, which is located on the X-chromosome. In mice, which carry both the mutant gene and express the Cre recombinase this artificial exon is removed, thereby restoring α3 subunit expression. Mice in which the α3 subunit is rescued globally have been generated by crossing α3KO mice to EIIa-Cre mice, which express the Cre recombinase in oocytes and preimplantation stages of the embryo.19 Mice carrying both the mutant gene and the Cre recombinase gene have been selected by PCR genotyping. These mice were crossed to C57BL/6J wild type mice. Hemizygote male mice carrying only the mutant gene were then selected for experiments to ensure that the rescue of the α3 subunit had been present in the germline.
Generation of neuron-specific rescue mice
Mice expressing the α3 subunit of the GABAA receptor exclusively in noradrenergic [dopamine β hydroxylase (DBH)-Rescue mice] and dopaminergic [dopamine transporter (DAT)-Rescue mice] neurons, respectively, have been generated by crossing female α3KO mice to male mice expressing the improved Cre (iCre) recombinase specifically in (nor)adrenergic (DBH-iCre mice) or dopaminergic neurons (DAT-iCre mice). DBH-iCre mice express the iCre recombinase under the control of the promoter of the dopamine-β-hydroxylase gene and have been described previously.20,21 DAT-iCre mice express the iCre recombinase under the control of the promoter of the dopamine transporter (DAT) gene and have been described previously.22 Male mice carrying both the α3KO gene and the respective iCre transgene have been selected by PCR genotyping.
Immunoperoxidase staining
4 wild type and 4 global rescue mice (9 weeks of age) were deeply anaesthetized with pentobarbital (Nembutal®, Abbott, Chicago, IL; 50 mg / kg, intraperitoneally) and perfused through the ascending aorta with fixative solution (4% paraformaldehyde in 0.15 M phosphate buffer and 15% saturated picric acid solution; pH 7.4). Brains were postfixed in fixative solution for 3 h and incubated in citrate buffer (0.2M Na2HPO4dihydrate, 0.1M citric acid; pH 4.5) overnight. The next day brains were cut parasagittally in about 8 mm thick blocks and irradiated in citrate buffer at 650 watt in the microwave for 90 seconds for antigen retrieval.23 Brains were cryoprotected in 10% dimethyl sulfoxide in phosphate buffered saline (PBS) for at least 2 hours prior to sectioning them transversally at 40 μm with a sliding microtome. The sections were collected in PBS. Free-floating sections were incubated overnight at 4 °C with a primary antibody directed against the α3 subunit of the GABAA receptor (1:5,000; antibody was developed in-house and is described in detail9 in Tris-Triton buffer (0.05M Tris, 0.15M NaCl, 0.2% Triton X-100; pH 7.4) containing 2% normal goat serum. The next day sections were washed three times in Tris-Triton buffer and incubated for 30 min at room temperature in biotinylated secondary antibody (1:300; Jackson Immunoresearch, West Grove, PA) in Tris-Triton buffer containing 2% normal goat serum. After three washes in Tris-Triton buffer sections were incubated in the Avidin-Peroxidase (ABC)-Solution (1:100 in Tris-Triton buffer) for 30 min (Vectastain Elite Kit; Vector Laboratories, Burlingame, CA) and after another wash finally reacted with diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St Louis, MO) in Tris-Triton buffer (pH 7.7) containing 0.015% hydrogen peroxide. After 15 min the color reaction was stopped with ice-cold PBS. After two washes in PBS at room temperature sections were mounted on gelatin-coated slides and air-dried. Finally, they were dehydrated with an ascending series of ethanol, cleared in xylene, and coverslipped with Eukitt (Erne Chemie, Dällikon, Switzerland). Brains and sections from wild type and global rescue mice were processed in parallel under identical conditions to minimize variability in staining intensity. Images were taken with a stereo microscope (Zeiss, Jena, Germany).
Immunofluorescence staining
DAT-Rescue (n=4), DBH-Rescue (n=3), wild type (n=3) and α3KO (n=2) mice (8-14 weeks of age) were examined to confirm selective expression of the α3 subunit in dopaminergic and noradrenergic neurons, respectively. For optimal detection of tyrosine hydroxylase, a neuronal marker for dopaminergic and noradrenergic neurons, and the α3 subunit of the GABAA receptor simultaneously a novel protocol was used, which has been described in detail previously.24,25 In brief, mice were anaesthetized with isoflurane and decapitated. The brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid (composition in mM: NaCl, 125; NaHCO3, 26; NaH2PO4, 1.25; KCl, 2.5; MgCl2, 1; CaCl2, 2.5; and glucose, 11; oxygenated with 95% O2- 5% CO2). The brain was affixed to a vibratome stage with cyanoacrylate and kept in the ice-cold ACSF for slicing. Parasagittal 300 μm-thick slices were prepared and incubated at 33 °C for 20 min in oxygenated ACSF. They were then fixed by immersion for 10 min in 4% paraformaldehyde in 0.15 M phosphate buffer, washed with PBS, cryoprotected overnight in 30% sucrose in PBS and frozen on a flat surface in the cryostat. Parasagittal sections were cut at a thickness of 16 μm, mounted onto gelatin-coated glass slides, air-dried at room temperature for 30 s, and stored at 20 °C for at least 1 h. Sections were preincubated for 1 h at room temperature in PBS containing 10% normal goat serum and 0.2% Triton X-100, followed by overnight incubation at 4 °C with primary antibodies against tyrosine hydroxylase raised in rabbit (1:5,000; Merck Millipore) and against the α3 subunit of the GABAA receptor raised in guinea pig (1:3,000)9 diluted in the same solution. Sections were then washed extensively in PBS and incubated for 1 h at room temperature in corresponding affinity-purified goat immunoglobulin G coupled to Cy3 (1:500) or Alexa 488 (1:1000; Jackson Immunoresearch). Sections were washed again, and coverslipped with aqueous mounting medium (Dako, Carpinteria, CA). Immunofluorescence staining was visualized by confocal laser scanning microscopy (LSM-510 Meta; Zeiss) with a 40× objective (N.A. 1.3) and sequential acquisition of separate color channels. Image acquisition settings were adjusted to cover the entire dynamic range of the photomultipliers. The pinhole size was set to 1.0 Airy units for each channel, and stacks of 12 sections (512×512) spaced by 0.4 μm were acquired. For display, images were processed with the image-analysis program Imaris (Bitplane, Zurich, Switzerland). Images from both channels were overlaid, background was subtracted and a low pass filter (“edge preserving” filter) was applied. Images are displayed as extended projection of the entire stack.
Western Blotting
Membrane preparation and western blotting have been conducted as described previously.26 Fresh frozen brains from 5 wild type and 5 global rescue mice (10 weeks of age) were homogenized in 5 ml sucrose buffer (0.32 M sucrose, 5 mM EDTA, 10 mM Tris pH 7.5, 0.02% NaN3, PMSF 1:500, complete mini (Roche Diagnostics, Mannheim, Germany)) and centrifuged for 10 min at 1,000 × g. The supernatant was collected and the step repeated. Both supernatants were combined and centrifuged for 20 min at 40,000 × g to obtain the crude membrane fraction. The pellet was resuspended in 3 ml sucrose buffer. Protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL). Samples were incubated for 5 min at 95 °C with an equal volume of 125 mM Tris-HCl pH 6.8, 20% glycerol, 0.002% bromphenol blue, 10% β-mercaptoethanol, 4% SDS and stored at −20 °C. Before use samples were incubated for 10 min at 60 °C and diluted to a concentration of 1 mg/ml. Aliquots with increasing protein content (2.5, 5, 7.5 and 10 μg) were subjected to SDS-PAGE using 10% mini-gels (Mini protean II; Bio-Rad, Hercules, CA). Proteins were transferred onto nitrocellulose membranes using a Trans Blot Mini Cell (Bio- Rad). The blots were blocked for 1-2 h in 10 mM Tris-HCl pH 8, 0.15 M NaCl, 0.05% Tween 20 containing 5% non-fat dry milk at room temperature, followed by incubation with affinity-purified antiserum against the α3 subunit of the GABAA receptor (1:500)9 together with a monoclonal antibody directed against β-actin (1:20,000, Merck Millipore, Billerica, MA) overnight at 4 °C in 10 mM Tris-HCl pH 8, 0.15 M NaCl, 0.05% Tween 20 / 5% non-fat dry milk. The blots were washed once with 20 mM Tris pH 7.5, 60 mM NaCl, 2 mM EDTA, 0.4% SDS, 0.4% Triton-X 100, 0.4% deoxycholate and four times with 10 mM Tris-HCl pH 8, 0.15 M NaCl, 0.05% Tween 20. Incubation with the appropriate horse radish peroxidase-conjugated secondary antibodies was carried out for 1 h at room temperature. Following extensive washing immunoreactivity was detected by the enhanced chemoluminescence method (Super Signal West Pico chemoluminescence, Pierce, Rockford, IL,). Images were captured using a Fujifilm (Tokyo, Japan) LAS-1000 Plus Gel Documentation System, and immunoreactive bands were quantified with the AIDA software (Version 3.25, Raytest, Pforzheim, Germany). Actin immunoreactivity was used to monitor equal sample loading. The specificity of the antibody against the α3 subunit has been confirmed previously using α3KO mice.18
Drugs
Ketamine-HCl/Xylazine-HCl solution (Sigma-Aldrich) was administered at a volume of 20 ml/kg. Ketamine-HCl (Sigma-Aldrich), medetomidine-HCl solution (Domitor®, Pfizer Animal Health, New York, NY) and pentobarbital-Na (Sigma-Aldrich) were administered at a volume of 10ml/kg. Etomidate (Amidate®, Hospira, Lake Forest, IL) was administered at a volume of 15 ml/kg. Midazolam-HCl solution (Bedford Laboratories, Bedford, Ohio) was administered at a volume of 16.5 ml/kg. All drugs were dissolved or diluted in physiological (0.9%) saline where necessary and drug doses were calculated as mg/kg base. All drugs were administered by intraperitoneal injection. The doses administered are reported in the results section.
Behavioral testing
Tests were conducted during the dark phase (0900-2100 h) and each test was conducted at approximately the same time of day. The tester was blind to the genotype of the animals. Animals were tested in 3 cohorts. Cohort 1 containing wild type, α3KO, DBH-Rescue and DBH-iCre mice received a single injection of ketamine/xylazine and anesthetic properties were observed. Cohort 2 containing wild type and α3KO mice were first tested for medetomidine-induced sedation in the locomotor activity test, 3 days later for medetomidine-induced hypothermia, and 4-6 days later for anesthetic properties of ketamine, medetomidine or pentobarbital. Animals received increasing doses of ketamine, medetomidine or pentobarbital every 7 days. Mice were counterbalanced for drug experience. Cohort 3 containing wild type and α3KO mice were first tested for anesthetic properties of midazolam and 7 days later for anesthetic properties of etomidate.
Locomotoracitivity
Animals were moved into the testing room at least one hour before testing. Animals were injected with medetomidine (30 μg/kg or 60 μg/kg) or vehicle immediately before testing. The testing apparatus consisted of four evenly illuminated (about 130 lux) clear plexiglas (J. Freeman, Inc., Dorchester, MA)_boxes (41 × 41 × 31 cm) separated by white cardboard. Boxes were cleaned with 70% ethanol between animals. Locomotor activity was recorded for 60 min using the video tracking system EthoVisionXT (Noldus Information Technology, Wageningen, The Netherlands).
Medetomidine-induced hypothermia
Animals were moved into the testing room directly before testing. Body temperature was determined using a TH-5 digital thermometer (Physitemp Instruments, Clifton, NJ) with a RET3-Iso thermal probe for mice (inserted 2cm deep) immediately before drug injection (vehicle, 60 μg/kg, 120 μg/kg medetomidine) and 30 min, 60 min, 90 min and 120 min thereafter. Mice were kept together in their home cage throughout the experiment.
Anesthetic endpoints
The assessment of anesthetic end points was adapted from published protocols.27-29 Animals were moved into the testing room directly before testing. Each animal was placed in a clean holding cage without bedding material immediately after drug injection. Body temperature was determined using a TH-5 digital thermometer (Physitemp Instruments) with a RET3-Iso thermal probe for mice (inserted 2cm deep) immediately before drug injection and every 30 minutes thereafter. Once the animal stopped moving it was turned on its back. Beginning of LORR was defined as the time-point when the animal was placed on its back. End of LORR was defined as the time-point when the animal righted itself spontaneously from dorsal to sternal recumbency with all four extremities touching the ground. The time between beginning and end of LORR was defined as duration of LORR. After regaining the righting reflex animals were turned on their back every minute and time to righting was scored: less than 2 seconds (Score=1), between 2 and 10 seconds (Score=2), more than 10 seconds (Score=3). Animals were defined as having fully recovered when they were able to right themselves for 3 consecutive times within 2 seconds. Time to recovery was defined as the time between beginning of LORR and full recovery. A total LORR score was calculated by adding the score values for every minute from beginning of LORR until full recovery. The loss of hindlimb withdrawal reflex (LHWR) was determined every 5 minutes by pinching the paw firmly with an atraumatic forceps alternating between the left and right hindpaw until animals regained the righting reflex. LHWR was defined as present if no signs of reaction to the pinch were observed.
Anesthetic properties of medetomidine were assessed using a different protocol. Animals were moved into the testing room directly before testing and each animal was placed in a clean holding cage without bedding material immediately after drug injection. Animals were gently rolled on their side 30 min, 60 min, 90 min and 120 min after injection and time to righting defined as completely returning to the prone position with all four extremities touching the ground was noted. LORR was defined as present if animals were not able to roll back to the prone position within 10 seconds.
Statistical analysis
Data were analysed using the appropriate statistical test (Student's t-test, ANOVA followed by post hoc Dunnett's t-test, ANOVA with repeated measures followed by post hoc t-tests with Bonferroni correction) or Sheffe's post-hoc test. Hypothesis testing was two-tailed. Data for medetomidine-induced LORR were analyzed by fitting standard least squares mixed models with subject as a random effect. Drug dose and genotype were included as fixed effects. All effects were assessed by F-tests based on the fitted regression models. This analysis was followed by post hoc Mann-Whitney U tests. Data are expressed as the mean ± SEM. P<0.05 was considered to be statistically significant. Statistical analysis was performed using the SPSS and GraphPad software packages.
Results
When performing stereotaxic surgeries for implantation of electrodes into the medial forebrain bundle under ketamine/xylazine (139/21 mg/kg intraperitoneally) anesthesia, we unexpectedly observed that using the same doses of anesthetics α3KO mice were anesthetized longer than wild type mice (approximately 130 min compared to 80 min), prompting us to investigate the role of α3-containing GABAA receptors in the action of intravenous general anesthetics.
Anesthetic endpoints with etomidate, midazolam and pentobarbital
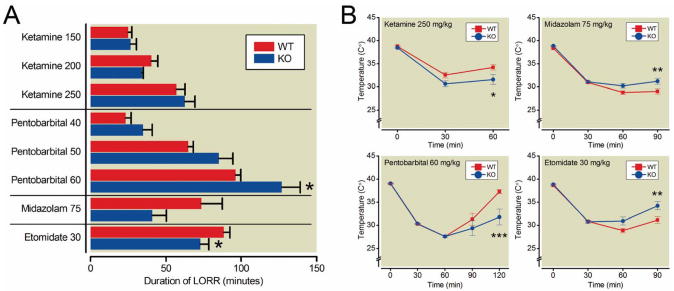
The anesthetic effects of etomidate, midazolam and pentobarbital, three compounds known to act at least in part via GABAA receptors, were assessed by determining the following end points: duration of LORR, time to recovery and total LORR score as a measure of hypnosis, LHWR as a measure of immobility and body temperature as a measure of hypothermic effects of the drug (Fig. 1 and Table 1).
Figure 1. Effect of ketamine, pentobarbital, midazolam, and etomidate on duration of LORR (A) and core body temperature (B).
A. Duration of loss of righting reflex (LORR) was significantly increased in α3KO mice by 60 mg/kg pentobarbital (repeated measures ANOVA followed by t-tests with Bonferroni correction) and significantly decreased by 30 mg/kg etomidate (unpaired t-test).
B. Repeated measures ANOVA showed a mean effect of genotype on core body temperature for 250mg/kg ketamine, 75mg/kg midazolam and 30 mg/kg etomidate. t-tests with Bonferroni correction revealed that the temperature decrease was more pronounced in α3KO mice compared to wild type mice 60 minutes and 120 minutes after administration of ketamine and pentobarbital, respectively and that the temperature decrease was less pronounced in α3KO mice compared to wild type mice 90 minutes after administration of midazolam or etomidate. Data represent the mean ± SEM; *, P<0.05, **, P<0.01 and ***, P<0.001, n=9 wild type and n=7 α3KO for ketamine; n=9 for pentobarbital; n=11 for etomidate and midazolam. WT, wild type; KO = α3KO.
Table 1. Anesthetic Properties (Ketamine, Pentobarbital, Midazolam, Etomidate).
| Drug | Dose (mg/kg) | Genotype | n | duration of LORR (min) | time to recovery (min) | Total LORR score | LHWR (min) |
|---|---|---|---|---|---|---|---|
| Ketamine | 150 | WT | 9 | 25.0 ± 2.4 | 36.1 ± 2.4 | 94.8 ± 5.6 | 0.0 ± 0.0 |
| α3KO | 7 | 26.7 ± 4.4 | 42.0 ± 5.3 | 110.1 ± 14.4 | 0.0 ± 0.0 | ||
| 200 | WT | 9 | 40.3 ± 4.4 | 61.7 ± 3.7 | 168.3 ± 10.3 | 0.0 ± 0.0 | |
| α3KO | 7 | 34.1 ± 1.1 | 61.3 ± 3.0 | 161.6 ± 7.1 | 0.0 ± 0.0 | ||
| 250 | WT | 9 | 56.8 ± 5.9 | 83.0 ± 4.1 | 231.3 ± 11.7 | 0.0 ± 0.0 | |
| α3KO | 7 | 62.6 ± 6.7 | 97.3 ± 5.8 | 269.6 ± 18.3 | 0.0 ± 0.0 | ||
| Pentobarbital | 40 | WT | 9 | 23.2 ± 3.7 | 26.3 ± 3.5 | 75.2 ± 10.6 | 0.0 ± 0.0 |
| α3KO | 9 | 34.8 ± 6.0 | 36.8 ± 5.7 | 107.6 ± 17.0 | 0.0 ± 0.0 | ||
| 50 | WT | 9 | 64.7 ± 3.6 | 65.9 ± 3.5 | 194.4 ± 10.6 | 0.0 ± 0.0 | |
| α3KO | 9 | 85.0 ± 9.5 | 86.0 ± 9.5 | 255.2 ± 28.5 | 0.0 ± 0.0 | ||
| 60 | WT | 9 | 96.2 ± 3.6 | 98.2 ± 3.6 | 290.3 ± 10.7 | 0.0 ± 0.0 | |
| α3KO | 9 | 126.8 ± 12.2* | 129.1 ± 12.3* | 382.1 ± 36.7* | 25.0 ± 11.6 | ||
| Midazolam | 75 | WT | 11 | 73.5 ± 14.1 | 101.1 ± 7.5 | 298.8 ± 23.6 | 0.0 ± 0.0 |
| α3KO | 11 | 40.8 ± 9.4 | 53.8 ± 9.9** | 159.4 ± 27.3*** | 0.0 ± 0.0 | ||
| Etomidate | 30 | WT | 11 | 88.3 ± 4.3 | 91.1 ± 4.5 | 273.7 ± 10.3 | 0.0 ± 0.0 |
| α3KO | 11 | 72.7 ± 5.8* | 77.2 ± 5.8 | 224.9 ± 17.6* | 0.0 ± 0.0 |
Data represent the mean ± SEM. Duration of LORR, time to recovery and the global anesthetic score were significantly increased in α3KO mice compared to wild type mice following 60mg/kg pentobarbital (2-way ANOVA with repeated measures followed by post hoc t-tests with Bonferroni correction). Time to recovery and the global anesthetic score were significantly decreased following 75mg/kg midazolam and the duration of LORR and the global anesthetic score were significantly decreased following 30mg/kg etomidate in α3KO mice compared to wild type mice (unpaired t-test)
P<0.05,
P<0.01 and
P<0.001
Footnote: LORR, loss of righting reflex; LHWR, loss of hindlimb withdrawal reflex; WT, wild type.
Etomidate
Etomidate (30 mg/kg) was less effective in α3KO mice compared to wild type mice. Duration of LORR (WT: 88.3+/−4.3 min, α3KO: 72.7+/−5.8 min) and total LORR score (WT: 273.7+/−10.3, α3KO: 224.9+/−17.5) were significantly decreased in α3KO mice (unpaired t-test, P=0.0439 and P=0.0263, respectively), while time to recovery was not different (WT: 91.1+/−4.5 min, α3KO: 77.2+/−5.8 min, unpaired t-test, P=0.0710). Anesthetic-induced decrease in body temperature was smaller in α3KO mice compared to wild type controls (2-way ANOVA with repeated measures of time: significant effect of time F(2,40), P<0.0001; significant effect of genotype F(1,20)=4.90, P=0.0386; significant time × genotype interaction F(2,40)=8.12, P=0.0011). Post hoc t-tests with Bonferroni correction showed that the temperature differed significantly 90 minutes after injection (P<0.01).
Midazolam
The hypnotic action of midazolam (75 mg/kg) was decreased in α3KO mice compared to wild type mice. Although the difference in the duration of LORR failed to reach significance (unpaired t-test, P=0.0691), the time to recovery (WT: 101.1+/-7.5 min, α3KO: 53.8+/-9.9 min) and the total LORR score (WT: 298.8+/-23.6, α3KO: 159.4+/-27.3) were significantly lower in α3KO mice (unpaired t-test, P=0.0011 and P=0.001, respectively). Similarly, the reduction in body temperature was smaller in α3KO mice (2-way ANOVA with repeated measures of time: significant effect of time F(2,40)=17.65, P<0.0001; significant effect of genotype F(1,20), P=0.0395 and significant time × genotype interaction F(2,40), P=0.0008). Post hoc t-tests with Bonferroni correction showed that the temperature difference was significant 90 minutes after injection (P<0.01).
Pentobarbital
For pentobarbital 2-way ANOVAs using drug dose and genotype as factors revealed main effects of drug and genotype on LORR (drug F(2,32)=160.92, P<0.0001; genotype F(1,16)=5.64, P=0.304), on time to recovery (drug F(2,32)=163.15, P<0.0001; genotype F(1,16)=5.51, P=0.0321) and on total LORR score (drug F(2,32)=158.06, P<0.0001, genotype F(1,16)=5.63, P=0.305). Furthermore, 4 out of 9 α3KO mice lost the hindlimb withdrawal reflex at the highest dose of pentobarbital (60 mg/kg), whereas none of the wild type mice lost the reflex. This difference was not statistically significant (Fisher's exact probability test: P=0.0824). At the highest dose of pentobarbital (60 mg/kg), 2-way ANOVA with repeated measures of time showed a significant effect of time F(3,48)=27.19, P<0.0001 but no effect of genotype F(1,16)=3.60, P=0.0761). However, with respect to body temperature, there was a significant time × genotype interaction (F(3,48)=5.56, P=0.0023) and t-tests with Bonferroni correction showed that body temperature was significantly lower in α3KO mice 120 minutes after injection (P<0.001).
Anesthetic endpoints with ketamine and medetomidine
Ketamine
There were no differences in duration of LORR, time to recovery, total LORR score or LHWR between wild type and α3KO mice at any dose of ketamine, although two α3KO mice died after administration of 150 mg/kg and 250 mg/kg, respectively. However, the reduction in body temperature was significantly larger in α3KO mice compared to wild type mice by 250 mg/kg ketamine (ANOVA with repeated measures of time: significant effect of time F(1,14)=7.99 P=0.0134; significant effect of genotype F(1,14)=7.99 P=0.025). Post hoc t-tests with Bonferroni correction showed that body temperature was significantly different 60 minutes after injection (P<0.05).
Medetomidine
Sedative effects of medetomidine
The sedative effects of the α2-adrenergic agonist medetomidine in α3KO and wild type mice were assessed by measuring the locomotor activity in a novel open field over 60 minutes following drug injection. The total distance traveled was decreased by medetomidine (30 or 60 μg/kg) (Fig. 2A) but there were no differences between α3KO and wild type mice (2-way ANOVA: significant effect of drug (F(2,48)=32.25, P<0.0001) (insert in Fig. 2A). Analysis with 2-way ANOVA with repeated measures of 5-minute time bins also did not reveal any difference between genotypes, although a general reduction in activity over time was observed in both genotypes (Vehicle: significant effect of time F(11,176)=32.41, P<0.0001; 30 μg/kg: significant effect of time F(11,176)=41.85, P<0.0001; 60 μg/kg: significant effect of time F(11,176)=47.54, P<0.0001) (Fig. 2A).
Figure 2. Effects of medetomidine on locomotor activity (A), duration of LORR (B) and body temperature (C) in α3KO and wild type mice.
A. Locomotor activity was comparable in both genotypes at all medetomidine doses over the whole 60 minute time period (insert) and looking at 5-minute time bins.
B. Duration of loss of righting reflex (LORR) was significantly increased in α3KO mice by 250 μg/kg medetomidine compared to wild type mice (Sheffe post-hoc tests).
C. Body temperature was comparable between α3KO mice and wild type mice. Data represent the mean ± SEM; n=9 per genotype.
Hypnotic effects of medetomidine
The hypnotic effects of medetomidine in α3KO and wild type mice were assessed 60 minutes after injection by rolling the mice on their side and noting the time to righting (Fig. 2B). When righting did not occur within 30 minutes the observation was stopped. Data were analyzed by fitting standard least squares mixed models with subject as a random effect. Drug dose and genotype were included as fixed effects. Effects were assessed by F-tests based on the fitted regression models. This revealed a significant overall effect of genotype (F(1,16)=8.90, P=0.0088) and dose (F(1,34)=36.96, P<0.0001). Post hoc Sheffe tests showed that the LORR was significantly increased in α3KO mice (298±192sec) compared to wild type mice (6±4sec) by 250 μg/kg medetomidine (P=0.0098). At this dose only 2 out of 9 wild type mice lost the righting reflex (defined by the inability to right within 10 seconds) in contrast to 7 out of 9 α3KO mice.
Hypothermic effects of medetomidine
The hypothermic effects of medetomidine in α3KO and wild type mice were assessed by determining the rectal body temperature immediately before drug or vehicle injection and every 30 minutes thereafter over a period of 120 minutes (Fig. 2C).
The baseline body temperature before injection was elevated in α3KO mice compared to WT mice in the groups that received vehicle (WT=38.29+/-0.11°C, α3KO=39.01+/-0.18°C, unpaired t-Test, P<0.01) and 60μg/kg medetomidine (WT=38.297+/-0.15°C, α3KO=39.13+/-0.17°C, unpaired t-Test, P<0.01) in α3KO mice (39.0±0.2°C) , but not in the group that received 120μg/kg medetomidine (WT=38.82+/-0.14°C, α3KO=38.97+/-0.09°C, independent samples t-Test, P=0.398). This precluded direct statistical analysis of the hypothermic effects of 60 μg/kg medetomidine. For the 120 μg/kg medetomidine dose, a genotype × time 2-way ANOVA did not reveal a significant genotype main effect or genotype × time interaction. In the entire study, a difference in baseline temperature was only present in 2 out of the 11 experiments performed in total with midazolam, pentobarbital and ketamine. We therefore assume that extraneous undefined factors specific to the experiments with medetomidine are responsible for this baseline differences and that overall WT mice and α3KO mice do not display a difference in baseline body temperature. When we analyzed the change in body temperature from baseline, we still did not obtain any significant differences between genotypes both for 60 μg/kg and for 120 μg/kg medetomidine (not shown).
Generation of mice expressing the GABAA receptor α3 subunit exclusively in noradrenergic neurons
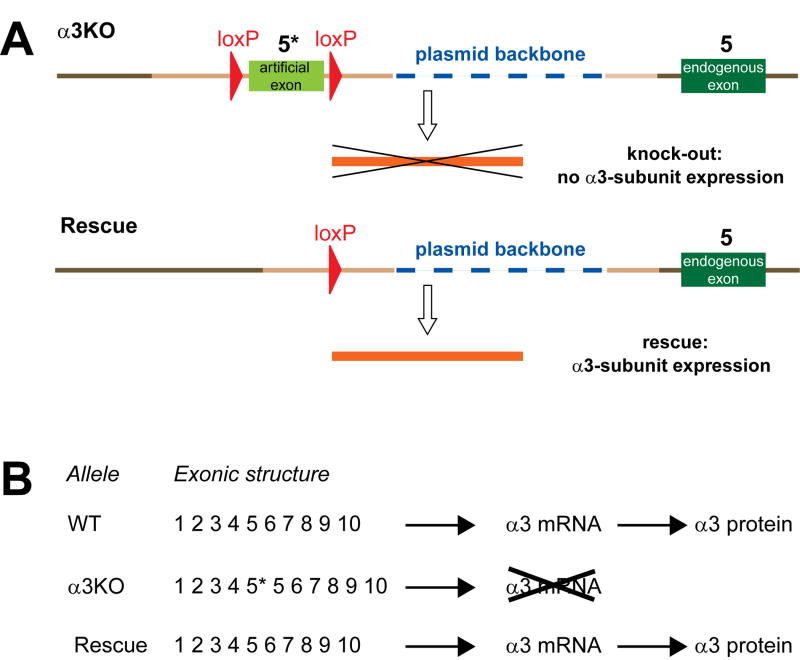
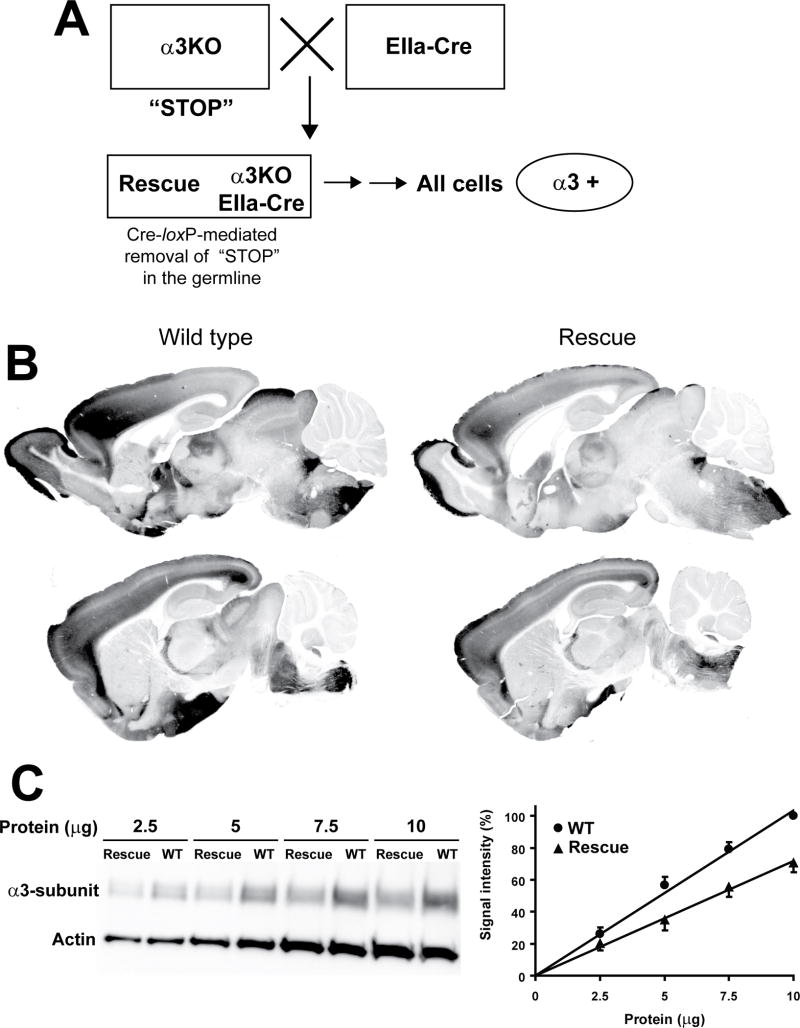
α3KO mice used in this study have been generated employing an insertion targeting strategy (see Fig. 1 in reference18) with an insertion-type targeting vector containing exon 5 of the Gabra3 gene flanked by two loxP sites. This vector was inserted into the target locus in its entirety by single reciprocal recombination resulting in a duplication of exon 5 (i.e. exon 4 in Fig. 1 in Yee et al.18). The resulting α3KO mice carry both an endogenous exon 5 and an artificial exon 5* (5* in Fig. 3A). This artificial exon 5* provides a “STOP” signal functionally disrupting the Gabra3 gene (Fig. 3A,B). We predicted that a cre-loxP-mediated excision of this loxP-flanked artificial exon 5* would functionally restore the Gabra3 gene. In a proof-of-principle experiment, α3KO mice were crossed to EIIa-cre mice, which express the cre transgene at early embryonic stages19 (Fig. 4A). As predicted, cre-loxP-mediated recombination resulted in elimination of the loxP-flanked artificial exon 5*. Subsequently, the Ella-Cre transgene was bred out to ensure that the rescue of the α3 subunit had been present in the germline. These global rescue mice were examined for α3 subunit expression. Immunohistochemical analysis revealed that α3 subunit expression is restored in these global rescue mice and the distribution pattern of α3 subunit expression is indistinguishable from wild type mice (Fig. 4B). Western blot analysis showed that α3 subunit expression levels in global rescue mice are 70±7% compared to wild type mice (Fig. 4C). Thus, the genomic rearrangement in the rescue allele, which includes the presence of a plasmid backbone, reduces the expression of the α3 subunit by approximately 30% without affecting its regional distribution. The precise mechanisms of this reduction are unknown.
Figure 3. Schematic of genetic rescue of the α3 knockout in noradrenergic neurons.
A. α3KO and Rescue alleles. theα3KO allele (top) has integrated the insertion-type gene targeting vector for single reciprocal recombination in its entirety18. It carries an artificial exon (light green, 5*) flanked by loxP sites (red), targeting vector-derived homologous genomic sequences (light brown) and the plasmid backbone of the targeting vector (blue). Genomic sequences that were not included in the targeting vector are shown as dark brown. The most relevant feature is that the α3KO allele contains the endogenous exon 5 (dark green, 5) and an artificial exon 5 (light green, 5*), which results messenger RNA (mRNA) degradation, which is the likely mechanism of the α3 knockout20. In the Rescue allele, elimination of the artificial exon by Cre-loxP-mediated recombination results in α3 mRNA (orange) being made and thus the expression of the α3 subunit being restored.
B. Exonic structure of predicted gene products from wild type (WT), α3KO and Rescue allele. Presence of the artificial exon 5, 5*, in the α3KO results in mRNA breakdown and thus a functional knockout. The exonic structure of wild type (WT) and Rescue alleles is identical.
Figure 4. Localization and quantification of the α3 knockout in global rescue mice.
A. Generation of global Rescue mice. An α3KO mouse (with artificial exon 5, the “STOP” signal) is bred with an EIIa-Cre mouse19. Some of the offspring will carry both the α3KO allele and the EIIa-Cre transgene. This mouse is bred with a wild type mouse to breed out the EIIa-Cre transgene (depicted by two sequential horizontal arrows) to confirm that the artificial exon 5 (the “STOP” signal) has been removed from the germline, so that the α3 subunit is expected to be expressed in all cells in which it is naturally expressed.
B. Immunoperoxidase staining of perfusion-fixed parasagittal brain sections: α3 subunit distribution pattern is equivalent in wild type and global rescue mice. Representative sections from 2 wild type and 2 global rescue mice are shown.
C. Left panel: Representative Western Blot: α3 subunit expression level is decreased in global rescue mice compared to wild type mice. Right panel: Quantification of Western Blot signal: signals were normalized to the α3 subunit signal at 10 μg protein in wild type mice (100%). α3 subunit expression level in global rescue mice is 70±7% of wild type expression. Data represent the mean±SEM of five experiments.
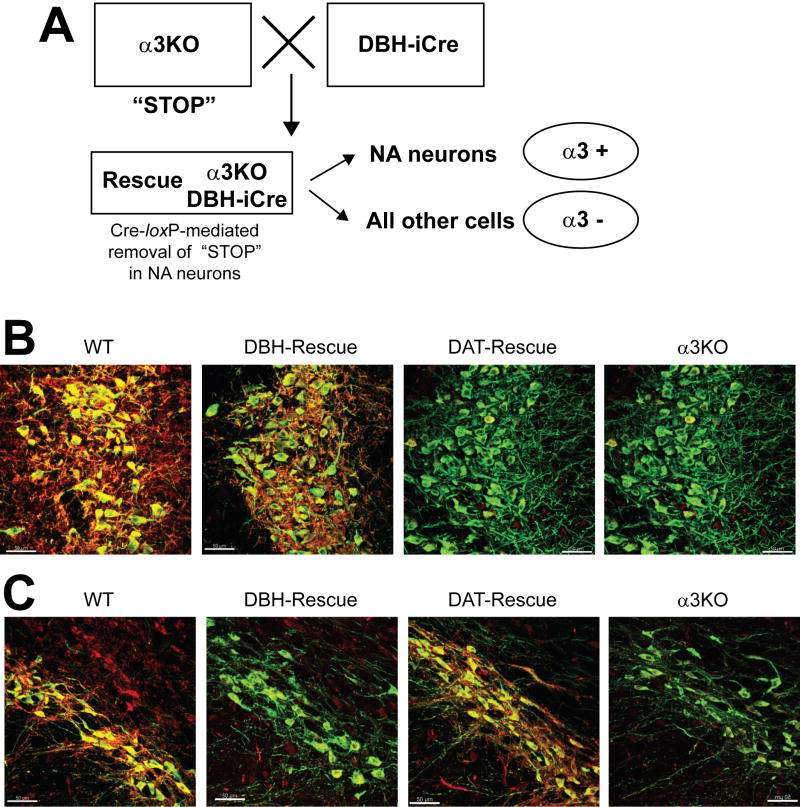
In order to generate mice expressing the α3 subunit exclusively in noradrenergic neurons, mice carrying the α3 knockout allele and a dopamine β hydroxylase iCre transgene (DBH-iCre) were generated (Fig. 5A). In addition, mice carrying the α3 knockout allele and a dopamine transporter iCre transgene (DAT-iCre) were generated to demonstrate the specificity of the rescue approach.
Figure 5. Recue of α3 expression in defined neuronal cell types.
A. An α3KO mouse is crossed with a dopamine β hydroxylase impoved Cre (DBH-iCre) mouse to obtain DBH-iCre rescue mice (in our studies hemizygous males) containing both the α3 knockout allele and the DBH-iCre transgene. The artificial exon 5 in the α3 knockout allele functions as a “STOP” signal resulting in messenger RNA degradation18. In noradrenergic neurons of the rescue mouse, this “STOP” signal is removed by cre-loxP-mediated recombination. Thus, in the Rescue mice, the α3 subunit is expressed only in the noradrenergic neurons. Similar considerations apply to the generation of the dopamine transporter (DAT)-Rescue mice using the DAT-iCre transgene expressing iCre specifically in dopaminergic neurons.
B. Neuron-specific rescue of α3 subunit expression in the locus coeruleus: Immunofluorescence double-labeling of the α3 subunit (red) and tyrosine hydroxylase (green) shows that the α3 subunit is highly expressed in the locus coeruleus (LC) in wild type mice (WT). In α3KO mice expressing the iCre recombinase selectively in noradrenergic neurons (DBH-Rescue) α3 subunit expression is restricted to noradrenergic neurons. It is not detectable in the locus coeruleus of α3KO mice expressing the iCre recombinase exclusively in dopaminergic neurons (DAT-Rescue), nor in α3KO mice. Scale bar: 50μm.
C. Neuron-specific rescue of α3 subunit expression in the substantia nigra pars compacta: Immunofluorescence double-labeling of the α3 subunit (red) and tyrosine hydroxylase (green) shows that α3 subunit expression is found in dopaminergic neurons of the substantia nigra pars compacta (SNpc) of wild type mice (WT) and in α3KO mice expressing the iCre recombinase selectively in dopaminergic neurons (DAT-Rescue). It is not detectable in the substantia nigra pars compacta of α3KO mice expressing the iCre recombinase selectively in noradrenergic neurons (DBH-Rescue), nor in α3KO mice. Scale bar: 50μm.
Immunofluorescence analysis confirmed that the α3 subunit is exclusively expressed in noradrenergic neurons in α3KO/DBH-iCre (DBH-Rescue) mice (Fig. 5B), while it is exclusively expressed in dopaminergic neurons in α3KO/DAT-iCre (DAT-Rescue) mice (Fig. 5C). Wild type mice show intense expression of the α3 subunit on noradrenergic neurons of the locus coeruleus and on unidentified neurons in the proximity, while DBH-Rescue mice express the α3 subunit only on noradrenergic neurons (Fig. 5B). α3 subunit expression is not detectable in α3KO mice and DAT-Rescue mice in this brain region (Fig. 5B). To confirm the specificity of the rescue approach α3 subunit expression in DAT-Rescue mice was examined further. In the dopaminergic neurons of the substantia nigra pars compacta the α3 subunit protein is absent in α3KO and DBH-Rescue mice, while high levels of α3 subunit expression are found in wild type mice and expression is specifically restored in dopaminergic neurons in DAT-Rescue mice (Fig. 5C). In other brain regions where the α3 subunit is normally expressed such as the cerebral cortex or the hippocampus but where no noradrenergic or dopaminergic neurons are present, no α3 subunit protein was detectable in DBH- or DAT-rescue mice (not shown). Overall, these data demonstrate that the α3 subunit can be rescued specifically and to a large extent in noradrenergic neurons.
Anesthetic end points with ketamine/xylazine and rescue of the phenotype in mice expressing the GABAA receptor α3 subunit exclusively in noradrenergic neurons
In order to confirm the unexpected observation that ketamine/xylazine-induced anesthesia is significantly prolonged in α3KO mice and to address the question whether α3 subunit-containing GABAA receptors on noradrenergic neurons modulate these anesthetic effects, we studied several anesthetic end points and the body temperature decrease during anesthesia in α3KO mice, wild type mice and in DBH-Rescue mice (Fig. 6 and Table 2). To exclude the possibility that the iCre transgene, which is also present in the DBH-Rescue mice, is causing any effects by itself (also referred to as potential “Cre toxicity”), control mice expressing the iCre transgene in noradrenergic neurons (DBH-iCre) were included.
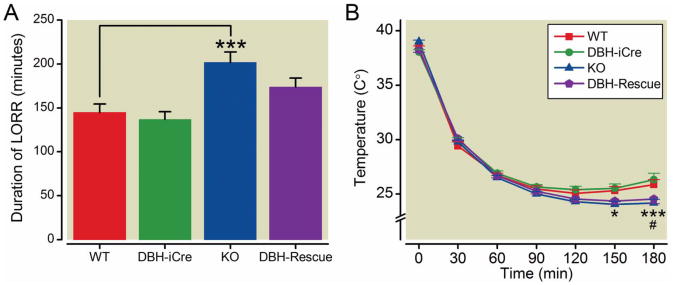
Figure 6. Effect of Ketamine (139 mg/kg)/Xylazine (21 mg/kg) on duration of LORR (A) and core body temperature (B).
A. Duration of loss of righting reflex (LORR) was significantly increased in α3KO mice compared to wild type mice, whereas mice expressing the α3 subunit exclusively in noradrenergic neurons [dopamine β hydroxylase (DBH)-Rescue] and mice carrying the improved cre (iCre) transgene (DBH-iCre) did not differ from wild type mice (1-way ANOVA followed by post hoc Dunnett's t-tests). B. Body temperature was significantly lower in α3KO mice compared to wild type mice 150 minutes and 180 minutes after injection. Body temperature in DBH-Rescue mice was significantly lower 180 minutes after injection compared to wild type mice (2-way repeated measures ANOVA followed by post hoc t-tests with Bonferroni correction). Data represent the mean ± SEM; ***, P<0.001 (α3KO compared to wild-type), *, P<0.05 (α3KO compared to wild-type), #, P<0.05 (DBH-Rescue compared to wild type); n=15 wild type and DBH-iCre, n=14 α3KO and DBH-Rescue. WT, wild type.
Table 2. Anesthetic end points with ketamine (139 mg/kg) / xylazine (21 mg/kg).
| Genotype | n | duration of LORR (min) | time to recovery (min) | Total LORR score | LHWR (min) |
|---|---|---|---|---|---|
| WT | 15 | 144.7 ± 9.5 | 159.9 ± 9.7 | 466.3 ± 28.7 | 95.0 ± 7.8 |
| DBH-iCre | 15 | 136.9 ± 8.5 | 153.9 ± 9.4 | 448.3 ± 27.5 | 83.3 ± 5.0 |
| α3KO | 14 | 201.7 ± 11.9*** | 218.2 ± 11.2*** | 641.3 ± 33.9*** | 137.1 ± 8.3*** |
| DBH-Rescue | 14 | 174.0 ± 9.8 | 201.9 ± 10.6* | 588.4 ± 31.5* | 113.2 ± 7.1 |
All anesthetic parameters were significantly increased in α3KO mice compared to wild type. Time to recovery and total anesthetic score were significantly increased in DBH-Rescue mice compared to wild type (1-way ANOVAs followed by post hoc Dunnett's t-tests). Data represent the mean ± SEM.
P<0.05 and
P<0.001 (compared to wild type).
Footnote: LORR, loss of righting reflex; LHWR, loss of hindlimb withdrawal reflex; WT, wild type; DBH, dopamine β hydroxylase, iCre, improved Cre.
One-way ANOVA showed a significant effect of genotype for all anesthetic parameters: duration of LORR (F(3,54)=8.81, P<0.0001), time to recovery (F(3,54)=9.52, P<0.0001), total LORR score (F(3,54)=9.51, P<0.0001) and LHWR (F(3,54)=10.76,P<0.0001) (Table 2). Post hoc Dunnett's tests revealed that α3KO mice show significantly increased measures on all anesthetic parameters (LORR, total LORR score, LHWR, P<0.001 and time to recovery P<0.01) compared to wild type mice. In contrast, DBH-Rescue mice showed an increase only in the time to recovery and the total LORR score (P<0.05) compared to wild type mice; they were not different from wild types in terms of LORR or LHWR times. In fact, the rescue mice were not different from either wild type or α3KO mice on these parameters, suggesting that they rank in between the two genotypes, leading to a “partial rescue” of the α3KO phenotype. The body temperature decrease caused by ketamine/xylazine was analyzed by a 2-way repeated measures ANOVA. A significant effect of genotype (F(3,54)=2.85, P=0.0456), significant effect of time (F(5,270)=475.04, P<0.0001) and a significant time × genotype interaction (F(15,270)=5.69, P<0.0001) were observed. Post hoc t tests with Bonferroni correction showed that the temperature was significantly lower in α3KO mice 150 minutes (P<0.05) and 180 minutes (P<0.001) after injection compared to wild type mice. DBH-Rescue mice showed a significantly lower temperature only 180 minutes (P<0.05) after injection compared to wild type mice. DBH-iCre mice did not differ from wild type mice in any anesthetic endpoint or in the body temperature indicating that the iCre transgene itself has no effect on the parameters measured. Overall, our results indicate that the hypnotic phenotype present in α3KO mice can be partially rescued by expression of the α3 subunit in noradrenergic neurons in DBH-Rescue mice, while the hypothermic phenotype appears to be independent of the α3 subunit in noradrenergic neurons.
Discussion
Clinically used intravenous general anesthetics are a structurally highly diverse class of drugs mediating their anesthetic effects through a variety of molecular targets.12 In this study, we evaluated the role of α3-containing GABAA receptors in the actions of intravenous anesthetics and found that while actions of GABAergic drugs like etomidate and midazolam are in part mediated by this receptor subtype, α3-containing GABAA receptors also constrain the actions of anesthetics which have partially (e.g., pentobarbital) or exclusively (Ketamine/xylazine, medetomidine) non-GABAergic targets. We also demonstrate that α3-containing GABAA receptors on noradrenergic neurons may constrain the hypnotic but not the hypothermic action of ketamine/xylazine action.
Studies using mice carrying β2(N265S) or β3(N265M) point mutations rendering the mutated subunit insensitive to etomidate or etomidate and propofol, respectively, have revealed that GABAA receptors containing the β2 or β3 subunits mediate the immobilizing (β3), hypnotic (β2 and β3), and hypothermic actions (mainly β2, minor role of β3 shown for etomidate and pentobarbital, but not for propofol) of etomidate, propofol, and pentobarbital.30,31,32 In line with previous reports showing that mice lacking the β3 subunit33 or the α1 subunit6 are less sensitive to the hypnotic effects of midazolam and etomidate, we found that α3KO mice are likewise less sensitive to these agents, collectively indicating that the α1, α3 and β3 subunits are involved in mediating the hypnotic response to GABAergic anesthetics. In contrast, α3KO mice showed an increase in the sensitivity to the hypnotic, immobilizing and hypothermic effects of pentobarbital. This is astonishing as GABAA receptors are a major target for pentobarbital.34 Consistent with this assumption α1KO mice6 and β3(N265M) point-mutant mice32 are less sensitive to the hypnotic effects of pentobarbital and moreover the immobilizing response to pentobarbital is completely absent in β3(N265M) point-mutant mice. On the other hand, the GABAA receptor antagonist gabazine attenuates the hypnotic response to pentobarbital to a much lesser extent than the hypnotic response to muscimol and propofol, pointing to the potential relevance of non-GABAA receptor targets for pentobarbital actions.35 Furthermore, in contrast to etomidate some effects of pentobarbital such as respiratory depression are similar in wild type and β3(N265M) point-mutant mice.32 Together with the fact that pentobarbital additionally acts on other drug targets such as nicotinic acetylcholine receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, kainate receptors, and glycine receptors36 it seems likely that at least some of the anesthetic effects of pentobarbital are mediated through non-GABAergic mechanisms. It is therefore possible that the increased sensitivity to pentobarbital in α3KO mice results from alterations in non-GABAergic pathways such as the cholinergic system where α3 subunit expression has been described37 or potentially from upregulation of non-GABAergic targets of pentobarbital or other compensatory mechanisms.
Based on the initial observation that ketamine/xylazine anesthesia is prolonged in α3KO mice and actions of such compounds would normally be expected to be unaltered in a GABAA receptor knockout mouse, investigating the contribution of N-methyl-D-aspartate receptor antagonism and of α2-adrenergic receptor agonism to this phenomenon was a major goal in this study. We therefore examined the action of ketamine and of the α2-adrenergic agonist medetomidine. Medetomidine was chosen for two reasons: 1) Essential pharmacologically relevant data, e.g. with respect to binding to α2-adrenergic receptor subtypes were not available for xylazine, and 2) there is a significant body of experimental studies on the sedative, hypnotic and hypothermic actions with medetomidine.38 Strikingly, our studies revealed a clear dissociation between the sedative, hypothermic and hypnotic effects induced by medetomidine. While the sedative and hypothermic effects were indistinguishable between wild type and α3KO mice, α3KO mice were more sensitive to the hypnotic effects of medetomidine. However, it is interesting to note that the unspecific blockade of all GABAA receptor subtypes by the antagonist gabazine is attenuating the hypnotic effects of medetomidine16. This indicates that the hypnotic response to medetomidine may be modulated in different directions by different GABAA receptor subtypes. Interestingly, α3KO mice did not differ from wild type mice in their hypnotic response to ketamine. However, the decrease in body temperature was more pronounced. Even though ketamine is typically not exhibiting synergistic effects in combination with other anesthetic agents,39 our findings suggest that the increased sensitivity to the hypnotic effects of α2-adrenergic agents combined with the increased sensitivity to the hypothermic effects of ketamine might cause the significant phenotype observed in α3KO mice in response to the drug combination ketamine/xylazine in a synergistic manner.
Another objective of this study was to evaluate whether α3-containing GABAA receptors on noradrenergic neurons constrain ketamine/xylazine action. For this purpose, we generated “rescue” mice expressing the α3 subunit exclusively in noradrenergic neurons. In these mice, the α3 subunit is expressed under the control of its endogenous promoter, thus limiting expression to cells in which expression occurs naturally. Compared to the wild type allele the expression level of the rescue allele was approximately 70%. We assume that transcriptional processes may be partially disrupted due to the presence of foreign DNA (i.e. plasmid backbone of the targeting vector) in the allele. Presumably α3 expression levels in noradrenergic neurons will not reach wild type expression levels in the α3-DBH-Rescue mice either, although this has not been formally examined. As a consequence a rescue of the α3KO phenotype may not restore function to wild type levels. Indeed, this is what we observed in this study: the presence of α3-containing GABAA receptors on noradrenergic neurons partially abolishes the increased hypnotic and immobilizing actions of ketamine/xylazine observed in the α3KO mice. The increased hypothermic actions, however, were not affected. This indicates that while noradrenergic neurons are involved in modulating the hypnotic and immobilizing actions of ketamine/xylazine, they may not be involved in mediating their hypothermic action.
Our findings are consistent with a model in which the noradrenergic neurons naturally express both α2-adrenergic receptors and α3-containing GABAA receptors11. Activation of both receptors would be predicted to decrease the activity of the noradrenergic neurons. Similar to previous findings in the reticular nucleus of the thalamus,40 currently unexplained compensations to preserve inhibitory function may be present in α3KO mice . At the same time increased sensitivity to α2-adrenergic agonists might occur in α3KO mice to compensate for the lack of GABAergic inhibition and designed to keep the inhibitory modulation of these neurons constant. This would explain why the hypnotic and hypothermic actions of compounds such as midazolam and etomidate, which act mainly or exclusively via GABAA receptors without any effect on α2-adrenergic receptors, are reduced in the α3KO mice, while the hypnotic action of medetomidine, which has no effect on GABAA receptors, is increased.
MS #201201024 Final Box Summary.
What we already know about this topic
The GABAA receptor system mediates actions of many general anesthetics in the central nervous system. However, the contributions of the different GABAA receptor subtypes and the role of noradrenergic neurons require further clarification.
What this article tells us that is new
GABAA receptors containing the alpha3 subunit mediate anesthetic responses to etomidate and midazolam. Lack of the alpha3 subunit results in enhanced responses to the combination ketamine/xylazine, an effect that is rescued in part by expression of the alpha3 subunit selectively in noradrenergic neurons, indicating an involvement of noradrenergic neurons in modulating hypnosis.
Acknowledgments
We thank Drs. Alo C. Basu (College of the Holy Cross, Worcester, MA) and Elif Engin (McLean Hospital, Belmont, MA) for advice on statistical analysis and Dr. Elif Engin for critically reading the manuscript.
The research described in this publication was supported by institutional funds from the University of Zurich, Zurich, Switzerland and from McLean Hospital, Belmont, MA. UR's work on the manuscript was supported in part by award number R01-GM086448 from the National Institute of General Medical Sciences of the National Institutes of Health (Bethesda, MD). The views supported in this manuscript do not necessarily represent the views of the National Institute of General Medical Sciences or of the National Institutes of Health.
Footnotes
The work was received from the Laboratory of Genetic Neuropharmacology, McLean Hospital and the Department of Psychiatry, Harvard Medical School.
Conflict of Interest: In the last three years, Uwe Rudolph provided professional services to Sunovion Pharmaceuticals (Marlbourough, MA) and to Concert Pharmaceuticals (Lexington, MA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph U, Knoflach F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 10:685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 4.Bonin RP, Orser BA. GABAA receptor subtypes underlying general anesthesia. Pharmacol Biochem Behav. 2008;90:105–12. doi: 10.1016/j.pbb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 6.Kralic JE, Wheeler M, Renzi K, Ferguson C, O'Buckley TK, Grobin AC, Morrow AL, Homanics GE. Deletion of GABAA Receptor α1 Subunit-containing Receptors Alters Responses to Ethanol and Other Anesthetics. J Pharmacol Exp Ther. 2003;305:600–7. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- 7.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. α5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–20. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi ZP, Wang XH, Grayson DR, Firestone LL. Gene knockout of the α6 subunit of the γ-aminobutyric acid type A receptor: Lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997;51:588–96. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- 9.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: Differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–94. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Pallares J, Caruncho HJ, Lopez-Real A, Wojcik S, Guerra MJ, Labandeira-Garcia JL. Rat brain cholinergic, dopaminergic, noradrenergic and serotonergic neurons express GABAA receptors derived from the α3 subunit. Receptors Channels. 2001;7:471–8. [PubMed] [Google Scholar]
- 11.Corteen NL, Cole TM, Sarna A, Sieghart W, Swinny JD. Localization of GABAA receptor alpha subunits on neurochemically distinct cell types in the rat locus coeruleus. Eur J Neurosci. 34:250–62. doi: 10.1111/j.1460-9568.2011.07740.x. [DOI] [PubMed] [Google Scholar]
- 12.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 13.Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar VM, Vetrivelan R, Mallick HN. Noradrenergic afferents and receptors in the medial preoptic area: Neuroanatomical and neurochemical links between the regulation of sleep and body temperature. Neurochem Int. 2007;50:783–90. doi: 10.1016/j.neuint.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The α2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, anα2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, Dong Y, Malenka RC, Fritschy JM, Bluethmann H, Feldon J, Möhler H, Rudolph U. A schizophrenia-related sensorimotor deficit links α3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–9. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parlato R, Otto C, Begus Y, Stotz S, Schütz G. Specific ablation of the transcription factor CREB in sympathetic neurons surprisingly protects against developmentally regulated apoptosis. Development. 2007;134:1663–70. doi: 10.1242/dev.02838. [DOI] [PubMed] [Google Scholar]
- 21.Parlato R, Cruz H, Otto C, Murtra P, Parkitna JR, Martin M, Bura SA, Begus-Nahrmann Y, von Bohlen und Halbach O, Maldonado R, Schutz G, Luscher C. Effects of the cell type-specific ablation of the cAMP-responsive transcription factor in noradrenergic neurons on locus coeruleus firing and withdrawal behavior after chronic exposure to morphine. J Neurochem. 2010;115:563–73. doi: 10.1111/j.1471-4159.2010.06709.x. [DOI] [PubMed] [Google Scholar]
- 22.Parlato R, Rieker C, Turiault M, Tronche F, Schütz G. Survival of DA neurons is independent of CREM upregulation in absence of CREB. Genesis. 2006;44:454–64. doi: 10.1002/dvg.20236. [DOI] [PubMed] [Google Scholar]
- 23.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- 24.Schneider Gasser EM, Duveau V, Prenosil GA, Fritschy JM. Reorganization of GABAergic circuits maintains GABAA receptor-mediated transmission onto CA1 interneurons in α1-subunit-null mice. Eur J Neurosci. 2007;25:3287–304. doi: 10.1111/j.1460-9568.2007.05558.x. [DOI] [PubMed] [Google Scholar]
- 25.Schneider Gasser EM, Straub CJ, Panzanelli P, Weinmann O, Sassoe-Pognetto M, Fritschy JM. Immunofluorescence in brain sections: Simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nat Protoc. 2006;1:1887–97. doi: 10.1038/nprot.2006.265. [DOI] [PubMed] [Google Scholar]
- 26.Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Mohler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–81. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato Y, Seo N, Kobayashi E. Genetic background differences between FVB and C57BL/6 mice affect hypnotic susceptibility to pentobarbital, ketamine and nitrous oxide, but not isoflurane. Acta Anaesthesiol Scand. 2006;50:553–6. doi: 10.1111/j.1399-6576.2006.001002.x. [DOI] [PubMed] [Google Scholar]
- 28.Irifune M, Sato T, Kamata Y, Nishikawa T, Dohi T, Kawahara M. Evidence for GABAA receptor agonistic properties of ketamine: Convulsive and anesthetic behavioral models in mice. Anesth Analg. 2000;91:230–6. doi: 10.1097/00000539-200007000-00043. [DOI] [PubMed] [Google Scholar]
- 29.Arras M, Autenried P, Rettich A, Spaeni D, Rülicke T. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med. 2001;51:443–56. [PubMed] [Google Scholar]
- 30.Cirone J, Rosahl TW, Reynolds DS, Newman RJ, O'Meara GF, Hutson PH, Wafford KA. γ-aminobutyric acid type A receptor β2 subunit mediates the hypothermic effect of etomidate in mice. Anesthesiology. 2004;100:1438–45. doi: 10.1097/00000542-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U. Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J. 2005;19:1677–9. doi: 10.1096/fj.04-3443fje. [DOI] [PubMed] [Google Scholar]
- 32.Zeller A, Arras M, Jurd R, Rudolph U. Identification of a molecular target mediating the general anesthetic actions of pentobarbital. Mol Pharmacol. 2007;71:852–9. doi: 10.1124/mol.106.030049. [DOI] [PubMed] [Google Scholar]
- 33.Quinlan JJ, Homanics GE, Firestone LL. Anesthesia sensitivity in mice that lack the β3 subunit of the γ-aminobutyric acid type A receptor. Anesthesiology. 1998;88:775–80. doi: 10.1097/00000542-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 34.Peters JA, Kirkness EF, Callachan H, Lambert JJ, Turner AJ. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988;94:1257–69. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 36.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao B, Hornung JP, Fritschy JM. Identification of distinct GABAA-receptor subtypes in cholinergic and parvalbumin-positive neurons of the rat and marmoset medial septum-diagonal band complex. Neuroscience. 1995;65:101–17. doi: 10.1016/0306-4522(94)00480-s. [DOI] [PubMed] [Google Scholar]
- 38.Gilsbach C, Röser C, Beetz N, Brede M, Hadamek K, Haubold M, Leemhuis J, Philipp M, Schneider J, Urbanski M, Szabo B, Weinshenker D, Hein L. Genetic dissection of α2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Mol Pharmacol. 2009;75:1160–70. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- 39.Hendrickx JF, Eger EI, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506. doi: 10.1213/ane.0b013e31817b859e. [DOI] [PubMed] [Google Scholar]
- 40.Winsky-Sommerer R, Knapman A, Fedele DE, Schofield CM, Vyazovskiy VV, Rudolph U, Huguenard JR, Fritschy JM, Tobler I. Normal sleep homeostasis and lack of epilepsy phenotype in GABAA receptor α3 subunit-knockout mice. Neuroscience. 2008;154:595–605. doi: 10.1016/j.neuroscience.2008.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]