SUMMARY
The high renal oxygen (O2) demand is associated primarily with tubular O2 consumption (QO2) necessary for solute reabsorption. Increasing O2 delivery relative to demand via increased blood flow results in augmented tubular electrolyte load following elevated glomerular filtration, which, in turn, increases metabolic demand. Consequently, elevated kidney metabolism results in decreased tissue oxygen tension.
The metabolic efficiency for solute transport (QO2/TNa) varies not only between different nephron sites, but also under different conditions of fluid homeostasis and disease. Contributing mechanisms include the presence of different Na+ transporters, different levels of oxidative stress and segmental tubular dysfunction.
Sustained hyperglycaemia results in increased kidney QO2, partly due to mitochondrial dysfunction and reduced electrolyte transport efficiency. This results in intrarenal tissue hypoxia because the increased QO2 is not matched by a similar increase in O2 delivery.
Hypertension leads to renal hypoxia, mediated by increased angiotensin receptor tonus and oxidative stress. Reduced uptake in the proximal tubule increases load to the thick ascending limb. There, the increased load is reabsorbed, but at greater O2 cost. The combination of hypertension, angiotensin II and oxidative stress initiates events leading to renal damage and reduced function.
Tissue hypoxia is now recognized as a unifying pathway to chronic kidney disease. We have gained good knowledge about major changes in O2 metabolism occurring in diabetic and hypertensive kidneys. However, further efforts are needed to elucidate how these alterations can be prevented or reversed before translation into clinical practice.
Keywords: diabetes, hypertension, hypoxia, kidney, oxygen consumption, tissue oxygenation
INTRODUCTION
Renal oxygenation is based on a balance between oxygen (O2) supply and consumption (QO2). Under physiological steady state conditions the O2 supply to the renal tissues is well in excess of the O2 demand. Renal O2 extraction in the healthy kidney is only 10–15%1,2; in most other organs it is closer to 45%. Under pathological conditions the balance of O2 supply compared with demand is disturbed due, in part, to the unique arrangement of the renal microvasculature and its diffusive shunting pathways.3–5 The high O2 demand is associated with the tubular QO2 necessary for solute exchange6 and the high rate of aerobic glycolysis.7 Even though the kidney is only 0.5% of total bodyweight, it uses approximately 7% of the O2 consumed by the body.8
“High O2 demand due to high solute exchange”
The vast majority of QO2 is due to reabsorption of approximately 99.5% of filtered sodium (Na+).9 This further drives the cellular and paracellular transport of solutes and water. The amount of filtered Na+ is the product of the glomerular filtration rate (GFR) and the plasma Na+ concentration. With approximations of GFR of 125 mL/min and plasma Na+ concentrations of 140 mmol/L, the amount of Na+ reabsorbed is in the order of 1 mol/h. Because there is little variation in plasma Na+ concentrations in healthy individuals, renal QO2 is related directly to GFR because the latter determines the sodium load. The estimated energy required to reabsorb 1 mol Na+ against an electric potential of −70 mV in the cytoplasm and the chemical gradient is approximately 7 kJ (using Faraday’s constant). To give further appreciation of the amount of energy required, this corresponds to lifting 1 mol Na+ (≈20 g) to a height of approximately 70 km. To perform such an effort it is obvious that a large amount of ATP is required continuously, meaning a high QO2. Most of the ATP produced by the kidney (some 95%) is through aerobic mechanisms,10 whereas some nephron segments, particularly in the medulla, can use anaerobic metabolism efficiently.7
“QO2 is a determinant for kidney tissue PO2”
The ratio between the GFR (QO2 related to active transport) and renal blood flow (RBF; O2 delivery) is the filtration fraction, which does not vary to any large extent in humans under normal physiological conditions.11, 12 If the filtration fraction does not vary significantly from day to day, then a near-constant tissue O2 tension (PO2) should prevail in the tissue. This infers that the kidney PO2 will vary primarily based on QO2. A deranged QO2 with consequences for tissue PO2 has been suggested as an important parameter leading to kidney dysfunction in several major disease conditions.13–15 For example, increased kidney metabolism is associated with diabetic nephropathy16 and diabetes is associated with decreased kidney PO2 in both animals and patients.17–22 Fine et al. proposed that initial glomerular injury decreases blood flow through peritubular capillaries and results in decreased PO2 of the kidney, promoting tubulointerstitial fibrosis and progression to kidney damage.23 Importantly, chronic tubulointerstitial hypoxia is acknowledged as a common pathway to end-stage renal disease.24–28 The two pathways may seem contradictory, however both scenarios are plausible and do not exclude each other. The metabolic changes in experimental diabetes occur before the structural changes appear and the loss of capillaries can follow. However, in any kidney damage scenario a loss of capillaries will also lead to tissue hypoxia.
In most tissues an increased demand for O2 is followed by increased perfusion and delivery of O2. However, in the kidney increased O2 delivery (i.e. increased RBF) is likely to increase GFR and, thus, sodium load. This will increase the work load for reabsorption and the beneficial effect on oxygen homeostasis is unclear. In the kidney, both perfusion and tubular transport activity are governed mainly by a number of hormones and substances. As metabolic products, carbon dioxide (CO2) and protons act as vasodilators in precapillary resistance vessels, thereby increasing perfusion in tissues like skeletal muscle during work. In the kidney, increased perfusion will not necessarily give rise to a similar increase in PO2 as in most other organs. This is due to the fact that increased perfusion will also increase the filtered load of solutes, which, in turn, will increase the tubular workload (i.e. QO2). For example, giving a vasodilator like atrial natriuretic peptide (ANP) to rats does not increase renal oxygenation but, in contrast, reduces both cortical and medullary PO2.29 This occurs through an elevation in the filtered load of sodium, which, in turn, increases QO2 leading to reduced PO2. Conversely, if QO2 is reduced in rats by, for example, giving the loop diuretic furosemide, which reduces sodium transport in the thick ascending limb of the loop of Henle (mTAL), tissue PO2 increases.29,30 Furthermore, acute hypotension paradoxically increases medullary PO2, which can be abolished using furosemide,31 suggesting reduced filtered load as a plausible mechanism. This emphasizes the importance of QO2 as a determinant for kidney tissue PO2.
Kidney perfusion and QO2 are heterogeneous. From this follows that regions of the kidney may be susceptible to hypoxia. Only some 10–15% of renal perfusion transits the medullary region. At the same time, the mTAL is a major O2-consuming site in this region owing to Na+–K+–2Cl− transporter-mediated solute uptake. Furthermore, the countercurrent exchange system (i.e. the vasa recta) also contributes to reduced delivery through shunting between closely lying descending and ascending vessels and because they also contain a lower haematocrit than systemic blood.32 Together, these factors will result in a lower PO2 in the medulla than in the cortex. This is supported by studies showing a PO2 gradient from the cortex and along the medullary structures.33–35 Furthermore, high expression of hypoxia inducible factor (HIF)-1α in the medullary region36 is a marker of a low tissue PO2 in this region.
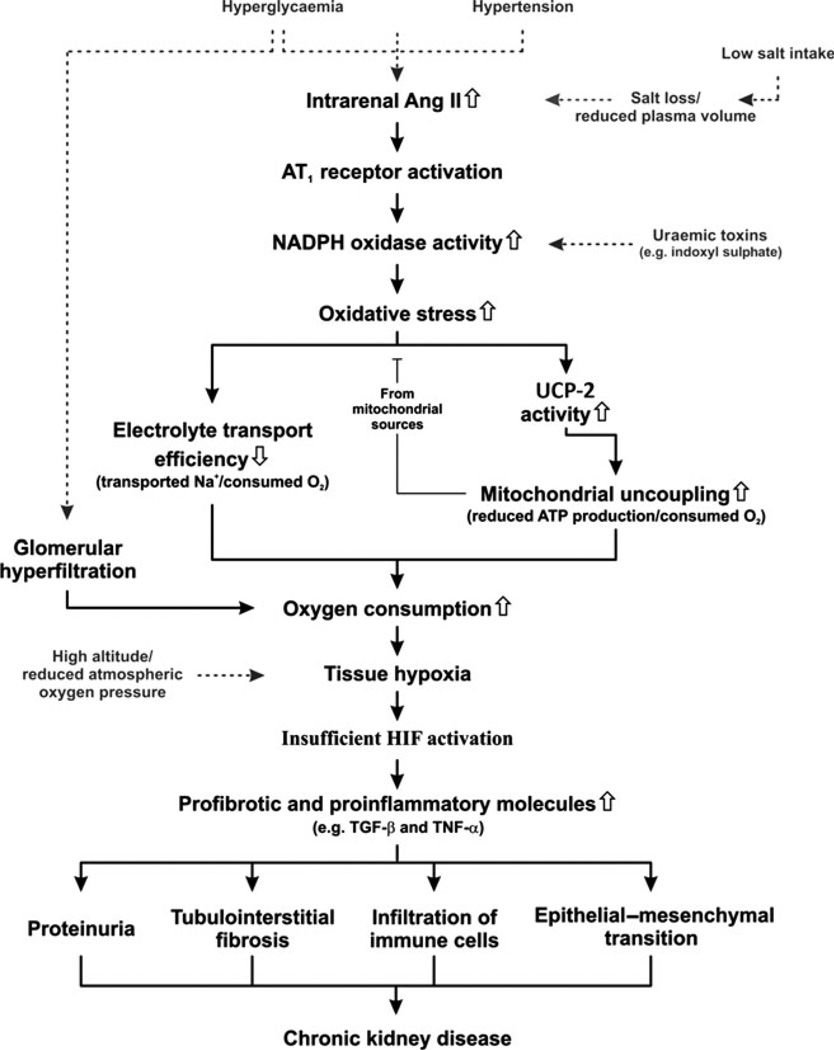
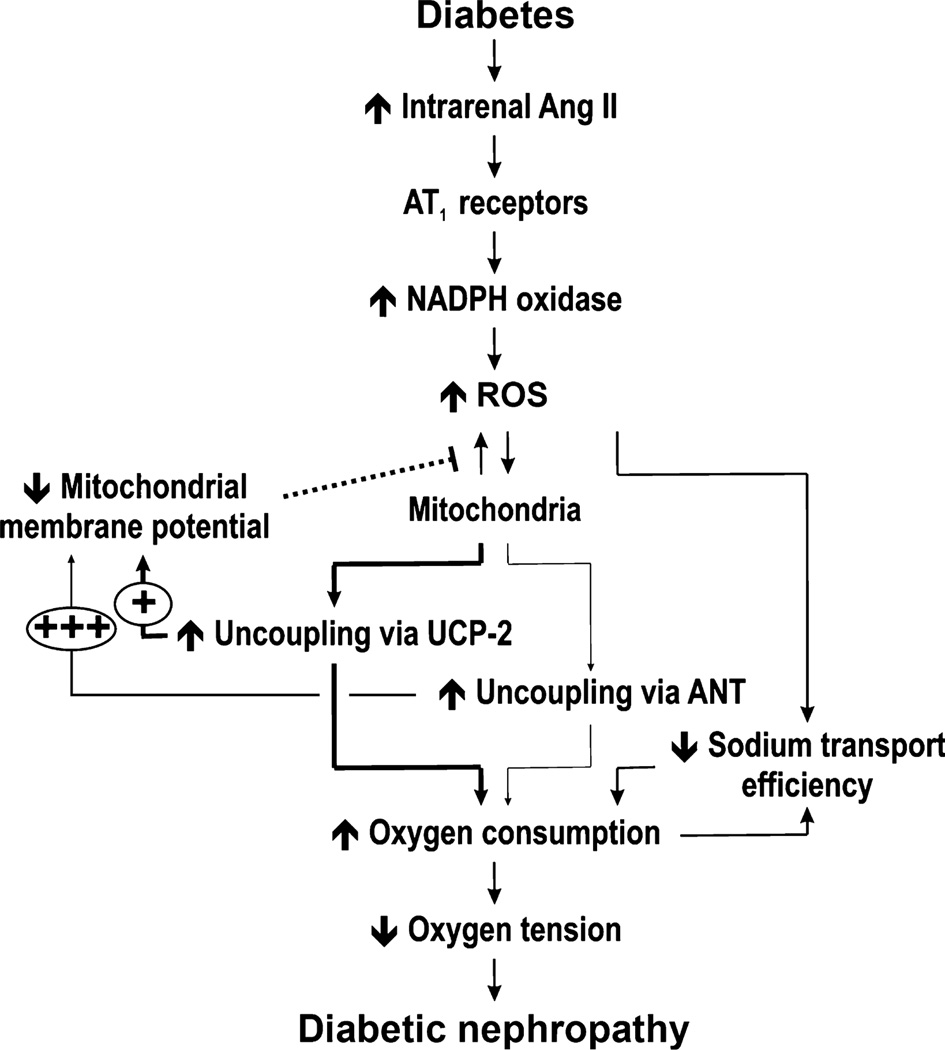
In 2003, the first report demonstrating kidney tissue hypoxia in diabetic rats was published.35 This finding has since been verified by several international laboratories in animals and humans.17–22,37 We propose a hypothesis that will provide a mechanistic explanation for the development of chronic kidney disease (CKD) under conditions associated with increased risk of the progressive loss of kidney function (Fig. 1). Briefly, elevated intrarenal angiotensin (Ang) II activates NADPH oxidase via AT1 receptors to produce superoxide radicals ().38 The resulting oxidative stress reduces electrolyte transport efficiency39 and causes mitochondrial uncoupling via activation of uncoupling protein (UCP)-2.40 Reduced tubular electrolyte transport efficiency is a result of an intricate interplay of several different mechanisms, including altered paracellular electrolyte permeability, direct effects on Na+/K+-ATPase, the shift of Na+ transport to less-efficient nephron segments and ‘slipping’ of the Na+ pumps. The mitochondrial uncoupling is manifested as increased kidney QO2. It can be speculated that the increase in QO2 serves to maintain adequate ATP production.41 Both these mechanisms are tightly regulated by oxidative stress and result in increased kidney QO2. Because the kidney, in contrast with the brain, for example, does not match increased metabolic demand (i.e. increased QO2) with increased O2 delivery, the result is a mismatch between supply and demand that causes intrarenal tissue hypoxia.35 Normally the cellular response to sustained tissue hypoxia includes a counteracting activation of the HIF system resulting in the transcription of genes involved in angiogenesis, cellular energy production and oxidative stress defence.42 However, for currently unknown reasons, HIF is not adequately activated in hypoxic CKD and the expression of HIF-regulated genes, such as erythropoietin, vascular endothelial growth factor or heme oxygenase-1, are not elevated in these kidneys.43 Rather, the hypoxia induces elevated levels of several detrimental profibrotic and proinflammatory molecules such as transforming growth factor (TGF)-β and tumour necrosis factor (TNF)-α. Finally, sustained intrarenal tissue hypoxia results in proteinuria, tubulointerstitial fibrosis and infiltration of immune cells,44,45 all hallmarks of developing CKD.
Fig. 1.
Unifying hypothesis for the development of chronic kidney disease (see text for details). AngII, angiotensin II; UCP, uncoupling protein; HIF, hypoxia-inducible factor; TGF-β, transforming growth factor-β; TNF-α, tumour necrosis factor-α.
The cascade of events described in Fig. 1 can be initiated at several levels. Hyperglycaemia, hypertension and low salt intake are associated with increased intrarenal AngII levels,46–48 the uraemic toxin indoxyl sulphate induces production by NADPH oxidase,14 whereas reduced O2 content in the inhaled air results directly in reduced intrarenal tissue PO2.49
“Mismatch between metabolic demand and O2 delivery”
VARIABILITY IN METABOLIC EFFICIENCY DURING TUBULAR REABSORPTION
The kidney exhibits an extraordinarily efficient autoregulation relating to the myogenic and tubuloglomerular feedback, resulting in relative constancy of blood flow and glomerular ultrafiltration.50–52 Experimental findings suggest that the tubuloglomerular feedback system normally provides exquisite coordination between: (i) kidney blood flow and the load of glomerular filtration; and (ii) tubular reabsorption, predominantly Na+, and an accompanying anion. This highly regulated process may maintain a balance of metabolic or O2-related supply and demand, especially because the kidney PO2 is low and the dominant tubular segment for reabsorption exhibits an obligate aerobic dependency for its energy supply.53,54 In support of such a link between filtered load and tubular O2 requirements for reabsorption, it has been observed that the oscillatory frequency of the tubuloglomerular feedback system is identical to the oscillations in PO2 within the kidney cortex.55 Therefore, it has been logical to describe the metabolic efficiency or QO2 as factored by the major ‘work’ of the kidney, namely Na+ reabsorption (TNa).56,57 Recent evidence shows that the slope of this relationship (QO2/TNa), or the metabolic efficiency of the kidney, is variable among physiological and pathophysiological conditions and that it is affected by a variety of factors, including hormonal influences.39,57 In fact, the normal index of metabolic efficiency of the kidney (QO2/TNa) is not constant and can increase by over 100%, either acutely or chronically, indicating a marked reduction in the metabolic efficiency of the kidney and indicating a large increase in kidney QO2.57,58 This is usually accompanied by a significant reduction in kidney tissue PO2. However, this variability in QO2/TNa under physiological and pathophysiological conditions can be caused by multiple mechanisms. It should be noted that in computation of QO2/TNa the fixed cost (i.e. basal metabolism) should not be included. Otherwise QO2/TNa approaches infinity as TNa is reduced. Let us consider some possibilities prior to examining the evidence for variability in metabolic efficiency:
a shift in the site of TNa along the nephron
altered efficiency of the process of TNa, such as altered permeability of tight junctions in the epithelium
loss of passive TNa or the addition of active Na+ reabsorptive processes with the accompanying ATP and O2 costs
mitochondrial changes, such as the action of uncoupling proteins that increase QO2 without generation of ATP
reabsorption of molecules other than Na+, such as filtered proteins reabsorbed by the proximal tubule
induction of gluconeogenesis and glucose generation from predominantly lactate, glutamine or glycerol; this could occur under conditions of starvation or even insulin resistance, and possibly as a result of the actions of AngII
activation of other oxidases, such as NADPH oxidase, which contributes to increases in QO2 independent of any changes in TNa
nitric oxide (NO) deficiency from a variety of causes
reductions in phosphorylated AMP-activated protein kinase (AMPK) possibly related to insulin status
a change in the balance of aerobic metabolism and glycolysis.
The simplest explanation for a major increase in QO2/TNa is a shift in the site of TNa to more distal sites within the nephron. However, the exact contribution of such a shift to increased O2 requirements has not been firmly quantified. It is clear the QO2/TNa increases as it moves along the nephron, with the proximal tubule being the most metabolically efficient segment. The exact computation of the metabolic costs for each nephron segment has varied over the years. Early studies estimated that the costs were 0.36 calories/mEq Na+ in the proximal tubule, 1.4 calories/mEq Na+ in the thick ascending limb (TAL), 2.7 calories/mEq Na+ in the distal connecting tubule and 4.6 calories/mEq Na+ in the collecting duct.59 Later estimates of segmental ratios have been somewhat less varied, with ratios of 1 : 2 : 6 for the proximal : TAL : distal tubule.60 There are at least two examples whereby an increase in QO2 and a reduction in metabolic efficiency could be explained. in part, by such a shift in the site of Na+ reabsorption. Studies by Brezis et al.61 and De Nicola et al.62 have examined the effects of non-selective NO synthase (NOS) blockade. These studies showed a reduction in proximal reabsorption with a shift into the loop of Henle and distal tubule. Since then, studies have clearly shown that NOS blockade markedly increases QO2 and increases QO2/TNa. Brezis et al.61 demonstrated an increase in medullary QO2 with NOS blockade. Below, we discuss how NOS blockade exerts other effects that can increase QO2 and decrease metabolic efficiency. The contribution of this shift in reabsorption is potentially significant, but has not been quantified precisely. As discussed later, subtotal nephrectomy, a model of CKD, also demonstrates a shift in Na+ reabsorption from the more proximal to distal tubular segment and exhibits increased QO2, tissue hypoxia and a marked decrease in metabolic efficiency.57,58 Potential contributors to increased QO2 or treatments that have been shown to normalize QO2, such as AngII blockade, do not shift the site of TNa to more proximal nephron segments, making this mechanism less likely to be the sole cause of altered metabolic efficiency.57
“Variability in metabolic efficiency along nephron”
Specific factors influencing reabsorptive efficiency
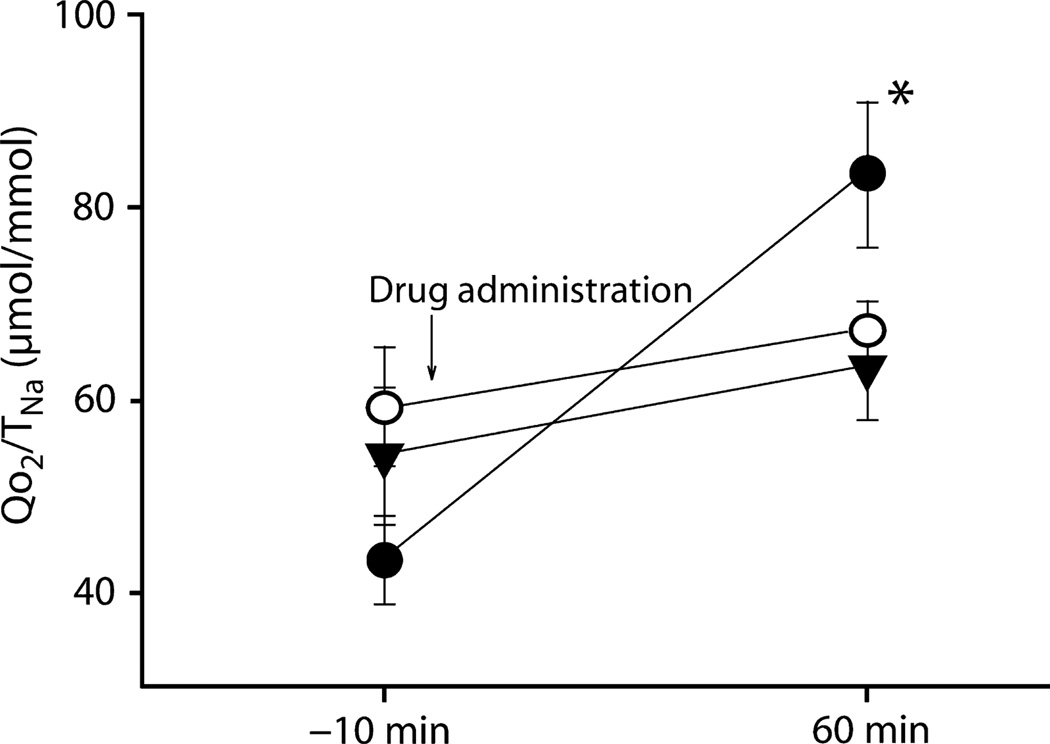
Changes in the structure of the tubule may also decrease the efficiency of TNa. After periods of kidney ischaemia, an increase in QO2 has been documented.63,64 This could be due to at least two reasons relating to changes in the tight junctions, particularly in the proximal tubule. Not only may back-leak of Na+ be increased, but Molitoris et al. have shown that the apical and basolateral locations of the transporters are significantly altered, thereby markedly decreasing the efficiency of vectorial NaCl reabsorption.65 Any other process that alters the Na+ or anionic permeability of the tubule could potentially reduce the efficiency of reabsorption and increase QO2. Evidence for specific mechanisms contributing to changes in the metabolic efficiency of the kidney (that are usually reversible) has been accumulating. For example, circumstances under which there is loss of passive reabsorption of Na+ or additional active transport can also increase QO2/TNa. Benzolamide is a carbonic anhydrase inhibitor that decreases proximal tubular reabsorption by approximately 50% and activates tubuloglomerular feedback in the rat.66 This effect should shift reabsorption into the distal nephron, but major reductions in TNa may decrease QO2. In fact, QO2 increased by >50% despite the major reductions in GFR and TNa, and QO2/TNa increased by >80% (Fig. 2).67 Benzolamide causes a major reduction in proximal tubular luminal pH.67 When we applied agents that inhibited proton secretion in the proximal tubule, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) and adenosine A1 receptor antagonists, these agents normalized QO2 and QO2/TNa. In vitro studies in isolated proximal tubules gave identical results, whereby benzolamide increased QO2 and this effect was prevented by inhibition of Na+/H+ exchanger isoform 3 and proton secretion.67 Weinstein et al.68 had observed similar findings earlier, but attributed the greater QO2 to elimination of the chloride gradients that promoted passive reabsorption. However, this did not explain fully the documented increase in QO2 that we observed. We documented an absolute increase in chloride reabsorption by induction of active transport that was due to the marked reduction in luminal pH, which led to activation of chloride/formate exchange and promoted the recycling of formate across the proximal tubular apical membrane.69
Fig. 2.
Inhibition of proximal tubular reabsorption by benzolamide (BNZ) increased the cost of Na+ reabsorption (TNa), as determined by the ratio of tubular O2 consumption (QO2)/TNa (●). Concurrent application of BNZ with either the adenosine A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (○) or the sodium/hydrogen exchange blocker 5-(N-ethyl-N-isopropyl)-amiloride (▼) prevented the BNZ-induced increase in QO2/TNa, suggesting that the BNZ-induced increase in oxygen consumption requires proton secretion and luminal acidification. Data are the mean ± SEM. *P < 0.01 compared with control (10 min before drug administration). Reproduced with permission from Deng et al.67
There are several other potential mechanisms for which there is even less evidence for a contribution to changes in metabolic efficiency. Uncoupling proteins are expressed in mitochondria and function as proton channels to allow proton leakage back across the inner mitochondrial membrane without creating ATP.70 The uncoupling could be a defence system against oxidative stress because superoxide activates UCP-2.71 Uncoupling proteins could certainly increase QO2, possibly as a regulatory event that is completely separate from the function of TNa. Leakage of protons through UCP results in elevated QO2. Although the sensing mechanisms for this remain unclear, it can be speculated that the initially reduced production of ATP elevates QO2 to sustain similar ATP production. There is more evidence that other oxidases contribute to QO2 sufficiently to contribute to reduced metabolic efficiency. For example, NADPH oxidase is greatly increased during subtotal nephrectomy, possibly due to increased AngII activity, and is normalized following combined therapy with angiotensin-converting enzyme (ACE) inhibitors and AngII receptor blockers (ARB).72 The exact molar contribution of NADPH oxidase to the overall increase in QO2 has not been quantified exactly, but it is likely that these oxidases make up a significant portion of the increase in QO2. Glomerular protein leakage and reabsorption of proteins and peptides by the proximal tubule may also require significant energy and O2. However, in subtotal nephrectomy studies, we found that inhibition of tubular protein reabsorption did not significantly reduce QO2.72
Metabolic changes independent of tubular reabsorption
Another potential contribution to increased QO2 and the apparent decrease in metabolic efficiency is the induction of gluconeogenesis.59,60 This is not an easy contribution to quantify in the in vivo rat kidney except through indirect approaches using blockers of gluconeogenesis. Lactate is reabsorbed and secreted by the tubule, so quantification of lactate used to synthesise glucose requires complex in vivo analysis. Under certain conditions the kidney can rival the liver in its contribution of glucose to the circulation.59,60 Major gluconeogenesis is usually not the case, but under conditions of starvation and with certain acid–base conditions, glucose is synthesised, usually from either lactate or glutamine, but at a significant cost of ATP and O2. There are few data regarding the contribution of gluconeogenesis to increased QO2 and QO2/TNa under normal physiological conditions. We have examined the effects of acute insulin administration in the subtotal nephrectomy model and observed a tendency for QO2 to decrease towards normal values. This effect could be related to reductions in gluconeogenesis, but that has not yet been proven (RC Blantz, unpubl. obs., 2012). Although AngII blockade has no observable effect on kidney QO2 in the normal rat,57 we have found that combined AngII blockade (ARB + ACE inhibitors or ARB + HIF-1 induction) does normalize QO2/TNa in the subtotal nephrectomy model of CKD.58,72 We have shown that AngII can produce a form of insulin resistance in the kidney73 and that it is possible that under pathophysiological conditions glucose production via gluconeogenesis may be elevated as a by-product of AngII-induced insulin resistance.
“NO a critical modulator of QO2”
Nitric oxide and other factors influencing oxygen consumption
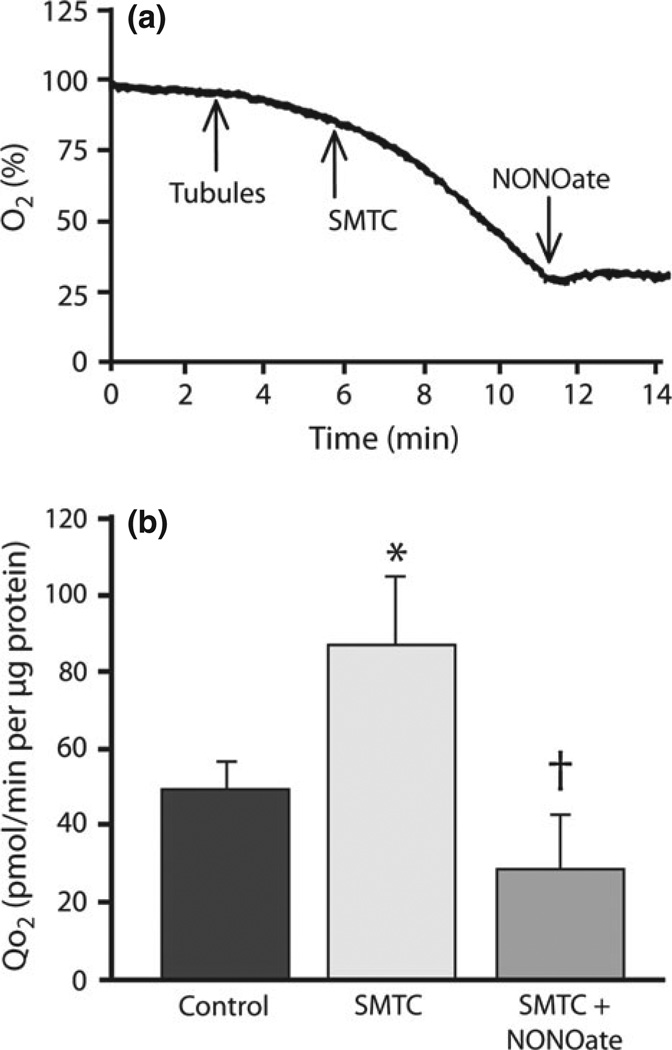
Nitric oxide is a critical modulator of tubuloglomerular feedback activity and for the temporal adaptation of tubuloglomerular feedback.74 Given the multiple vascular, tubular and metabolic effects of NO, this substance has been a logical candidate for modulation of the metabolic efficiency of kidney function. Laycock et al.75 have demonstrated in the dog that application of non-selective NOS inhibitors produces major increases in kidney QO2. Koivisto et al.76 also demonstrated an effect of NO on QO2 in isolated kidney proximal tubules in vitro. We have recently demonstrated an important effect of NO derived from NOS-1 in both the in vivo kidney and freshly harvested isolated proximal tubules (Fig. 3).57 It is of interest that NOS-1 also mediates much of the modulation of tubuloglomerular feedback function.74 The effects of NOS-1 inhibition were not dependent on changes in kidney blood flow and were not influenced by an intermediary action of AngII.57 In vitro studies in freshly harvested proximal tubules have shown that application of S-methyl-l-thiocitrulline (SMTC), an inhibitor of NOS-1, produced an immediate increase in QO2 (Fig. 3). The addition of NO donors immediately reverses this process and normalizes QO2.57 Nitric oxide has several biochemical interactions that influence oxidative metabolism. For example, NO suppresses the citric acid cycle enzyme aconitase, as does superoxide, and inhibits mitochondrial pyruvate uptake. However, the major ‘braking’ effect of NO on oxidative metabolism is accomplished via the inhibition of cytochrome c oxidase.77–81 Studies have shown that NO can inhibit mitochondrial respiration in vitro by up to 85% and that it prevents progressive loss of mitochondrial membrane potential and apoptosis.77–81 It has been suggested that NO inhibits not only these systems, but also critical mitochondrial enzymes in complex I and complex II and cytochrome b.81 Moncada and Erusalimsky showed that, during sepsis, increased generation of NO acts to reduce QO2 in a variety of tissues.81 In subtotal nephrectomy, the increase in QO2 observed by several laboratories is accompanied by substantial evidence for NO deficiency in this model of CKD.24,72–81 There are multiple reasons for the reduction in NO activity, including increased reactive oxygen species (ROS) and inactivation of NO, reduced NOS activity, increased NOS inhibitors, such as asymmetric dimethylarginine. We have observed decreased functional NOS activity in the subtotal nephrectomy model.72 Combined AngII blockade does normalize metabolic efficiency and kidney QO2 and, concurrently, NOS and NO functional activity are normalized, implying that reduced NO activity contributes to the increase in oxygen consumption and reduced metabolic efficiency.
“Metabolic efficiency changes in pathophysiology”
Fig. 3.
Effects of nitric oxide (NO) and the NO synthase 1 blocker S-methyl-l-thiocitrulline (SMTC) on oxygen consumption (QO2) in freshly harvested isolated proximal tubules. (a) Profile of declining O2% in a metabolic chamber containing proximal tubules. When the NOS-1 inhibitor SMTC is added, QO2 increases (as evidenced by the increase in the slope of decline). When the NO donor NONOate is applied, QO2 decreases significantly. (b) Absolute QO2 is depicted in control tubules and in tubules after the addition of SMTC alone or with NONOate. The increase in QO2 after NOS-1 blockade is totally reversed by application of the NO donor, suggesting NO specificity to the phenomenon. These data suggest a major role for intracellular NOS activity in the regulation of QO2. Data are the mean ± SEM. *P < 0.05 compared with control; †P < 0.05 compared with SMTC. Figure partly based on data in Deng et al.57
An understudied area relates to factors that determine the shift between aerobic or oxidative metabolism and glycolytic metabolism in the kidney.59,60 Obviously shifts in the site of TNa will influence this shift in metabolism, but factors such as HIF-1 and phosphorylation of AMPK may also exert an impact.58 We have noted that, in subtotal nephrectomy, phosphorylation of AMPK is reduced and therapies that restore normal haemodynamics and QO2 tend to increase AMPK phosphorylation (RC Blantz, unpubl. obs., 2012). At this stage, there are many metabolic influences, including insulin resistance, that have the potential to change the metabolic efficiency of the kidney or, more specifically, QO2/TNa. Some of the designated causal influences may not be additive or independent variables, but may be acting in series. However, further investigations are required to determine the exact relationships among the multiple mechanisms influencing the metabolic efficiency of TNa.
In summary, recent studies have made it clear that the metabolic efficiency of the kidney can change significantly and that under pathophysiological conditions, such as CKD, this may make an important contribution to the progressive decline in kidney function and fibrosis that characterizes this important disease.
OXYGEN CONSUMPTION IN DIABETES AND ITS RELATIONSHIP TO TISSUE OXYGEN AVAILABILITY
Today, diabetes mellitus is the most common cause of end-stage renal disease. The prevalence of diabetes mellitus in the industrialized world continues to increase and is presently approximately 7%. Type 1 diabetes accounts for 5–10% of all diagnosed cases of diabetes (see http://diabetes.niddk.nih.gov, accessed 10 Dec 2012). Despite intense research, the mechanisms underlying the development of diabetic nephropathy remain largely unknown. An early theory ascribed diabetic nephropathy to sustained barotraumas of the glomerular capillaries due to a combination of preglomerular vasodilatation, which increased glomerular capillary pressure, and to glomerular hypertrophy, which increased capillary wall tension.82 A competing theory states that critical changes in the cytokine and growth factor environment in the glomerular capillary result in kidney damage.83 Neither theory alone is presently fully compatible with the available evidence. Recently, focus has increased on the involvement of diabetes-induced formation of ROS, increased QO2 and altered renal energy metabolism.84 Diabetes is primarily a disease of metabolism and diabetes-induced changes in metabolism are important in the development of other secondary complications of diabetes, such as retinopathy and neuropathy.85 In accordance with the hypothesis that diabetes-induced changes in renal O2 metabolism mediate the development of altered kidney function, we and others have found severe alterations in O2 metabolism that have been related to increased oxidative stress.35,86 It is well known that mitochondrial function is deranged in diabetes due, at least in part, to increased oxidative stress, but it has also been proposed that the altered mitochondrial function per se increases formation and augments the progression of diabetic nephropathy via increasing QO2.27,87
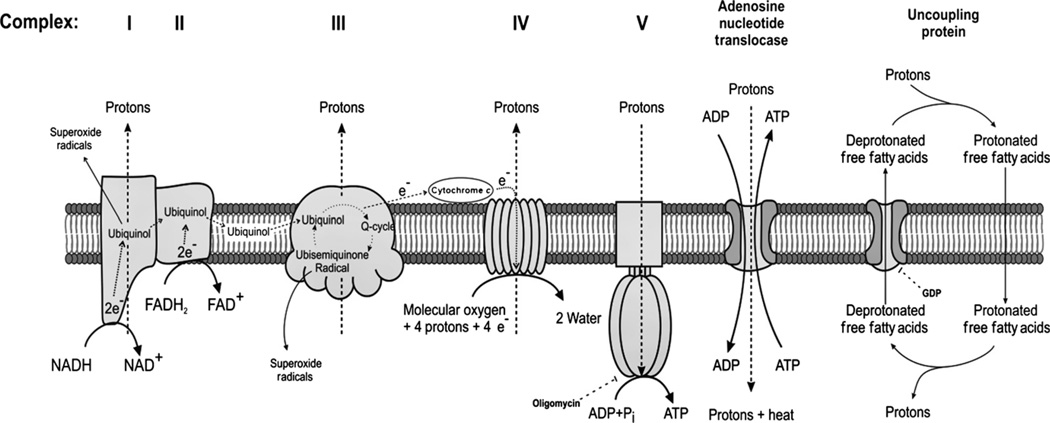
Fat, carbohydrates and proteins are metabolised through β-oxidation, glycolysis and deamination to enter the Krebs’ cycle located in the mitochondrial matrix. This creates NADH and FADH2, which are used by the mitochondria to produce energy (ATP) via oxidative phosphorylation. Oxidative phosphorylation takes place in the mitochondrial electron transport chain (ETC), which consists of four complexes located in the mitochondrial inner membrane. During oxidation of NADH and FADH2, electrons are transported via these complexes and donated to O2, the ultimate electron acceptor. Protons (H+) are pumped across the inner membrane to the intermembrane space during this process, which creates an electrochemical gradient, commonly referred to as the membrane potential. Thereafter, protons are released back across the membrane via channels referred to as ATP synthase because this process produces ATP from ADP and inorganic phosphate (Pi). The mitochondrial membrane potential is also used to transport ions and metabolites in and out of the mitochondria.
The ETC. complexes are regulated by their redox status (i.e. by the electrochemical membrane). During the creation and maintenance of a high and stable mitochondrial electrochemical membrane potential, is produced. Different complexes react differently to changes in membrane potential, which results in a complex regulation of radical production. The vast majority of radicals are formed by complex I and complex III (Fig. 4).88 However, is rapidly converted to hydrogen peroxide by superoxide dismutase under normal physiological conditions. Catalase further metabolises H2O2 to water and O2.85 During excessive substrate load to the ETC. (e.g. intracellular hyperglycaemia), the mitochondrial membrane potential is elevated, which induces excessive formation of .89 This will result in increased damage by the radicals formed, including the formation of protein carbonyls, lipid peroxidation and direct DNA damage. The role of mitochondrial formation in the development of diabetes-related pathologies has been clearly demonstrated by Brownlee.90 Inhibition of mitochondrial formation totally inhibits all the classical pathways known to result in diabetes-related diseases.89
“Diabetes-induced formation of ROS increases QO2”
Fig. 4.
Electron transport chain and the proposed mechanisms of mitochondrial uncoupling via adenosine nucleotide translocase and uncoupling proteins. e−, electrons. Reproduced with permission from O’Rourke et al.88
A pivotal prerequisite for the proposed hypothesis (Fig. 1) is that intrarenal AngII is elevated in diabetes. An early study by Machimura demonstrated beneficial effects on serum creatinine levels after ACE inhibition in patients with diabetic nephropathy.92 Furthermore, Onozato et al.46 showed that streptozotocin-diabetic rats have elevated intrarenal AngII levels. Importantly, elevated AngII levels increase mitochondrial H2O2 production and reduce the respiratory control ratio.92 Notably, NADPH oxidase activity is increased by AngII, but all the AngII-induced alterations are prevented by treatment with the mitochondria-targeting anti-oxidant mitoTempo,92 including increased NADPH oxidase activity. Indeed, chronic treatment with apocynin to inhibit NADPH oxidase activity during the entire 4-week duration of diabetes in rats resulted in improved kidney PO2,93 establishing a link between increased NADPH oxidase activity and deranged O2 metabolism in the diabetic kidney. This further supports the hypothesis of an intimate interplay between NADPH oxidase and the mitochondria.
It has been shown recently that acute inhibition of NADPH oxidase significantly improves intrarenal PO2 via inhibition of active tubular Na+ transport.94 Because the diabetes-induced glomerular hyperfiltration was unaffected by both chronic and acute apocynin treatment,93,94 it can be concluded that changes in GFR merely play a minor role in increased kidney QO2 and subsequent tissue hypoxia in the diabetic kidney. This is in good agreement with our previous report showing that anti-oxidant treatment in diabetic rats normalizes kidney PO2 independent of any effects on GFR.35
Role of UCP-2
To decrease mitochondrial membrane potential under conditions of excess substrate availability, it has been hypothesized (and shown from in vitro studies) that cells can increase the H+ leak across the mitochondrial membrane by synthesising and incorporating ion channels.95 Activation of UCP results in H+ leak across the inner membrane that reduces the mitochondrial membrane production and thus limits oxidative stress, but also uncouples QO2 from the production of ATP.96 This results in increased QO2 for fixed ATP production. This alters the redox status of the ETC. complexes and several studies have shown that this results in decreased production,40 which implies an important regulatory function of UCPs. Uncoupling protein-1 is present in brown adipose tissue, where it has a solely thermogenic effect, whereas UCP-2 is present in tissues with significant levels of ATP production, including the kidneys.97 Furthermore, increases have been demonstrated in UCP-2 and UCP-3 when oxidative stress and lipid peroxidation increase.98 It has been hypothesized that altered regulation of UCPs could be a significant part of the pathology of arteriosclerosis, hypertension and diabetes.99,100 The reported increased expression of UCP-2 during hyperglycaemia101 could be viewed as a cellular defence against excessive formation, but with a significant increase in QO2 as a noticeable side-effect (Fig. 4). Indeed, we have shown recently that UCP-2 expression and activity are increased in diabetic kidneys.41,97,102
“Activation of UCP-2 results in increased QO2”
It should be noted that the mitochondrial membrane potential is normal as long as UCP-2 function is maintained, but increased when GDP is present.96 However, administration of short interference (si) RNA against UCP-2, which reduced UCP-2 protein expression by 62%, paradoxically increased uncoupling, evident from increased glutamate-stimulated QO2 and significantly lower membrane potential.96 Importantly, GDP did not affect the uncoupling in mitochondria from rats administered siRNA against UCP-2, indicating no involvement of UCPs.96 The addition of ADP in the absence of ATP and during blockade of ATP synthase by oligomycin reduced QO2,96 indicating a pivotal role of the adenosine nucleotide transporter (ANT). It is known that, under certain conditions, ANT has uncoupling properties and it was therefore proposed that this is a backup mechanism controlling mitochondrial membrane potential during UCP-2 dysfunction.96 The ANT-mediated uncoupling was far more potent in reducing both mitochondrial membrane potential and kidney tissue oxidative stress, but resulted in a further accelerated QO2. Thus, it seems that the system favours the regulation of mitochondrial membrane potential and radical formation at the expense of elevated QO2 and the risk of intrarenal tissue hypoxia (Fig. 5).
Fig. 5.
Simplified hypothesis for how diabetes, via mitochondrial uncoupling mediated by either uncoupling protein (UCP)-2 and adenosine nucleotide translocase (ANT) increases kidney oxygen consumption (QO2), which results in kidney tissue hypoxia and the development of diabetic nephropathy. So far, it has only been demonstrated that mitochondrial uncoupling via ANT occurs when normal UCP-2 function is reduced.102 AngII, angiotensin II; ROS, reactive oxygen species. Modified from Welch et al.114 and Welch et al.117
Link between increased QO2 and diabetic nephropathy involves intrarenal tissue hypoxia
Previously, we showed that diabetic rats have decreased PO2 throughout their kidney tissue due to increased QO2.35,86 Lower oxygen levels in diabetic kidneys have also been found in patients.22,37 Functional impairment of the kidney is well correlated with the degree of tubulointerstitial damage and this pathological finding has led to the broad recognition that the final common pathway of progression of kidney failure operates principally in the tubulointerstitium.103,104 The chronic hypoxia hypothesis emphasizes chronic hypoxic damage in the tubulointerstitium as a final common pathway in end-stage renal disease and has been intensively investigated by many.105,106 It should be noted that the decreased intrarenal PO2 was independent of GFR (i.e. it occurred under conditions of both hyperfiltration35 and normal GFR86, that is independent of altered TNa). Isolated proximal tubular cells from diabetic rats exhibit increased QO2 compared with controls, which is totally prevented by intense insulin treatment.41 This highlights sustained hyperglycaemia as a pivotal factor for the development of the increased QO2 in diabetic animals. Furthermore, anti-oxidant treatment throughout the course of diabetes totally prevented the increase in QO2, suggesting a close relationship between increased radical formation and elevated QO2 in the kidneys of diabetic rats.35 The increased QO2 resulted in decreased intrarenal tissue PO2, which has been proposed to be involved in the development of diabetes-induced hypoxic kidney injury.27,87 Until recently, the mechanisms mediating the altered O2 metabolism in the diabetic kidney have been largely unknown, but we have now described in detail at least one mechanism responsible for the diabetes-induced increase in QO2.41 Increased UCP-2 levels in the diabetic mitochondria result in uncoupling, which was demonstrated as glutamate-stimulated QO2 during complete blockade of ATP production by oligomycin QO2.41 The specific involvement of UCP-2 was inferred by the abolished mitochondrial uncoupling either in the presence of the specific UCP inhibitor GDP or removal of the free fatty acids that are required for normal UCP function. Because only UCP-2 has been identified in rat kidneys,97 the GDP-induced abolishment of uncoupling strongly suggests involvement of UCP-2.
Echtay et al., using kidney mitochondria from both rat and mouse, have shown that UCP-2 is activated by .107 Importantly, the absolute requirement for UCP-2 for mediating the H+ leak and reducing mitochondrial membrane potential was demonstrated using kidney mitochondria from UCP-2−/− mice. In contrast with the study of Echtay et al., in which an exogenous source was used, Krauss et al. demonstrated that endogenous activates UCP-2 in a similar way.108 Indeed, in a recent study, we confirmed that db/db mice, a model of Type 2 diabetes, also exhibit pronounced mitochondrial uncoupling in the kidney, which is prevented by anti-oxidant treatment with coenzyme Q10. Importantly, coenzyme Q10 not only prevented diabetes-induced oxidative stress, but also normalized mitochondrial morphology and function and normalized glomerular filtration and urinary protein leakage.109 These findings expand the hypothesis beyond insulinopenic diabetes to also include Type 2 diabetes, the prevalence of which is increasing rapidly and which is far more frequent than Type 1 diabetes.
“Link between increased QO2 and diabetic nephropathy”
In summary, sustained hyperglycaemia increases intrarenal AngII levels, resulting in increased oxidative stress due to activation of NADPH oxidase. This results in increased mitochondrial uncoupling via UCP-2 and decreased electrolyte transport efficiency. The commonly occurring diabetes-induced glomerular hyperfiltration, with subsequent increased TNa, together with reduced tubular electrolyte transport efficiency and increased mitochondrial uncoupling result in increased QO2. These events result in intrarenal tissue hypoxia and the development of clinical hallmarks of diabetic nephropathy, because no compensatory increase in oxygen delivery (i.e. RBF) occurs. However, these findings from experimental animal models need to be verified in the clinical setting before they can be translated into new therapeutic approaches for diabetic patients.
OXYGEN CONSUMPTION AND TISSUE OXYGENATION IN HYPERTENSION
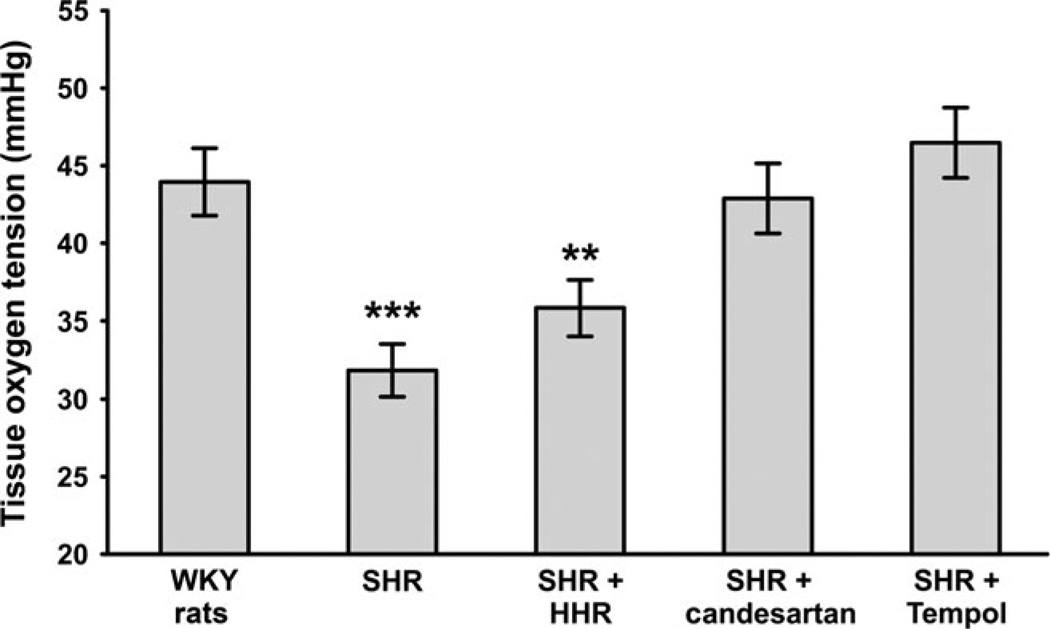
Welch et al.39 were the first to show that PO2 was lower in both the cortex and medulla of kidneys from spontaneously hypertensive rats (SHR) compared with their normotensive genetic control, Wistar-Kyoto rats. In the outer cortex of kidneys from SHR, PO2 was 8–10 mmHg lower than in normotensive rats. The differences were fractionally higher in the inner cortex and outer medulla, with PO2 as low as 12 mmHg in kidneys from SHR. The GFR in the SHR was similar to that in the appropriate control rats, suggesting disruption of the normal relationship between O2 and GFR. The ARB candesartan reduced mean arterial blood pressure (MAP) and normalized PO2 in SHR (Fig. 6). Although the antioxidant tempol also normalized PO2 in SHR, it lowered, but did not normalize, MAP. Because AngII receptor activation is linked to the generation of superoxide,110 the normalizing effects of the ARB could be partially linked to oxidative stress. Supporting the role of AngII and oxidant pathways, the combination of hydralazine, hydrochlorothiazide and reserpine (HHR), which reduces MAP, but does not block AngII or reduce oxidative stress,111 had no effect on renal PO2.39 However, Dworkin showed that HHR was just as effective in preventing renal damage as other antihypertensive agents, although that study included uninephrectomized SHR, which may have complicated matters.112 Subsequently, Welch et al.113 showed that renal PO2 was lower in animals made hypertensive by AngII infusion. Renal cortical and medullary PO2 was lower in AngII-infused rats, which raised blood pressure by 20–25 mmHg, compared with vehicle-infused rats. This reduction was normalized by the anti-oxidant tempol.113
“PO2 lower in kidneys from SHR”
Fig. 6.
Renal oxygen tension was lower in spontaneously hypertensive rats (SHR) compared with Wistar-Kyoto (WKY) rats and was normalized by the angiotensin receptor blocker candesartan and the anti-oxidant tempol, but not by the antihypertensive combination of hydralazine, hydrochlorothiazide and reserpine (HHR).113,117 Data are the mean ± SEM. **P < 0.01, ***P < 0.001 compared with WKY rats.
Recent studies using pimonidazole have confirmed these Clarke electrode studies. For example, AngII increased the number of pimonidazole-positive cells in kidneys of AngII-infused rats compared with control in two studies.114,115 Renal hypoxia in humans has been difficult to assess, but in a recent study using blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI),116 renal medullary PO2 was lower in hypertensive African American subjects compared with normotensive controls. Typically these patients have elevated markers of oxidative stress.116 The hypoxic medulla was normalized by acute treatment with a loop diuretic, suggesting an overly active transport system in the mTAL in these patients and confirming PO2 in this section reflects active Na+ transport.
The relationship between QO2 and TNa has been used to define O2 metabolism in multiple models. We reported that tubular transport efficiency was altered in hypertensive rats, with higher levels of QO2 per Na+ transported, suggesting inefficient renal O2 usage in SHR.117 For a given amount of Na+ transported, nearly twice as much O2 was consumed. This suggests that the major energy-requiring function of the kidney is not limited by O2 delivery. This is consistent with previous suggestions that the kidney is unique in that function is not tied to O2 delivery.56 Because these rats were in steady state and excreted the same levels of Na+ as the control rats, we speculated that the kidney maintains normal Na+ uptake requirements, primarily by maintaining local O2. Therefore, the excess use of O2 in hypertensive rats suggests that tissue O2 will be lower, reflecting this presumed inefficiency. However, as these data suggest, this may be part of the kidney’s ability to maintain sufficient Na+ reabsorption and should not be considered a lack of efficiency, per se.
What are the consequences of the reduced efficiency found in hypertensive kidneys? The immediate and obvious effect is the lower PO2 levels observed in SHR and other models of hypertension,113,114,118,119 as well as in some hypertensive patients.3,116 Renal tissue hypoxia is linked to tubulointerstitial fibrosis.120 The hypoxia, along with barotrauma of high blood pressure on preglomerular vasculature, may be considered as the precursor to the renal damage that accompanies hypertension. In addition, systemic hypertension is accompanied by the release of multiple vasoconstrictors that also impact on the effects of hypoxia on renal damage. These include higher levels of the renin–angiotensin–aldosterone system121,122 and higher levels of constrictor prostaglandins123 and endothelin.124 However, as these studies suggest, oxidants released during hypertension have major effects on blood pressure.
OXIDATIVE STRESS AND HYPERTENSION
Oxidative stress occurs when pro-oxidants systems, such as enzymes and mitochondria, generate levels of ROS that are not quenched by endogenous anti-oxidant systems. Multiple markers of ROS are elevated in SHR,15,125,126 AngII-induced hypertension,114 renovascular hypertension127,128 and hypertension induced by blockade of NO.129 Renal levels of ROS are obviously elevated during hypertension.130 Blockade of AngII receptors corrected the hypoxia and the inefficient use of O2 in SHR, whereas lowering of blood pressure alone has only minor effects on these parameters.131 Furthermore, the common pathway seemed to be elevated ROS, because exogenous anti-oxidants were as effective as ARBs. Reduction of p22phox, the critical component of NADPH oxidases, also corrected hypoxia and the associated renal dysfunction. Laycock et al.75 have reported that NO inhibition, which increases superoxide, reduced tubular electrolyte transport efficiency even in normotensive animals. This suggests that regulation of tubular transport efficiency is closely linked to superoxide.
Activation of AngII receptors stimulates oxidant pathways by activating NADPH oxidase and mitochondria, and many of the prohypertensive effects of AngII may be linked to oxidative stress.129 Elevated ROS are associated with end-organ damage not only in hypertensive animals and humans, but also in experimental models of and patients with diabetes, as well as in ageing humans.19 Therefore, we propose that increased oxidants during hypertension, combined with tissue hypoxia, are critical elements in the early stages of tubulointerstitial fibrosis and renal damage.
The absence of endogenous anti-oxidant enzymes, such as superoxide dismutase (SOD), can also affect oxidative stress and renal O2 balance. Multiple markers of oxidative stress, including superoxide, are elevated in extracellular superoxide dismutase (EC-SOD) knockout mice.132 These mice also have lower renal PO2 and lower tubular electrolyte transport efficiency compared with wild-type controls.132 Both renal hypoxia and O2 efficiency are normalized by tempol treatment.132
Hypoxia generated by shifts in Na+ uptake and the inefficient use of O2 is part of the overall dysfunction that occurs in hypertensive kidneys. The resulting hypoxia can lead to tissue damage in both vascular and tubular cells. The increased levels of superoxide and related oxidants, such as H2O2, peroxynitrite and hydroxyl radicals, exacerbate the effects of hypoxia. Therefore, prolonged exposure to hypoxia, oxidative stress and increased levels of vasoconstrictor hormones lead to renal injury, which is often seen in older SHR.133 Expression of the prooxidant enzyme NADPH oxidase is higher in kidneys of older compared with young SHR.134 Conversely, expression of the anti-oxidant enzymes SOD1 and SOD3 is lower in older SHR. The loss of the anti-oxidant NO, generated by NOS isoforms, is also associated with renal injury in SHR. Long-term treatment with ARBs has been shown to enhance renal NOS and reduce renal injury in SHR.135 Furthermore, long-term treatment with exogenous anti-oxidants reduces blood pressure136 and improves GFR137 in SHR. These results, combined with observations that ARBs and tempol both restore renal hypoxia and the inefficient use of O2 in SHR and other forms of hypertension,39,113,117 suggest that renal injuries induced by hypertension are preceded by hypoxia as a contributing factor.
In summary, the kidney is relatively hypoxic in experimental models of hypertension, which may exacerbate renal damage associated with the disease. The changes in oxygen use along the nephron may contribute to renal tissue hypoxia. The kidney adjusts to the resulting hypoxia and maintains sodium balance.
“AngII receptors stimulates oxidant pathways”
CONCLUSION
An increasing number of experimental studies are providing evidence that intrarenal hypoxia is a unifying pathway to CKD. To be able to find new treatment modalities in, for example, diabetic nephropathy and hypertension, it is first necessary to verify the findings of deranged oxygen metabolism in the clinical setting. The need for non-invasive methodology is crucial for such a translation and, in the paper by Francis et al. in this series, such methodology is presented. The use of magnetic resonance imaging for the approximation of tissue oxygenation and blood flow is already in use in patients and is promising for the verification of experimental data.
ACKNOWLEDGEMENTS
Financial support from the Swedish Science Council - Medicine (to FP and PH), National Institutes of Health (NIH) grants HL089583 (WJW) and HL068686 (WJW), R01DK-28602 (RCB), the O’Brien Kidney Center for Acute Kidney Injury Grant P30DK-079337 (RCB), the Department of Veterans Affairs Research Service.
REFERENCES
- 1.Levy MN. Effect of variations of blood flow on renal oxygen extraction. Am. J. Physiol. 1960;199:13–18. doi: 10.1152/ajplegacy.1960.199.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Persson PB, Ehmke H, Kirchheim HR, et al. Autoregulation and non-homeostatic behaviour of renal blood flow in conscious dogs. J. Physiol. 1993;462:261–273. doi: 10.1113/jphysiol.1993.sp019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckardt KU, Bernhardt WM, Weidemann A, et al. Role of hypoxia in the pathogenesis of renal disease. Kidney Int. Suppl. 2005;99:S46–S51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 4.Eckardt KU, Rosenberger C, Jurgensen JS, Wiesener MS. Role of hypoxia in the pathogenesis of renal disease. Blood Purif. 2003;21:253–257. doi: 10.1159/000070698. [DOI] [PubMed] [Google Scholar]
- 5.Leong CL, Anderson WP, O’Connor PM, Evans RG. Evidence that renal arterial–venous oxygen shunting contributes to dynamic regulation of renal oxygenation. Am. J. Physiol. Renal Physiol. 2007;292:F1726–F1733. doi: 10.1152/ajprenal.00436.2006. [DOI] [PubMed] [Google Scholar]
- 6.Rosen S, Epstein FH, Brezis M. Determinants of intrarenal oxygenation: Factors in acute renal failure. Ren. Fail. 1992;14:321–325. doi: 10.3109/08860229209106636. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JJ. Is the function of the renal papilla coupled exclusively to an anaerobic pattern of metabolism? Am. J. Physiol. 1979;236:F423–F433. doi: 10.1152/ajprenal.1979.236.5.F423. [DOI] [PubMed] [Google Scholar]
- 8.Valtin H. Renal Function: Mechanisms Preserving Fluid and Solute Balance in Health. 2nd edn. Boston: Little Brown; 1983. [Google Scholar]
- 9.Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am. J. Physiol. 1981;240:F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- 10.Soltoff SP. ATP and the regulation of renal cell function. Annu. Rev. Physiol. 1986;48:9–31. doi: 10.1146/annurev.ph.48.030186.000301. [DOI] [PubMed] [Google Scholar]
- 11.Fliser D, Zeier M, Nowack R, Ritz E. Renal functional reserve in healthy elderly subjects. J. Am. Soc. Nephrol. 1993;3:1371–1377. doi: 10.1681/ASN.V371371. [DOI] [PubMed] [Google Scholar]
- 12.Fesler P, Du Cailar G, Ribstein J, Mimran A. Left ventricular remodeling and renal function in never-treated essential hypertension. J. Am. Soc. Nephrol. 2003;14:881–887. doi: 10.1097/01.asn.0000057855.93268.9f. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2010;19:43–50. doi: 10.1097/MNH.0b013e3283328eed. [DOI] [PubMed] [Google Scholar]
- 14.Palm F, Nangaku M, Fasching A, et al. Uremia induces abnormal oxygen consumption in tubules and aggravates chronic hypoxia of the kidney via oxidative stress. Am. J. Physiol. Renal Physiol. 2010;299:F380–F386. doi: 10.1152/ajprenal.00175.2010. [DOI] [PubMed] [Google Scholar]
- 15.Welch WJ. Intrarenal oxygen and hypertension. Clin. Exp. Pharmacol. Physiol. 2006;33:1002–1005. doi: 10.1111/j.1440-1681.2006.04478.x. [DOI] [PubMed] [Google Scholar]
- 16.Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43:629–633. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 17.Haidara MA, Mikhailidis DP, Rateb MA, et al. Evaluation of the effect of oxidative stress and vitamin E supplementation on renal function in rats with streptozotocin-induced Type 1 diabetes. J. Diabetes Complications. 2009;23:130–136. doi: 10.1016/j.jdiacomp.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Edlund J, Hansell P, Fasching A, et al. Reduced oxygenation in diabetic rat kidneys measured by T2* weighted magnetic resonance micro-imaging. Adv. Exp. Med. Biol. 2009;645:199–204. doi: 10.1007/978-0-387-85998-9_31. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberger C, Khamaisi M, Abassi Z, et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008;73:34–42. doi: 10.1038/sj.ki.5002567. [DOI] [PubMed] [Google Scholar]
- 20.dos Santos EA, Li LP, Ji L, Prasad PV. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest. Radiol. 2007;42:157–162. doi: 10.1097/01.rli.0000252492.96709.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J. Magn. Reson. Imaging. 2003;17:104–113. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J. Am. Soc. Nephrol. 2011;22:1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int. Suppl. 1998;65:S74–S78. [PubMed] [Google Scholar]
- 24.Nangaku M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 25.Nangaku M. Hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. Nephron Exp. Nephrol. 2004;98:e8–e12. doi: 10.1159/000079927. [DOI] [PubMed] [Google Scholar]
- 26.Singh DK, Winocour P, Farrington K. Mechanisms of disease: The hypoxic tubular hypothesis of diabetic nephropathy. Nat. Clin. Pract. Nephrol. 2008;4:216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 27.Palm F, Nordquist L. Renal tubulointerstitial hypoxia: Cause and consequence of kidney dysfunction. Clin. Exp. Pharmacol. Physiol. 2011;38:474–480. doi: 10.1111/j.1440-1681.2011.05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mimura I, Nangaku M. The suffocating kidney: Tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 29.Brezis M, Heyman SN, Epstein F. Determinants of intrarenal oxygenation II. Hemodynamic effects. Am. J. Physiol. 1994;267:F1063–F1068. doi: 10.1152/ajprenal.1994.267.6.F1063. [DOI] [PubMed] [Google Scholar]
- 30.Liss P, Nygren A, Ulfendahl HR, Erikson U. Effect of furosemide or mannitol before injection of a non-ionic contrast medium on intrarenal oxygen tension. Adv. Exp. Med. Biol. 1999;471:353–359. doi: 10.1007/978-1-4615-4717-4_42. [DOI] [PubMed] [Google Scholar]
- 31.Brezis M, Agmon Y, Epstein F. Determinants of intrarenal oxygenation I. Effects of diuretics. Am. J. Physiol. 1994;267:F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen SN. Red cell and plasma volume flows to the inner medulla of the rat kidney: Determinations by means of a step function input indicator technique. Pflüugers Arch. 1978;373:153–159. doi: 10.1007/BF00584854. [DOI] [PubMed] [Google Scholar]
- 33.Aukland K, Krog J. Renal oxygen tension. Nature. 1960;188:671. doi: 10.1038/188671a0. [DOI] [PubMed] [Google Scholar]
- 34.Liss P, Nygren A, Revsbech NP, Ulfendahl H. Intrarenal oxygen tension measured by a modified Clark electrode at normal and low blood pressure and after injection of X-ray contrast media. Pflüugers Arch. 1997;434:705–711. doi: 10.1007/s004240050455. [DOI] [PubMed] [Google Scholar]
- 35.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 36.Zou AP, Cowley AW., Jr Reactive oxygen species and molecular regulation of renal oxygenation. Acta Physiol. Scand. 2003;179:233–241. doi: 10.1046/j.0001-6772.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- 37.Yin WJ, Liu F, Li XM, et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur. J. Radiol. 2012;81:1426–1431. doi: 10.1016/j.ejrad.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 39.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron PO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001;59:230–237. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 40.Friederich M, Hansell P, Palm F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr. Diabetes Rev. 2009;5:120–144. doi: 10.2174/157339909788166800. [DOI] [PubMed] [Google Scholar]
- 41.Friederich M, Fasching A, Hansell P, Nordquist L, Palm F. Diabetes-induced up-regulation of uncoupling protein-2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim. Biophys. Acta. 2008;1777:935–940. doi: 10.1016/j.bbabio.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Nangaku M, Rosenberger C, Heyman SN, Eckardt KU. HIF regulation in kidney disease. Clin. Exp. Pharmacol. Physiol. 2013;40:148–157. doi: 10.1111/1440-1681.12005. [DOI] [PubMed] [Google Scholar]
- 43.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp. Nephrol. 2008;110:e1–e7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- 44.Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou CY, Miyata T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol. Dial. Transplant. 2008;23:1166–1172. doi: 10.1093/ndt/gfm715. [DOI] [PubMed] [Google Scholar]
- 45.Manotham K, Tanaka T, Matsumoto M, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004;65:871–880. doi: 10.1111/j.1523-1755.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- 46.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: Effects of angiotensin II receptor blockers. Diabetes. 2008;57:172–180. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- 47.Redfors B, Bragadottir G, Sellgren J, Sward K, Ricksten SE. Acute renal failure is not an ‘acute renal success’: A clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit. Care Med. 2010;38:1695–1701. doi: 10.1097/CCM.0b013e3181e61911. [DOI] [PubMed] [Google Scholar]
- 48.Stillman IE, Brezis M, Heyman SN, Epstein FH, Spokes K, Rosen S. Effects of salt depletion on the kidney: Changes in medullary oxygenation and thick ascending limb size. J. Am. Soc. Nephrol. 1994;4:1538–1545. doi: 10.1681/ASN.V481538. [DOI] [PubMed] [Google Scholar]
- 49.Wilcox CS, Palm F, Welch WJ. Renal oxygenation and function of the rat kidney. Effects of inspired oxygen and preglomerular oxygen shunting. Adv. Exp. Med. Biol. 2013;765:329–334. doi: 10.1007/978-1-4614-4989-8_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guyton AC, Langston JB, Navar G. Theory for renal autoregulation by feedback at the juxtaglomerular apparatus. Circ. Res. 1964;15(Suppl.):187–197. [PubMed] [Google Scholar]
- 51.Blantz RC. Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53:512–523. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x. [DOI] [PubMed] [Google Scholar]
- 52.Tucker BJ, Blantz RC. An analysis of the determinants of nephron filtration rate. Am. J. Physiol. 1977;232:F477–F483. doi: 10.1152/ajprenal.1977.232.6.F477. [DOI] [PubMed] [Google Scholar]
- 53.Blantz RC, Weir MR. Are the oxygen costs of kidney function highly regulated? Curr. Opin. Nephrol. Hypertens. 2004;13:67–71. doi: 10.1097/00041552-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Schurek HJ. [Kidney medullary hypoxia: A key to understanding acute renal failure?] Klin Wochenschr. 1988;66:828–835. doi: 10.1007/BF01728943. (in German). [DOI] [PubMed] [Google Scholar]
- 55.Schureck H-J, Johns O. Is tubuloglomerular feedback a tool to prevent nephron oxygen deficiency. Kidney Int. 1997;51:386–392. doi: 10.1038/ki.1997.51. [DOI] [PubMed] [Google Scholar]
- 56.Deetjen P, Kramer K. [The relation of O2 consumption by the kidney to Na re-resorption.] Pflüugers Arch Gesamte Physiol. Menschen Tiere. 1961;273:636–650. (in German). [PubMed] [Google Scholar]
- 57.Deng A, Miracle CM, Suarez JM, et al. Oxygen consumption in the kidney: Effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int. 2005;68:723–730. doi: 10.1111/j.1523-1755.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 58.Deng A, Arndt MA, Satriano J, et al. Renal protection in chronic kidney disease: Hypoxia-inducible factor activation vs. angiotensin II blockade. Am. J. Physiol. Renal Physiol. 2010;299:F1365–F1373. doi: 10.1152/ajprenal.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen JJ, Kamm DE. Renal metabolism: Relation to renal function. In: Brenner BM, Rector FC, editors. The Kidney. Philadelphia: WB Saunders; 1985. pp. 144–248. [Google Scholar]
- 60.Klahr S, Hamm LL, Hammerman MR, Mandel LJ. Renal metabolism: Integrated responses. In: Orloff J, Berliner RW, editors. Handbook of Physiology. Ch. 8. Oxford: Oxford University Press; 1989. [Google Scholar]
- 61.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J. Clin. Invest. 1991;88:390–395. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Nicola L, Blantz RC, Gabbai FB. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J. Clin. Invest. 1992;89:1248–1256. doi: 10.1172/JCI115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuramochi G, Homma S. Postischemic recovery process of renal oxygen consumption in normal and streptozotocin diabetic rats. Ren. Fail. 1993;15:587–594. doi: 10.3109/08860229309069408. [DOI] [PubMed] [Google Scholar]
- 64.Legrand M, Almac E, Mik EG, et al. l-NIL prevents renal microvascular hypoxia and increase of renal oxygen consumption after ischemia–reperfusion in rats. Am. J. Physiol. Renal Physiol. 2009;296:F1109–F1117. doi: 10.1152/ajprenal.90371.2008. [DOI] [PubMed] [Google Scholar]
- 65.Molitoris BA, Falk SA, Dahl RH. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J. Clin. Invest. 1989;84:1334–1339. doi: 10.1172/JCI114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tucker BJ, Steiner RW, Gushwa LC, Blantz RC. Studies on the tubulo-glomerular feedback system in the rat. The mechanism of reduction in filtration rate with benzolamide. J. Clin. Invest. 1978;62:993–1004. doi: 10.1172/JCI109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng A, Miracle CM, Lortie M, et al. Kidney oxygen consumption, carbonic anhydrase, and proton secretion. Am. J. Physiol. Renal Physiol. 2006;290:F1009–F1015. doi: 10.1152/ajprenal.00343.2005. [DOI] [PubMed] [Google Scholar]
- 68.Weinstein SW, Klose R, Szyjewicz J. Proximal tubular Na, Cl, and HCO3 reabsorption and renal oxygen consumption. Am. J. Physiol. 1984;247:F151–F157. doi: 10.1152/ajprenal.1984.247.1.F151. [DOI] [PubMed] [Google Scholar]
- 69.Wang T, Yang CL, Abbiati T, Shull GE, Giebisch G, Aronson PS. Essential role of NHE3 in facilitating formate-dependent NaCl absorption in the proximal tubule. Am. J. Physiol. Renal Physiol. 2001;281:F288–F292. doi: 10.1152/ajprenal.2001.281.2.F288. [DOI] [PubMed] [Google Scholar]
- 70.Heaton GM, Wagenvoord RJ, Kemp A, Jr, Nicholls DG. Brown-adipose-tissue mitochondria: Photoaffinity labelling of the regulatory site of energy dissipation. Eur. J. Biochem. 1978;82:515–521. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- 71.Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J. Biol. Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 72.Deng A, Tang T, Singh P, et al. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int. 2009;75:197–204. doi: 10.1038/ki.2008.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruiz M, Singh P, Thomson SC, Munger K, Blantz RC, Gabbai FB. l-Arginine-induced glomerular hyperfiltration response. The roles of insulin and ANG II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1744–R1751. doi: 10.1152/ajpregu.00871.2007. [DOI] [PubMed] [Google Scholar]
- 74.Thomson SC, Bachmann S, Bostanjoglo M, et al. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J. Clin. Invest. 1999;104:1149–1158. doi: 10.1172/JCI5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laycock SK, Vogel T, Forfia PR, et al. Role of nitric oxide in the control of renal oxygen consumption and the regulation of chemical work in the kidney. Circ. Res. 1998;82:1263–1271. doi: 10.1161/01.res.82.12.1263. [DOI] [PubMed] [Google Scholar]
- 76.Koivisto A, Pittner J, Froelich M, Persson AE. Oxygen-dependent inhibition of respiration in isolated renal tubules by nitric oxide. Kidney Int. 1999;55:2368–2375. doi: 10.1046/j.1523-1755.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 77.Brown GC, Borutaite V. Nitric oxide, cytochrome c and mitochondria. Biochem. Soc. Symp. 1999;66:17–25. doi: 10.1042/bss0660017. [DOI] [PubMed] [Google Scholar]
- 78.Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc. Natl Acad. Sci. USA. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beltran B, Quintero M, Garcia-Zaragoza E, O’Connor E, Esplugues JV, Moncada S. Inhibition of mitochondrial respiration by endogenous nitric oxide: A critical step in Fas signaling. Proc. Natl Acad. Sci. USA. 2002;99:8892–8897. doi: 10.1073/pnas.092259799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersson U, Leighton B, Young ME, Blomstrand E, Newsholme EA. Inactivation of aconitase and oxoglutarate dehydrogenase in skeletal muscle in vitro by superoxide anions and/or nitric oxide. Biochem. Biophys. Res. Commun. 1998;249:512–516. doi: 10.1006/bbrc.1998.9171. [DOI] [PubMed] [Google Scholar]
- 81.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 82.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am. J. Med. 1982;72:375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 83.Sakharova OV, Taal MW, Brenner BM. Pathogenesis of diabetic nephropathy: Focus on transforming growth factor-beta and connective tissue growth factor. Curr. Opin. Nephrol. Hypertens. 2001;10:727–738. doi: 10.1097/00041552-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am. J. Physiol. Regul. Intergr. Comp. Physiol. 2002;282:R335–R342. doi: 10.1152/ajpregu.00605.2001. [DOI] [PubMed] [Google Scholar]
- 85.Pugliese G, Tilton RG, Williamson JR. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes Metab. Rev. 1991;7:35–59. doi: 10.1002/dmr.5610070106. [DOI] [PubMed] [Google Scholar]
- 86.Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia. 2004;47:1223–1231. doi: 10.1007/s00125-004-1434-3. [DOI] [PubMed] [Google Scholar]
- 87.Palm F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2006;33:997–1001. doi: 10.1111/j.1440-1681.2006.04473.x. [DOI] [PubMed] [Google Scholar]
- 88.O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: Gatekeepers of life and death. Physiology. 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishikawa T, Du XL, Edelstein D, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 90.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 91.Machimura H. Ameliorating effects of conventional therapy on declining renal function in patients with diabetic nephropathy. Tokai J. Exp. Clin. Med. 1991;16:187–192. [PubMed] [Google Scholar]
- 92.Dikalova AE, Bikineyeva AT, Budzyn K, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edlund J, Fasching A, Liss P, Hansell P, Palm F. The roles of NADPH-oxidase and nNOS for the increased oxidative stress and the oxygen consumption in the diabetic kidney. Diabetes Metab. Res. Rev. 2010;26:349–356. doi: 10.1002/dmrr.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Persson P, Hansell P, Palm F. NADPH oxidase inhibition reduces tubular sodium transport and improves kidney oxygenation in diabetes. Am. J. Physiol. Regul. Intergr. Comp. Physiol. 2012;302:R1443–R1449. doi: 10.1152/ajpregu.00502.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brookes PS. Mitochondrial H(+) leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 96.Friederich-Persson M, Aslam S, Nordquist L, Welch WJ, Wilcox CS, Palm F. Acute knockdown of uncoupling protein-2 increases uncoupling via the adenine nucleotide transporter and decreases oxidative stress in diabetic kidneys. PLoS One. 2012;7:e39635. doi: 10.1371/journal.pone.0039635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Friederich M, Nordquist L, Olerud J, Johansson M, Hansell P, Palm F. Identification and distribution of uncoupling protein isoforms in the normal and diabetic rat kidney. Adv. Exp. Med. Biol. 2009;645:205–212. doi: 10.1007/978-0-387-85998-9_32. [DOI] [PubMed] [Google Scholar]
- 98.Echtay KS, Esteves TC, Pakay JL, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 100.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 101.Friederich M, Olerud J, Fasching A, Liss P, Hansell P, Palm F. Uncoupling protein-2 in diabetic kidneys: Increased protein expression correlates to increased non-transport related oxygen consumption. Adv. Exp. Med. Biol. 2008;614:37–43. doi: 10.1007/978-0-387-74911-2_5. [DOI] [PubMed] [Google Scholar]
- 102.Fleury C, Neverova M, Collins S, et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 103.Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995;47:1546–1557. doi: 10.1038/ki.1995.218. [DOI] [PubMed] [Google Scholar]
- 104.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 105.Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int. Suppl. 2000;75:S22–S26. [PubMed] [Google Scholar]
- 106.Eckardt KU, Rosenberger C, Jurgensen JS, Wiesener MS. Role of hypoxia in the pathogenesis of renal disease. Blood Purif. 2003;23:253–257. doi: 10.1159/000070698. [DOI] [PubMed] [Google Scholar]
- 107.Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 108.Krauss S, Zhang CY, Scorrano L, et al. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J. Clin. Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Persson MF, Franzen S, Catrina SB, et al. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia. 2012;55:1535–1543. doi: 10.1007/s00125-012-2469-5. [DOI] [PubMed] [Google Scholar]
- 110.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dworkin LD, Grosser M, Feiner HD, Ullian M, Parker M. Renal vascular effects of antihypertensive therapy in uninephrectomized SHR. Kidney Int. 1989;35:790–798. doi: 10.1038/ki.1989.54. [DOI] [PubMed] [Google Scholar]
- 112.Dworkin LD. Impact of antihypertensive therapy on progression of experimental renal disease. J. Hum. Hypertens. 1996;10:663–668. [PubMed] [Google Scholar]
- 113.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: Role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H22–H28. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 114.Zhu Q, Wang Z, Xia M, et al. Silencing of hypoxia-inducible factor-1alpha gene attenuated angiotensin II-induced renal injury in Sprague-Dawley rats. Hypertension. 2011;58:657–664. doi: 10.1161/HYPERTENSIONAHA.111.177626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fujimoto S, Satoh M, Nagasu H, Horike H, Sasaki T, Kashihara N. Azelnidipine exerts renoprotective effects by improvement of renal microcirculation in angiotensin II infusion rats. Nephrol. Dial. Transplant. 2009;24:3651–3658. doi: 10.1093/ndt/gfp407. [DOI] [PubMed] [Google Scholar]
- 116.Textor SC, Gloviczki ML, Flessner MF, et al. Association of filtered sodium load with medullary volumes and medullary hypoxia in hypertensive African Americans as compared with Whites. Am. J. Kidney Dis. 2012;59:229–237. doi: 10.1053/j.ajkd.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat. Role of AT1 receptors. Kidney Int. 2003;63:202–208. doi: 10.1046/j.1523-1755.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- 118.Adler S, Huang H. Impaired regulation of renal oxygen consumption in spontaneously hypertensive rats. J. Am. Soc. Nephrol. 2002;13:1788–1794. doi: 10.1097/01.asn.0000019781.90630.0f. [DOI] [PubMed] [Google Scholar]
- 119.Gomez SI, Warner L, Haas JA, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am. J. Physiol. Renal Physiol. 2009;297:F981–F986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brezis M, Greenfeld Z, Shina A, Rosen S. Angiotensin II augments medullary hypoxia and predisposes to failure. Eur. J. Clin. Invest. 1990;20:199–207. doi: 10.1111/j.1365-2362.1990.tb02269.x. [DOI] [PubMed] [Google Scholar]
- 121.Schiffrin EL, Touyz RM. From bedside to bench to bedside: Role of renin–angiotensin–aldosterone system in remodeling of resistance arteries in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H435–H446. doi: 10.1152/ajpheart.00262.2004. [DOI] [PubMed] [Google Scholar]
- 122.Johnson RJ, Feig DI, Nakagawa T, Sanchez-Lozada LG, Rodriguez-Iturbe B. Pathogenesis of essential hypertension: Historical paradigms and modern insights. J. Hypertens. 2008;26:381–391. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tian XY, Wong WT, Leung FP, et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin F(2alpha) impairs endothelial function in renovascular hypertensive rats. Antioxid. Redox Signal. 2012;16:363–373. doi: 10.1089/ars.2010.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saleh MA, Pollock DM. Endothelin in renal inflammation and hypertension. Contrib. Nephrol. 2011;172:160–170. doi: 10.1159/000328696. [DOI] [PubMed] [Google Scholar]
- 125.Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: Role of angiotensin II. Semin. Nephrol. 2004;24:101–114. doi: 10.1016/j.semnephrol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 126.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am. J. Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 127.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension. 2008;51:345–351. doi: 10.1161/HYPERTENSIONAHA.107.097832. [DOI] [PubMed] [Google Scholar]
- 128.Palm F, Onozato M, Welch WJ, Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: Effects of tempol and angiotensin blockade. Hypertension. 2010;55:298–304. doi: 10.1161/HYPERTENSIONAHA.109.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension. 1998;32:958–964. doi: 10.1161/01.hyp.32.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K,1C kidney. Hypertension. 2003;41:692–696. doi: 10.1161/01.HYP.0000052945.84627.8F. [DOI] [PubMed] [Google Scholar]
- 131.Panico C, Luo Z, Damiano S, Artigiano F, Gill P, Welch WJ. Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats. Roles of superoxide and Na+/H+ exchanger 3. Hypertension. 2009;54:1291–1297. doi: 10.1161/HYPERTENSIONAHA.109.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Welch WJ, Chabrashvili T, Solis G, et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 133.Tolbert EM, Weisstuch J, Feiner HD, Dworkin LD. Onset of glomerular hypertension with aging precedes injury in the spontaneously hypertensive rat. Am. J. Physiol. Renal Physiol. 2000;278:F839–F846. doi: 10.1152/ajprenal.2000.278.5.F839. [DOI] [PubMed] [Google Scholar]
- 134.Simao S, Gomes P, Pinto V, et al. Age-related changes in renal expression of oxidant and antioxidant enzymes and oxidative stress markers in male SHR and WKY rats. Exp. Gerontol. 2011;46:468–474. doi: 10.1016/j.exger.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 135.Zhou XJ, Vaziri ND, Zhang J, Wang HW, Wang XQ. Association of renal injury with nitric oxide deficiency in aged SHR. Prevention by hypertension control with AT1 blockade. Kidney Int. 2002;62:914–921. doi: 10.1046/j.1523-1755.2002.00516.x. [DOI] [PubMed] [Google Scholar]
- 136.Racasan S, Braam B, van der Giezen DM, et al. Perinatal l-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–88. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]
- 137.Mihailovic-Stanojevic N, Miloradovic Z, Grujic-Milanovic J, Ivanov M, Jovovic D. Effects of angiotensin II type-1 receptor blocker losartan on age-related cardiovascular risk in spontaneously hypertensive rats. Gen. Physiol. Biophys. 2009;28:112–118. [PubMed] [Google Scholar]