Abstract
IMPORTANCE
Autism spectrum disorder (ASD) affects 1 in 88 children and is characterized by a complex phenotype, including social, communicative, and sensorimotor deficits. Autism spectrum disorder has been linked with atypical connectivity across multiple brain systems, yet the nature of these differences in young children with the disorder is not well understood.
OBJECTIVES
To examine connectivity of large-scale brain networks and determine whether specific networks can distinguish children with ASD from typically developing (TD) children and predict symptom severity in children with ASD.
DESIGN, SETTING, AND PARTICIPANTS
Case-control study performed at Stanford University School of Medicine of 20 children 7 to 12 years old with ASD and 20 age-, sex-, and IQ-matched TD children.
MAIN OUTCOMES AND MEASURES
Between-group differences in intrinsic functional connectivity of large-scale brain networks, performance of a classifier built to discriminate children with ASD from TD children based on specific brain networks, and correlations between brain networks and core symptoms of ASD.
RESULTS
We observed stronger functional connectivity within several large-scale brain networks in children with ASD compared with TD children. This hyperconnectivity in ASD encompassed salience, default mode, frontotemporal, motor, and visual networks. This hyperconnectivity result was replicated in an independent cohort obtained from publicly available databases. Using maps of each individual’s salience network, children with ASD could be discriminated from TD children with a classification accuracy of 78%, with 75% sensitivity and 80% specificity. The salience network showed the highest classification accuracy among all networks examined, and the blood oxygen–level dependent signal in this network predicted restricted and repetitive behavior scores. The classifier discriminated ASD from TD in the independent sample with 83% accuracy, 67% sensitivity, and 100% specificity.
CONCLUSIONS AND RELEVANCE
Salience network hyperconnectivity may be a distinguishing feature in children with ASD. Quantification of brain network connectivity is a step toward developing biomarkers for objectively identifying children with ASD.
Autism spectrum disorder (ASD), a neurodevelopmental disorder that affects language, social communication, motor behaviors, and sensory systems, affects nearly 1 in 88 children.1 Increasingly, ASD is understood to be associated with atypical development of multiple interconnected brain systems rather than isolated brain regions.2 Although converging lines of research have demonstrated altered brain connectivity in individuals with autism,3,4 inconsistency with respect to analytic techniques remains an issue.5 One prevailing theory posits that autism is caused by underfunctioning integrative circuitry, resulting in information integration deficits at the neural and cognitive levels.6 The underconnectivity theory is based largely on analysis of task-related changes in interregional connectivity during tasks that involve language,6,7 working memory,8 mental imagery,9 executive functions,10 cognitive control,11,12 and social cognition.13,14 However, several reports of brain hyperconnectivity in ASD also exist in the domains of visuomotor processing,15,16 visualsearch,17 emotionprocessing,18 memory,19 and language.20 More recently, evidence for both underconnectivity and hyperconnectivity in ASD has come from intrinsic connectivity studies21–24 using resting-state functional magnetic resonance imaging (fMRI). These contradictory findings leave open the question of whether and to what extent intrinsic functional connectivity of the brain is altered in autism and suggest that both hypoconnectivity and hyperconnectivity may underlie the complex phenotype of the disorder.25 At the cognitive and behavioral levels, ASD is characterized by difficulties with integration across domains, rigidity, repetitive behaviors, and hypersensitivity. How such behaviors may arise from hypoconnected or hyperconnected brain systems remains unknown. Although relatively few studies21,26 have directly linked aberrant intrinsic brain connectivity in ASD to specific symptoms, there is a growing literature that demonstrates that individual differences in intrinsic functional connectivity are systematically related to measures of empathy,27 IQ,28 and behavioral variability29 in neurotypical adults. To date, no studies have systematically explored whether connectivity is diminished or enhanced within specific large-scale functional brain systems30,31 in children with ASD.
Autism is a disorder with early life onset and variable developmental trajectory32; therefore, studies of young children are especially critical for developing accurate models of the underlying neurobiology of the disorder. Indeed, at earlier developmental time points, a quite different picture of brain connectivity in autism is observed. One of the earliest signs of autism is enlarged head circumference or macrocephaly.33 Infants and young children with ASD show signs of early brain overgrowth.34 Although the relationship between neuron number and brain size is complex, postmortem studies35 of children with ASD indicate that they have an overabundance or excess numbers of neurons in the prefrontal cortex. Some of these differences are diminished with development, such that older children,36 adolescents, and adults with autism do not differ from neurotypical individuals on measures of brain size.37 This finding suggests a different developmental trajectory in ASD, underscoring the point that group differences observed in adults cannot necessarily be extrapolated to understand the neurobiology of childhood autism. A study38 of functional connectivity during performance of a response inhibition task found no differences between children with ASD and typically developing (TD) children. The only published study24 examining intrinsic functional connectivity of the brain in children with ASD found hyperconnectivity between subcortical regions and heteromodal association cortex.
Accurate characterization of brain connectivity in children with autism is a first step toward developing brain-based biomarkers for the disorder. To date, only one study39 has used intrinsic functional connectivity to attempt to classify individuals with autism. For participants younger than 20 years, whole-brain functional connectivity measures could discriminate between individuals with ASD and control participants, but classification accuracies were much lower for older participants. This finding further highlights the need for investigations targeting younger individuals.
No previous studies have examined the complete range of large-scale brain networks30,31 in children with autism. We examine whole brain intrinsic functional connectivity in children with ASD using independent component analysis (ICA), a data-driven, unbiased approach for uncovering coherent and highly reproducible large-scale brain networks.40 We sought to determine whether children with ASD showed patterns of functional connectivity more consistent with hyperconnectivity or hypoconnectivity accounts and to test whether measures of functional connectivity could be used to discriminate children with ASD from TD children. On the basis of a recent systems-level theoretical model of social and cognitive dysfunction in ASD,41 we hypothesized that the salience network, composed of the anterior insular cortex and anterior cingulate cortex (ACC), would show aberrant patterns of brain connectivity and provide the greatest information regarding group membership. Regions within the salience network are implicated in multiple functions, ranging from attention to interoception and subjective awareness.42,43 The salience network integrates external sensory stimuli with internal states,44 and we have previously reported that it is critical for orchestrating brain network dynamics.45–47 Network analyses indicate that the anterior insula acts as a hub, mediating interactions between large-scale networks involved in externally and internally oriented cognitive processing,46 and a meta-analysis of neuroimaging studies of social cognition identifies the anterior insula as a consistent locus of hypoactivity in autism.48 Synthesizing previous work, we propose that a more general role of the salience network is attention allocation to stimuli that is salient to the individual49 and hypothesize that atypical development of the salience network may contribute to diminished interest in social interaction, a hallmark of ASD.41 The current study is the first to examine the salience network in this context.
Methods
Participants
Participants were recruited from the San Francisco Bay Area through advertisements. Children with ASD were also recruited from the Stanford Autism Clinic and the Lucille Packard Children’s Hospital. Children were evaluated using the Autism Diagnostic Interview–Revised (ADI-R)50 and the Autism Diagnostic Observation Schedule (ADOS)51 administered by a trained research assessor, and diagnoses were confirmed by a clinical psychologist (J.P.). All participants had scores in the autism range on the ADI-R with the exception of one child whose scores were slightly below the cutoffs for communication (2 points) and repetitive behavior (1 point) and one child whose score was 1 point below the cutoff for repetitive behavior. In both cases, these children fell within the ASD range on the ADOS and were therefore considered to fall within the ASD range using the combination of scores on these measures.52 On the ADOS, 9 of the participants had scores in the ASD range and 10 of the participants had scores in the autism range. The ADOS scores were not available for one of the participants, whose ADI-R score fell well within the autism range. All participants were verbal and high functioning, as evidenced by their relatively high IQ scores. All participants underwent standardized neuropsychological assessments, including the Wechsler Abbreviated Scale of Intelligence (eMaterials in the Supplement).
Neuroimaging data were collected from 33 children with ASD. Data from participants who exhibited greater than 5 mm of motion in any direction during fMRI were not considered for further analysis. The final sample included 20 children with ASD (16 boys and 4 girls; mean [SD] age, 9.96 [1.59] years; full-scale mean [SD] IQ, 112.6 [17.8]). Using an algorithm developed to select control participants from a preexisting data set (eMaterials), 20 children matched on age, sex, and IQ were selected (16 boys and 4 girls; mean [SD] age, 9.95 [1.60]; full-scale mean [SD] IQ, 112.1 [15.4]; Table 1). Participants gave written informed assent, and parents or guardians gave written informed consent. Additional information regarding current medications and comorbid psychopathologic conditions are provided in the eMaterials. The study was approved by the Stanford University Institutional Review Board.
Table 1.
Characteristics of the Study Participantsa
| Characteristic | Autism Spectrum Disorder (n = 20) | Typically Developing (n = 20) | P Value |
|---|---|---|---|
| Age, y | 9.96 (1.59) [7.52–11.88] | 9.95 (1.60) [7.75–12.43] | .99 |
| Sex, No. | |||
| Male | 16 | 16 | >.99 |
| Female | 4 | 4 | |
| Full-scale IQ | 112.6 (17.8) [78–148] | 112.1 (15.4) [79–136] | .98 |
| ADOS social scoreb | 8.2 (2.1) [4–11] | ||
| ADOS communication scoreb | 3.6 (1.5) [2–7] | ||
| ADI–social score | 20.4 (5.4) [10–29] | ||
| ADI–communication score | 15.9 (5.1) [6–23] | ||
| ADI–repetitive behavior scoreb | 5.8 (2.5) [2–11] | ||
Abbreviations: ADI, Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Schedule.
Data are presented as mean (SD) [range] unless otherwise indicated.
Score missing for one participant.
Data Acquisition
Functional MRI
Before resting-state fMRI, children viewed the following instructions: “Relax. Please keep your eyes closed but do not go to sleep.” They were also instructed to try not to move for the duration of the 6-minute scan. Functional images were acquired on a 3T GE Signa scanner (General Electric) using a custom-built head coil. Head movement was minimized during scanning by small cushions. A total of 29 axial sections (4.0-mm thickness, 0.5-mm skip) parallel to the anterior commissure—posterior commissure line and covering the whole brain were imaged using a T2-weighted gradient echo spiral in-out pulse sequence53 with the following parameters: repetition time, 2000 milliseconds; echo time, 30 milliseconds; flip angle, 80°; and 1 interleave, resulting in 180 volumes. The field of view was 20 cm, and the matrix size was 64 × 64, providing an in-plane spatial resolution of 3.125 mm. To reduce blurring and signal loss arising from field inhomogeneities, an automated high-order shimming method based on spiral acquisitions was used before acquiring functional scans.
Structural MRI
For each participant, a high-resolution T1-weighted spoiled grass gradient recalled inversion recovery 3-dimensional MRI sequence was acquired (inversion time, 300 milliseconds; repetition time, 8.4 milliseconds; echo time, 1.8 milliseconds; flip angle, 15°; 22-cm field of view; 132 sections in coronal plane; 256 × 192 matrix; 2 excitations, acquired resolution, 1.5 × 0.9 × 1.1 mm).
Data Processing
Preprocessing
The fMRI data were preprocessed using SPM statistical software, version 8 (http://www.fil.ion.ucl.ac.uk/spm). Details of preprocessing steps, results from analyses implementing the data “scrubbing” procedure for removing motion artifacts,54 and additional analyses are presented in the eMaterials, eFigure 1, eFigure 2, and eFigure 3 in the Supplement.
Dual Regression ICA
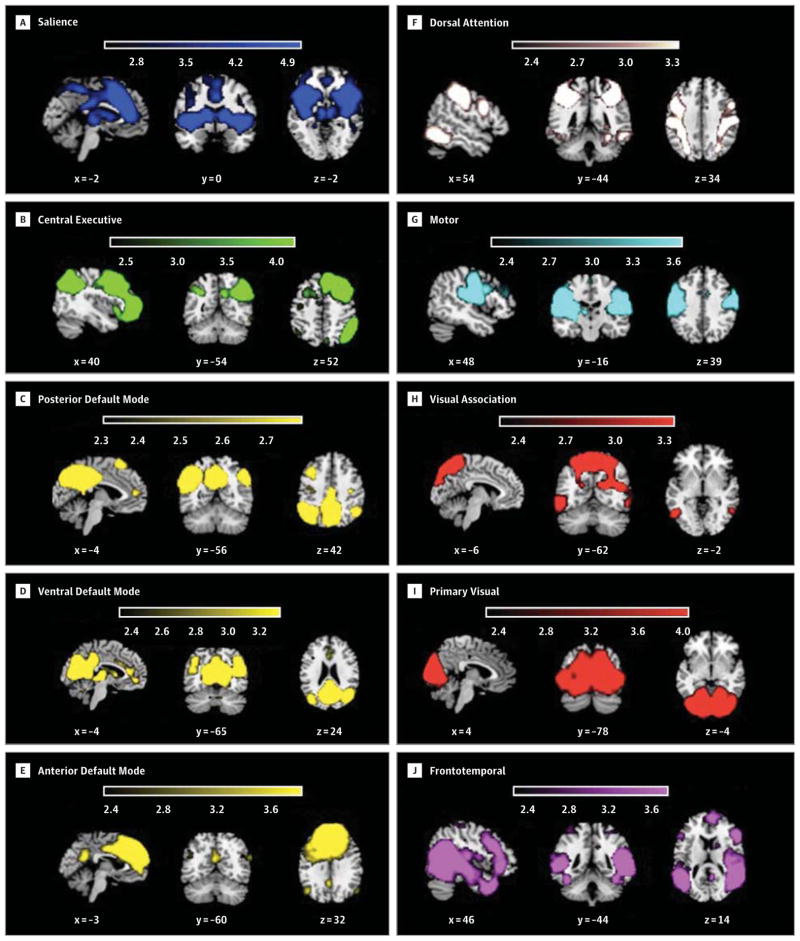
We used the dual regression approach implemented in FSL (www.fmrib.ox.ac.uk/analysis/dualreg/).55 This method has recently emerged as the preferred option for comparing large-scale brain networks between groups.56–59 First, preprocessed data from both groups (TD and ASD) were concatenated and entered into a group ICA to identify large-scale patterns of functional connectivity in the population. Data were decomposed into 25 independent components using MELODIC (http://fsl.fmrib.ox.ax.uk/fsl/fslwiki/MELODIC). Second, 10 components corresponding to previously described functional networks were selected (Figure 1). The salience network, with key nodes in the dorsal ACC and frontoinsular cortices, is thought to be a nexus that unites conflict monitoring, interoceptive-autonomic, and reward processing centers.44 The right central executive network is anchored in the dorsolateral prefrontal cortex and posterior parietal cortex and is involved in the maintenance and manipulation of information and decision making in the context of goal-directed behavior.46 We found 3 components resembling the default mode network (DMN), with key nodes in medial prefrontal cortex and posterior cingulate cortex. These components have previously been described as the posterior DMN, the ventral DMN, and the anterior DMN57 in studies of older populations. One study60 comparing children and adults demonstrated separation of the DMN into 2 components. DMN function encompasses a range of self-relevant and social cognitive processes.58 The dorsal attention network is a bilateral system with nodes in the intraparietal sulcus and frontal eye fields and is involved in top-down orienting of attention.61 The motor network comprises regions of precentral gyrus and supplementary motor area.62 Primary and association visual networks were observed,58 spanning medial and lateral surfaces of the occipital lobe, respectively. We also observed a frontotemporal network58 with nodes in bilateral superior temporal gyri and inferior frontal gyri. The remaining components corresponded to obvious noise and artifacts (eg, white matter, ventricles, eyes, and motion) and were not further analyzed.
Figure 1. Large-scale Brain Networks Identified Using Independent Component Analysis (ICA).
Data from 40 children (20 children with autism spectrum disorder [ASD] and 20 typically developing [TD] children) were combined in a group ICA to identify 25 independent components (networks) across all participants in a data-driven manner. Ten of these components correspond to previously identified functional networks: salience (A), central executive (B), posterior default mode (C), ventral default mode (D), anterior default mode (E), dorsal attention (F), motor (G), visual association (H), primary visual (I), and frontotemporal (J). Maps are displayed at z > 2.3 (P < .01).
Third, the dual-regression algorithm was applied to identify participant-specific time courses and spatial maps (eMaterials in the Supplement). Group difference maps from this statistical testing were thresholded using threshold-free cluster enhancement at the P < .05 level.
Network-Based Classification of ASD
The 10 components identified from each participant (described above) served as features to be input into classification analyses. We used a logistic regression classifier (implemented in the Matlab package GLMnet; http://www-stat.stanford.edu/~tibs/glmnet-matlab) to classify children with ASD and TD children (eMaterials in the Supplement). We report the cross-validation accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each of the 10 networks. Permutation tests were conducted to arrive at P values associated with classification accuracies for each network. Sensitivity measures the proportion of positive results that are correctly identified as such, in this case, the percentage of children with ASD who are correctly identified as having ASD. Specificity measures the proportion of negative results that are correctly identified, or the percentage of TD children who are correctly identified as TD.
Association With Symptom Severity
To investigate brain-behavior associations, we used a sparse regression algorithm (eMaterials in the Supplement). The sparse regression algorithm identifies voxels that predict symptom severity by modeling the relationship between the dependent variable (score on ADOS or ADI subscale) and the independent variables (voxels within a network). GLMnet (http://www-stat.stanford.edu/~tibs/glmnet-matlab/), a state-of-the-art sparse regression algorithm,63 was also used in this analysis. R2 was used to measure the performance of the regression algorithm in predicting symptom severity.
Results
Large-Scale Brain Networks In Children With ASD and TD Children
From the initial group ICA (n = 40), 10 of the 25 components were visually identified as corresponding to previously described functional networks40,64 (Figure 1, eFigure 1, and eFigure 2 in the Supplement). Significant group differences were found in 6 of the 10 functional networks examined: the salience, posterior DMN, frontotemporal, motor, and visual networks. For each of these networks, there was greater connectivity in children with ASD compared with TD children, or hyperconnectivity in ASD (Figure 2). Within the salience network, ASD greater than TD functional connectivity was observed in the ACC, superior frontal gyrus, thalamus, and bilateral insular cortices. Within the posterior DMN, ASD greater than TD functional connectivity was observed in the precuneus, posterior cingulate, and left angular gyrus. Within the frontotemporal network, there was ASD greater than TD functional connectivity in bilateral superior and middle temporal gyri. Within the motor network, there was ASD greater than TD functional connectivity in bilateral pre-central and postcentral gyri, left posterior insula, and thalamus. Within the 2 visual networks, there was ASD greater than TD functional connectivity in the left lateral occipital cortex, intracalcarine cortex, and occipital pole. All group differences were observed within component boundaries, and none of the networks examined had differences in any brain areas when we examined the opposite contrast (TD greater than ASD functional connectivity). This network hyperconnectivity result was replicated in an independent data set of 15 children with ASD and 15 TD children obtained from public databases (http://ndar.nih.gov/ and http://fcon_1000.projects.nitrc.org/indi/adhd200/; eMaterials, eFigure 4, and eFigure 5 in the Supplement).
Figure 2. Brain Network Hyperconnectivity in Children With Autism Spectrum Disorder (ASD) Compared With Typically Developing (TD) Children.
Autism spectrum disorder greater than TD functional connectivity was observed in 6 of the 10 networks examined: salience (A), posterior default mode (B), motor (C), visual association (D), primary visual (E), and frontotemporal (F). Group difference maps were thresholded using threshold-free cluster enhancement (P < .05).
Network-Based Classification of ASD
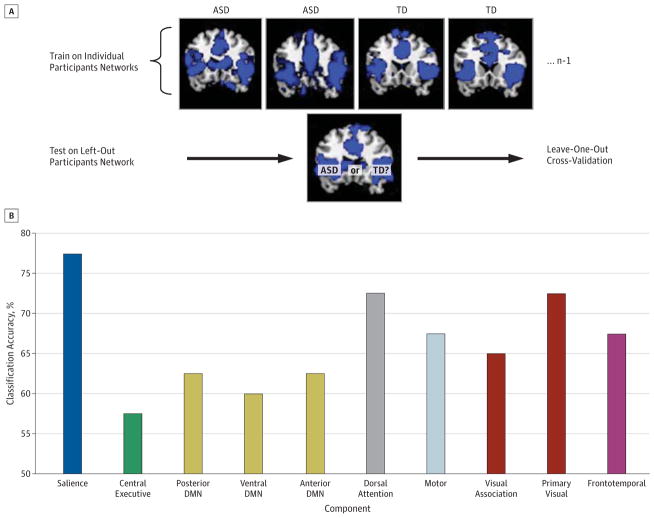
Maps corresponding to each of the 10 networks for each participant were individually evaluated for their ability to discriminate children with ASD from TD children using a classification algorithm based on a logistic regression framework63(Figure 3A). Classification accuracies, sensitivities, specificities, PPV, and NPV for each network a represented in Table 2. The salience network produced the highest classification accuracy of all networks, with 78% accuracy (P = .02), 75% sensitivity, and 80% specificity (PPV, 79%; NPV, 76%) (Figure 3B). Systematic variation of mask thresholds verified that the salience network produced the highest classification accuracy of all networks at all reasonable threshold values. Applying the salience network–based classifier to data from an independent cohort, 83% accuracy (P = .02), 67% sensitivity, and 100% specificity (PPV, 100%; NPV, 75%) were observed (eMaterials in the Supplement).
Figure 3. Classification Analysis and Accuracy.
A, Classification analysis flowchart. The 10 components identified from each participant served as features to be input into classification analyses. A linear classifier built using logistic regression was used to classify children with autism spectrum disorder (ASD) from typically developing (TD) children. B, Classification accuracy for brain networks. The salience network produced the highest classification accuracy at 78% (P = .02). DMN indicates default mode network.
Table 2.
Brain Network Connectivity Patterns for Children With Autism Spectrum Disorder From Typically Developing Children
| Network | Classification Accuracy, % | Sensitivity, % | Specificity, % | Positive Predictive Value, % | Negative Predictive Value, % | P Value |
|---|---|---|---|---|---|---|
| Salience | 78 | 75 | 80 | 79 | 76 | .02 |
| Salience (independent data set) | 83 | 67 | 100 | 100 | 75 | .02 |
| Dorsal attention | 73 | 75 | 70 | 71 | 74 | .06 |
| Primary visual | 73 | 60 | 85 | 80 | 68 | .06 |
| Motor | 68 | 60 | 75 | 71 | 65 | .15 |
| Frontotemporal | 68 | 60 | 75 | 71 | 65 | .16 |
| Visual association | 65 | 65 | 65 | 65 | 65 | .21 |
| Default mode | ||||||
| Anterior | 63 | 50 | 75 | 67 | 60 | .28 |
| Posterior | 63 | 65 | 60 | 62 | 63 | .29 |
| Ventral | 60 | 55 | 65 | 61 | 59 | .36 |
| Central executive | 58 | 55 | 60 | 58 | 57 | .47 |
Association With Symptom Severity
To examine the associations between ASD symptom severity and brain networks, we used a multivariate sparse regression analysis.63 On the basis of our a priori hypotheses41 and the finding that the salience network exhibited both strong group differences and the highest classification accuracy for discriminating ASD from TD, we focused on exploring relationships with this specific network. We found that the salience network was related to restricted and repetitive behaviors as measured by the ADI-R in that voxels within the network predicted severity of symptoms (R2 = 0.36, P = .007, Bonferroni corrected). No significant relationships were observed between the salience network and scores on the other subscales of the ADOS or ADI.
Discussion
Autism is a complex neurodevelopmental disorder that affects multiple cognitive domains and brain systems. Questions regarding the nature (hypoconnectivity or hyperconnectivity), extent (widespread or focal), and developmental timeframe (childhood or adulthood) of aberrant brain connectivity in autism are hotly debated. We address a critical gap in the literature by examining whole-brain intrinsic functional connectivity in children with ASD and identifying the specific brain network that most successfully discriminates children with ASD from TD peers. We found that childhood autism is characterized by hyperconnectivity of major large-scale brain networks and that the salience network may be a distinguishing feature in children with ASD.
Brain Network Hyperconnectivity in ASD
We used ICA, a data-driven approach for assessing group differences in functional brain networks, to analyze fMRI data from children with ASD and TD children. Across 6 of the 10 brain networks identified in the combined sample, children with ASD exhibited greater functional connectivity than TD children. The salience network, DMN, frontotemporal network, motor network, and visual network all demonstrated greater functional connectivity in children with ASD compared with TD children. The opposite pattern of results (TD greater than ASD functional connectivity) was not observed in any brain region for any of the networks examined. This pattern of results was replicated in an independent cohort of children with ASD and TD children.
The underconnectivity theory of autism posits that the disorder results from reduced functional connectivity between frontal and posterior cortical regions.6,65,66 It is not yet clear how connectivity measures can be affected by methodologic choices.5 In addition, few studies have addressed the question of how the brain is functionally organized in childhood autism, at times more proximal to the onset of the disorder.37 We report hyperconnectivity of several major large-scale brain networks in children with autism. We found that in the salience network, children with ASD showed hyperconnectivity, specifically in the ACC and bilateral insular cortices. This network is involved in interoceptive and affective processes and the identification of relevant internal and extrapersonal stimuli to guide behavior.44,49 We previously hypothesized that atypical functional connectivity of the insula may be part of the neuropathology of ASD.41 It has since been demonstrated that adolescents with ASD show decreased regional homogeneity (a measure of local synchronization of fMRI signal) in the right insula.67 Investigations using region of interest–based approaches have shown decreased resting-state functional connectivity between the insula and ACC in adolescents with autistic disorder.68 The insula-ACC pathway is of particular interest in ASD because it has previously been shown that this link undergoes significant functional and structural changes throughout typical development,46 and functional connectivity along this pathway is related to measures of social responsiveness in typical adults.69 We have proposed that the salience network may serve a general purpose to detect salient endogenous or exogenous events and initiate and mobilize resources for appropriate behavioral responses.49 Dysfunction of this network could in principle lead to some of the core features of ASD, including reduced attention to social stimuli.41
We also found evidence for hyperconnectivity of the posterior DMN in children with ASD. The DMN is involved in many of the processes that are compromised in individuals with autism, including social and interpersonal cognition.70,71 Our current findings contrast with previously published studies21–23,26 of DMN hypoconnectivity in adults and adolescents with ASD, underscoring the critical need for examining brain connectivity in younger children, where a different picture is beginning to emerge. We observed hyperconnectivity of the posterior DMN, which contains regions thought to be involved in episodic memory and self-related processing.72 Aberrant connectivity of the DMN in childhood autism may hinder the development of processes, such as reciprocal social interaction, that require flexible and adaptive responses and integration of current goals with past experience.
Brain hyperconnectivity in ASD was also observed within frontotemporal, motor, and visual networks. The superior temporal sulcus, involved in various aspects of biological motion processing, is a region that has been extensively implicated in ASD for its role in action understanding and social cognition73 and speech perception.74 Dyspraxia and other impairments of motor control have also been reported,75 as well as sensory processing abnormalities.76,77 We observed hyperconnectivity across brain systems supporting several cognitive functions known to be impaired in the complex autistic phenotype.
Taken together, these results suggest that brain network hyperconnectivity is a critical component of the underlying neurobiology of childhood ASD. Our work suggests that the large body of literature describing hypoconnectivity between brain regions in adult ASD may not be representative and obscures the full, complex developmental sequelae of the disorder. The functional implications of hyperconnectivity within brain networks are far-reaching. If enhanced within-network connectivity is characteristic of autism, this may limit dynamic interactions among networks, which are necessary for successful navigation of complex real-world scenarios. Thus, we speculate that network isolation may account for some of the core symptoms of ASD, namely, difficulty with adapting to change, restrictive and repetitive behavior,78 and increased sensitivity to visual, auditory, and tactile stimuli.79
The current results provide hints of an important developmental discontinuity in ASD, the underlying mechanisms of which are currently unknown. Although this idea is speculative, there might be a critical period in development, possibly during puberty, that affects brain organization in ASD differently than in TD children. It has been shown that ovarian hormones enhance both corticocortical and subcorticocortical functional connectivity, whereas androgens decrease subcorticocortical functional connectivity but increase functional connectivity among subcortical brain areas.80 These developmental factors have not yet received adequate attention. Whereas we find hyperconnectivity in 7- to 12-year-old children with ASD, most of the studies in the intrinsic connectivity16–18,26,81 and task-related connectivity literature6–8,10,12 find hypoconnectivity. Because almost all of the existing literature on functional brain connectivity in ASD reports data from adolescents and adults, rather than children with the disorder, it is possible that this critical developmental discontinuity has gone unrecognized.
Network-Based Classification of ASD
The current findings suggest that at earlier ages closer to onset of the disorder, the brain in ASD is largely hyperconnected. In addition, this hyperconnectivity can be quantified in ways that may eventually be used to discriminate children with ASD from TD children. Only one published study39 has evaluated resting-state functional connectivity–based biomarkers. Pairwise functional connectivity measures derived from 7266 regions of interest across gray matter could be used to distinguish adolescents and adults with autism from typically developing individuals. Our results suggest that specific, anatomically and functionally well-characterized brain networks82 contain more information regarding group membership than others. Previous work used measures of brain structure, such as cortical thickness,83 gray matter volume,84 or white matter integrity,85 to attempt to discriminate individuals with ASD from TD individuals with varying degrees of success. Because these studies have all examined adolescents or adults, their utility in identifying brain-based biomarkers in younger children remains unclear. We demonstrate that large-scale brain network connectivity can predict clinical category in children.
Salience Network as a Distinguishing Feature of ASD
Among the 10 networks examined, we found that the salience network had the greatest classification accuracy at 78%, with 75% sensitivity and 80% specificity. Furthermore, the classifier trained on the salience network in the current data set could generalize to discriminate children with ASD from TD children in an independent data set with 83% accuracy, 67% sensitivity, and 100% specificity. The salience network functions to identify relevant internal and extrapersonal stimuli to guide behavior.44 Dysfunction of this system may be part of the underlying neurobiology of autism.41 Increasingly, the anterior insula and ACC, the 2 main nodes of the salience network, are linked to behaviors affected in autism ranging from social perception86,87 to cognitive control.88 The other networks examined produced classification accuracies of 58% to 73%. Thus, although several networks exhibited hyperconnectivity in ASD, some were much more informative than others for providing information that may eventually be used in the development of brain-based biomarkers. The salience network was further linked to ASD symptoms. Information contained within the salience network could predict scores on measures of restricted and repetitive behaviors. The relationship between the salience network and restricted and repetitive behaviors might be due to the more general role of this network in attention allocation to stimuli that is salient to the individual.49 Regions that belong to this network are also involved in the maintenance of task sets during goal-directed behavior,89 an excess of which might contribute to restricted and repetitive behaviors. Thus, our findings link for the first time, to our knowledge, a novel description of the neurobiology of childhood ASD with the identification of a potential brain-based biomarker that is related to the core symptoms of the disorder.
Methodologic Issues
It is encouraging that the only other existing study of classification in autism using resting-state fMRI demonstrated that the brain regions most informative for classification included bilateral anterior insular cortices.39 This result is in line with our finding that the salience network discriminated groups with the highest classification accuracy of all networks examined. The authors of the previous study used a different approach for assessing group differences in functional connectivity, namely, pairwise correlations among regions of interest covering cortical gray matter. Despite considerable methodologic differences and differences in age and sample characteristics, these highly discriminating brain regions in ASD were identified across both studies. The various available methods for processing resting-state fMRI data each have their own strengths and weaknesses, and as a whole these methods can provide complementary information about brain functional organization in neurodevelopmental disorders.90
Limitations
Although the results of brain hyperconnectivity in ASD replicate across 2 independent data sets, a limitation of the current study is the sample size and the relatively high IQ of the children investigated. Future work is necessary to extend these findings to larger sample sizes that include children on both ends of the autism spectrum. The current samples may not be representative of the diagnostic category as a whole in a disorder as heterogeneous as ASD. In addition, some of the older children in the current study may be old enough to be considered adolescents, although data on pubertal stage are not available to verify this. An additional limitation is the fact that it is not possible to know whether children with ASD interpreted the instructions during the resting-state scan differently from TD children. In addition, future studies will need to control for medication effects and effects of comorbidities. Finally, the inclusion of data from children with other developmental disorders will be essential for determining to what extent the classifier developed here can discriminate ASD from other disorders. The current study is a first step toward uncovering distinguishing features in the brains of children with ASD.
In conclusion, characterizing the nature of aberrant brain connectivity in ASD is necessary for understanding its complex behavioral and cognitive phenotype. We demonstrate brain functional hyperconnectivity in childhood ASD and report that this may be a signature of the disorder. We identify the salience network as a candidate biomarker in ASD and provide evidence that atypical connectivity of this network is related to deficits characteristic of the disorder. Future work is necessary for extending this finding to even younger children, with the ultimate goal of developing brain-based biomarkers that may be used to aid diagnosis and guide targeted early intervention.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Institute of Mental Health Career Development Award K01MH092288 (Dr Uddin) and grants from the Singer Foundation, the Stanford Institute for Neuro-Innovation & Translational Neurosciences, and grants DC011095 and MH084164 from the National Institutes of Health (Dr Menon).
Role of the Sponsors: The funding organizations played no role in design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: Portions of these data were presented at the Annual Meeting of the Organization for Human Brain Mapping; June 14, 2012; Beijing, China.
Additional Contributions: Tianwen Chen, PhD, provided assistance with data processing and Maria Barth, BA, Christina Young, BA, Caitlin Tenison, BA, and Sangeetha Santhanam, BA, provided assistance with data collection. Data for the replication analyses were obtained from public databases (http://ndar.nih.gov/ and http://fcon_1000.projects.nitrc.org/indi/adhd200/) and were contributed by F. Xavier Castellanos, MD, and Michael P. Milham, MD, PhD.
Author Contributions: Study concept and design: Uddin, Supekar, Menon.
Acquisition of data: Uddin, Lynch, Khouzam, Phillips.
Analysis and interpretation of data: Uddin, Supekar, Lynch, Feinstein, Ryali, Menon.
Drafting of the manuscript: Uddin, Supekar, Lynch, Khouzam, Menon.
Critical revision of the manuscript for important intellectual content: Uddin, Phillips, Feinstein, Ryali, Menon.
Statistical analysis: Uddin, Supekar, Ryali.
Obtained funding: Uddin, Menon.
Administrative, technical, and material support: Lynch, Khouzam, Phillips, Menon.
Study supervision: Uddin, Phillips, Menon.
References
- 1.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 2.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy Behav. 2007;11(1):20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? a survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 7.Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon M, Ozonoff SJ, Ursu S, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinhans NM, Richards T, Sterling L, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 15.Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104(1):160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 16.Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller RA. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welchew DE, Ashwin C, Berkouk K, et al. Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biol Psychiatry. 2005;57(9):991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Noonan SK, Haist F, Müller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Müller RA. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48(10):2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng SJ, Wiggins JL, Peltier SJ, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Assaf M, Jagannathan K, Calhoun VD, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev. 2011;8(4):410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Monk CS, Peltier SJ, Wiggins JL, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc Cogn Affect Neurosci. 2012;7(6):727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29(23):7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 31.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Stefanatos GA. Regression in autistic spectrum disorders. Neuropsychol Rev. 2008;18(4):305–319. doi: 10.1007/s11065-008-9073-y. [DOI] [PubMed] [Google Scholar]
- 33.Lainhart JE, Piven J, Wzorek M, et al. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 35.Courchesne E, Mouton PR, Calhoun ME, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 36.Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry. 2009;66(4):320–326. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral DG. The promise and the pitfalls of autism research: an introductory note for new autism researchers. Brain Res. 2011;1380:3–9. doi: 10.1016/j.brainres.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee PS, Yerys BE, Della Rosa A, et al. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 2009;19(8):1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JS, Nielsen JA, Froehlich AL, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(pt 12):3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33(8):1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 43.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 44.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput Biol. 2012;8(2):e1002374. doi: 10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 51.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 52.Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 53.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 54.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging. 2012;33(4):828.e819–828.e830. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jolles DD, van Buchem MA, Crone EA, Rombouts SA. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21(2):385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 61.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 63.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Paakki JJ, Rahko J, Long XY, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 68.Ebisch SJ, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32(7):1013–1028. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Martino A, Shehzad Z, Kelly C, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166(8):891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 71.Uddin LQ. The self in autism: an emerging view from neuroimaging. Neurocase. 2011;17(3):201–208. doi: 10.1080/13554794.2010.509320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 73.Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008;32(1):123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 75.MacNeil LK, Mostofsky SH. Specificity of dyspraxia in children with autism. Neuropsychology. 2012;26(2):165–171. doi: 10.1037/a0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69(5 pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 78.Leekam SR, Prior MR, Uljarevic M. Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychol Bull. 2011;137(4):562–593. doi: 10.1037/a0023341. [DOI] [PubMed] [Google Scholar]
- 79.Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135(pt 9):2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 83.Ecker C, Marquand A, Mourão-Miranda J, et al. Describing the brain in autism in five dimensions: magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci. 2010;30(32):10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uddin LQ, Menon V, Young CB, et al. Multivariate searchlight classification of structural magnetic resonance imaging in children and adolescents with autism. Biol Psychiatry. 2011;70(9):833–841. doi: 10.1016/j.biopsych.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lange N, Dubray MB, Lee JE, et al. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3(6):350–358. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masten CL, Colich NL, Rudie JD, Bookheimer SY, Eisenberger NI, Dapretto M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Dev Cogn Neurosci. 2011;1(3):260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bolling DZ, Pitskel NB, Deen B, et al. Enhanced neural responses to rule violation in children with autism: a comparison to social exclusion. Dev Cogn Neurosci. 2011;1(3):280–294. doi: 10.1016/j.dcn.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52(1):336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.