Abstract
The function of cartilage in the adult is dependent upon a host of regulatory molecules such as growth factors, extracellular matrix, enzymes, signaling molecules and transcription factors. However, germline mutations in some genes that are expressed in adult cartilage lead to embryonic or perinatal lethality. To examine the function of these and other genes postnatally, we have generated a targeted mouse by homologous recombination that “knocks in” the inducible Cre recombinase construct, CreERT2, in the 3′ untranslated region (3′UTR) of the endogenous mouse aggrecan gene (Agc1tm(IRES-creERT2)). The properties and efficiency of the inducible cre recombinase were tested by examining X-gal staining of tissues from embryos as well as growing and adult Agc1tm(IRES-creERT2)/+; Rosa 26R mice. These mice were injected with the inducer, tamoxifen, at different time points during embryonic development and postnatally up to 6 months of age. Strong X-gal staining was observed in growth plate and articular cartilage as well as the fibrocartilage of meniscus, trachea, and intervertebral discs reproducing the pattern of endogenous aggrecan gene expression. In conclusion, we have generated a mouse model in which genes implicated in cartilage degenerative diseases can be inactivated in a spatial and temporal fashion in postnatal and adult mice.
Bone formation occurs through two distinct processes. Most of the skeleton is formed by endochondral ossification, a process that involves a cartilage intermediate. A limited number of mainly craniofacial bones are formed by intramembraneous ossification, a process by which bones form directly from mesenchymal condensations without a cartilage intermediate. Formation of the endochondral cartilage skeleton includes processes of proliferation as well as cell fate determination leading to the differentiation of chondrocytes, the only cell type in cartilage. These processes are orchestrated by several transcription factors and a number of signaling molecules (Karsenty, 2008; Kronenberg, 2006; Nakashima and de Crombrugghe, 2003). The function of many molecules involved in the formation of the endochondral skeleton has been elucidated by the identification and characterization of mutant genes responsible for human chondrodysplasias and also by the generation and characterization of mutant mice in which the genes for these proteins were experimentally manipulated.
Chondrocytes also constitute the sole cell type in articular cartilage, a tissue that covers the extremities of bones in the adult skeleton. These cells are also a major cell type in the intervertebral disc, a tissue that separates and cushions the individual skeletal elements in the vertebral column. Degenerative diseases of these tissues, including osteoarthritis and degenerative disc disease (Zhang et al., 2008), affect an important portion of the aging human population. To better understand the functions of specific extracellular and regulatory proteins in both articular cartilage and intervertebral discs of mice, in vivo, it is desirable to mutate the genes for these proteins in these tissues postnatally.
The conditional inactivation of genes in the adult mouse has become possible by the advent of the Cre-loxP recombination system (Lakso et al., 1992; Orban et al., 1992). The success of the Cre-loxP system is dependent upon two components – the conditional allele and the Cre driver. The conditional allele is typically generated by homologous recombination in mouse embryonic stem (ES) cells and hence is a targeted mouse mutation. The conditional allele harbors loxP recognition sites flanking critical exon(s) and/or regulatory elements of the gene of interest. Secondly, a transgenic mouse, typically referred to as a Cre driver, is generated in which the expression of cre recombinase is controlled by tissue specific regulatory elements. To more precisely control the timing of gene activation a fusion polypeptide between Cre recombinase and a mutant ligand binding domain of the estrogen receptor or CreERT2 has also been generated (Indra et al., 1999; Leone et al., 2003). In mice harboring the CreER transgene in their genome, the administration of tamoxifen activates the cre recombinase by translocation from the cytosol into the nucleus. Consequently, the excision of loxP containing alleles by the cre recombinase enzyme at specific times either during embryonic development or in adult animals is dependent upon the administration of the estrogen analog, tamoxifen (Hayashi and McMahon, 2002).
Aggrecan is a major extracellular matrix (ECM) protein of both growth plate and articular cartilage. In the latter tissue, the aggrecan gene is expressed more robustly than the gene for type II collagen, another cartilage ECM component, in adult mice (Chambers et al., 2002). Because transgenes may lack important regulatory elements or may lose their transcriptional activity postnatally (Pedram et al., 2006; Williams et al., 2008), we have introduced through homologous recombination in mouse embryonic stem cells a tamoxifen-inducible CreERT2 cassette in the 3′ untranslated region (3′UTR) of the endogenous aggrecan gene creating Aggrecan-CreERT2 mice. We report here that in offspring of crosses between and Aggrecan-CreERT2 and Rosa-26 reporter mice (R26R) (Soriano, 1999b), tamoxifen administration causes very efficient Cre reporter expression in the hyaline cartilage of the growth plate and articular cartilage as well as the fibrocartilage of the meniscus, trachea, and intervertebral disks of growing and adult mice.
We generated a targeted or “knock-in” allele in mouse ES cells in which an inducible Cre recombinase construct (CreERT2) is integrated in the 3′ UTR of the endogenous mouse aggrecan gene (Fig. 1). The construct consists of a 5′ internal ribosome entry sequence (IRES) followed immediately by the CreERT2 open reading frame (ORF) with a SV40 polyadenylation (pA) sequence. The IRES-CreERT2-pA construct is inserted 63 nucleotides downstream of the stop codon of the endogenous mouse aggrecan gene. Consequently, the aggrecan mRNA transcribed from the targeted allele incorporates the nucleotide sequence of IRES-creERT2 forming a bicistronic mRNA. The construct does not replace any nucleotide sequences at the endogenous aggrecan 3′ UTR and hence is an insertional mutation. The targeted mutation at the 3′ UTR of aggrecan is henceforth referred to as Agc1tm(IRES-CreERT2).
Figure 1.
Gene-targeting strategy for the Aggrecan-CreERT2 allele. Top panel displays the genomic structure of the mouse aggrecan gene. Middle panel shows the targeting vector. Bottom Panel depicts the targeted allele whereby IRES-CreERT2 and an FRT-flanked Pgk-Neo cassette (in reverse transcriptional orientation to aggrecan) is recombined into the 3′ untranslated region (3′ UTR) of the aggrecan gene. EcoRV restriction enzyme digestion is used in Southern blotting analysis to identify correctly targeted ES cell cones. Both the 5′ and 3′ probes (position shown in the middle panel) detect a 13.5 kb fragment with the wild-type allele. The targeted allele generates a 5.4 or 9.6 kb fragment detected by the 5′ or 3′ probe, respectively. At the bottom of the panel a targeted ES clone (3H11) is shown to the left in a Southern blot hybridized with the radiolabeled 5′ probe. The Southern blot of the 3′ probe with the same clone is shown to the right. RV: EcoRV sites. TK: MC1-TK. Neo: Pgk-Neo. FRT: FRT sites.
All heterozygous Agc1tm(IRES-creERT2)/+ mice were analyzed on a 129S6 ×C57BL/6 mixed genetic backround. They are healthy, grow normally, and are not smaller than wild-type littermates. Mice homozygous for the targeted allele (Agc1tm(IRES-CreERT2/IRES-CreERT2) are viable and fertile. In all experiments described in this paper, we used heterozygous Agc1tm(IRES-creERT2)/+ mice. To test the inducible properties of the Cre recombinase, male Agc1tm(IRES-creERT2)/+ mice were bred to female Rosa26 Cre reporter (R26R) mice (Soriano, 1999a). Pregnant mice were injected intraperitoneally once with the inducer, 4OH-tamoxifen (3mgs per female) in separate experiments at different time points during pregnancy, and sacrificed 24 hours later. The presence of Cre activity was monitored by detection of LacZ expression due to the excision of a transcriptional stop cassette flanked by LoxP sites located in the ROSA26 reporter locus resulting in LacZ expression (Soriano, 1999a). The Agc1tm(IRES-creERT2)/+; R26R embryos at embryonic day 12.5 (E12.5) harvested from the female injected with 4OH-tamoxifen at 11.5 days were X-gal negative. Positive X-gal cells were first detected in the forelimb (Fig. 2A) of E13.0 Agc1tm(IRES-creERT2)/+; R26R embryos harvested from pregnant mothers injected with 4OH-tamoxifen at E12.0. Strong X-gal staining was detected in the cartilage of the appendicular and axial skeleton of E13.5 embryos collected from females injected with tamoxifen at E12.5. To test for the possibility of leaky cre recombinase activity in the absence of the inducer, a control experiment was performed in which a pregnant mother was injected with the vehicle alone, oil. All E15.5 Agc1tm(IRES-creERT2)/+; R26R embryos stained with X-gal harvested from the female injected with oil alone at E14.5 were X-gal negative (Fig. 2B). On the other hand, in all E15.5 Agc1tm(IRES-creERT2)/+; R26R embryos stained with X-gal harvested from a different female injected with 4OH-tamoxifen at E14.5, all developing cartilages were X-gal positive (Fig. 2B). In a separate experiment, postnatal day 2 (P2) pups from a pregnant mother that had been injected with 4OH-tamoxifen one day prior to delivery were X-gal positive in all cartilage structures (Fig. 2C). Positive X-gal staining was observed in the proliferating and hypertrophic chondrocytes of a histological section of the ulna and radius growth plate from the E15.5 Agc1tm(IRES-creERT2)/+; R26R embryo harvested from the tamoxifen injected mother (Fig 2D). However, a RNA in situ hybridization experiment with a digoxigenin labeled aggrecan probe revealed that aggrecan mRNA was detected in the proliferating chondrocytes, but not in the hypertrophic chondrocytes of the ulna growth plate in an E15.5 Agc1tm(IRES-creERT2)/+; R26R embryo harvested from an oil injected mother (Fig 2E).
Figure 2.

Pregnant mothers were injected intraperitoneally once with 4OH-tamoxifen (total of 3mgs injected per mother) or vehicle control (oil). Females were sacrificed 24 hours later, and the embryos were collected and stained with X-gal. All embryos in the figure are genotype Agc1tm(IRES-CreERT2/+); R26R. A: No X-gal positive signal was detected at embryonic day 12.5 (E12.5), but X-gal positive signal was detected in the forelimb at E13.0 (arrow). At E13.5, signal was detected in the cartilage of the axial and appendicular skeleton. B: Left, Agc1tm(IRES-CreERT2/+); R26R E15.5 embryo harvested from a vehicle control (oil) injected mother and stained with X-gal; right, E15.5 embryo of the same genotype obtained from a different mother injected with 4OH-tamoxifen and stained with X-gal. Positive X-gal staining is detected in cartilage only in the embryo harvested from the 4OH-tamoxifen injected female. C: Dame injected one day prior to delivery, and pups were sacrificed at postnatal day 2 (P2). A P2 Agc1tm(IRES-CreERT2/+); R26R pup whose skin had been removed, was stained with X-gal showing positive signal in cartilage structures. D: Histological section of an X-gal stained ulna and radius growth plate from an E15.5 Agc1tm(IRES-CreERT2/+); R26R embryo harvested from a tamoxifen injected mother. Positive X-gal staining is detected in both the proliferating and hypertrophic chondrocytes. E: Histological section of an ulna from an E15.5 Agc1tm(IRES-CreERT2/+); R26R embryo harvested from an oil injected mother that was subjected to RNA in situ hybridization with a digoxigenin labeled aggrecan mRNA probe. Aggrecan mRNA was in detected in the proliferating chondrocytes of the ulna growth plate but was not detected in the hypertrophic chondrocytes.
Six-month-old Agc1tm(IRES-creERT2)/+;R26R mice were injected starting with three separate injections of either vehicle alone or tamoxifen (1.5 mg of tamoxifen/10 gm of animal weight) for three consecutive days, and sacrificed three days later. Tissues harvested from these mice revealed robust X-gal staining in multiple cartilaginous tissues. To improve penetration of X-gal, the skin, muscle, and other soft tissues were removed from the hindlimbs thus exposing the tibia-femoral joint. The joint from the 6-month-old Agc1tm(IRES-creERT2)/+; R26R joint injected with oil is shown from a lateral perspective at the top of Fig. 3A. The bottom two joints from 6-month-old Agc1tm(IRES-creERT2)/+; R26R mice injected with tamoxifen (Fig. 3A) exhibit intense X-gal staining in the growth plate and the articular cartilage. In Fig. 3B, right, the same bones are shown from a frontal view and display strong X-gal staining in the growth plate of the tibia, articular cartilage of tibial plateau, and femoral condyle, as well as the fibrocartilage of the meniscus. The bones from the oil injected mouse are X-gal negative and are shown in Fig. 3B.
Figure 3.

Tissues harvested from 6-month-old Agc1tm(IRES-CreERT2/+); R26R mice that were injected intraperitoneally with either tamoxifen (1.5 mgs tamoxifen/10 gms of mouse weight) or vehicle alone (oil), starting at 6 months for 3 consecutive days and sacrificed 3 days later. All tissues were mildly fixed and bathed in X-gal solution. A: Lateral view of the knee joints from two animals injected with tamoxifen (bottom two joints of panel) showing X-gal staining in the growth plate (*) and articular (arrow to tibial plateau) cartilage, but no positive X-gal staining is detected in the knee joint from the animal injected with oil (top of panel). B: Top view of tibia and femur from panel A showing the mouse tissue from oil injected mouse to the left, and tamoxifen injected mouse to the right. Positive X-gal staining is detected in the growth plate (*), articular cartilage of femoral condyle (arrow head), and tibial plateau (arrow), as well as the meniscus (“m”) from the tamoxifen-injected mouse. C: Lateral view of two ankles with skin and muscle removed (left, oil injected, -; right, tamoxifen, +). The positive X-gal staining at the tendon-bone junction is labeled with arrow. D: Dissected and cut trachea from a tamoxifen injected animal. E: Top view of rib cage from tamoxifen injected mouse bathed in X-gal (rib cartilage, arrow; trachea, arrow head; spine, asterisk). F: Cranial bones covering the skulls of either oil injected (left) or tamoxifen injected (right) animals were removed to expose the brain to X-gal solution. No X-gal staining was detected at the surface of the brain from the tamoxifen-injected animal, even though the nasal cartilage from the tamoxifen-injected animal was X-gal positive (right, arrow, +).
The inducible, cre recombinase activity from the aggrecan knockin allele is not restricted to hyaline cartilage of the growth plate and articular cartilage. Indeed, at the junction of the tendon with bone in the ankle region, localized X-gal staining is observed in tamoxifen injected 6-month-old Agc1tm(IRES-creERT2)/+; R26R mice (Fig. 3C); whereas, no positive is seen from the oil injected control littermate (Fig. 3C). Likewise, the fibrocartilage of the trachea from tamoxifen-injected 6-month-old Agc1tm(IRES-creERT2)/+; R26R mice is strongly X-gal positive (Fig. 3D). The cartilage of rib, trachea, and spine are also X-gal positive in the tamoxifen-injected 6-month-old Agc1tm(IRES-creERT2)/+; R26R mice (Fig. 3E). Since aggrecan has been reported to be expressed in the mouse brain (Bruckner et al., 2003; Costa et al., 2007), we removed skull bones to facilitate penetration of X-gal. However, no X-gal positive cells were observed at the surface of the brain, even though nasal cartilage was X-gal positive (Figure 3F). Histological sections of the brain also did not reveal X-gal positive cells (data not shown). However, we did not investigate the X-gal staining of the brain at several time points during development and postnatal growth. Similarly, the liver, kidney, and heart were negative for X-gal (data not shown).
The X-gal staining of knee joints was further investigated by histological sections. Two-week-old Agc1tm(IRES-creERT2)/+; R26R mice were injected once with oil and sacrificed three days later. In histological sections of these joints, no X-gal positive cells were observed in the growth plate and articular cartilage (Fig. 4A, B). Additionally, the mRNA expression pattern of aggrecan was examined by histological sections of uninjected 2.5-week-old animals by RNA in situ hybridization with an aggrecan digoxigenin probe. Aggrecan mRNA was detected in chondrocytes in the resting zone, proliferative zone, and higher hypertrophic zone (Fig. 4C). However, aggrecan mRNA was not detected in many chondrocytes in the lower hypertrophic zone. In histological sections of joints of tamoxifen-injected two-week-old Agc1tm(IRES-creERT2)/+; R26R mice sacrificed 3 days later, positive X-gal staining was observed in most of the chondrocytes in the growth plate, including the lower hypertrophic zone and the deep layers of the articular cartilage (Fig. 4D, E). Likewise, in sections of knee joints from four-week-old Agc1tm(IRES-creERT2)/+; Rosa26 mice injected once with tamoxifen and sacrificed three days later, the chondrocytes of the growth plate and articular cartilages intensely stained for X-gal (Fig. 4F), including all hypertrophic chondrocytes of the growth plate (Fig. 4G). However, in the articular cartilage, the positive X-gal staining of chondrocytes is largely restricted to the chondrocytes above the tidemark, a virtual line that separates the superficial non-mineralized cartilage from the deeper mineralized cartilage (Fig. 4H), and very few X-gal positive chondrocytes are observed in the zone of calcified cartilage below the tidemark.
Figure 4.

Histological sections of the knee joint from Agc1tm(IRES-CreERT2/+); R26R mice mouse injected with either tamoxifen or vehicle alone (oil) and stained with X-gal. A: Histological section of the knee joint from a 2.5 week old Agc1tm(IRES-CreERT2); Rosa26 mouse injected once with oil at 2 weeks and stained with X-gal. B: View of the articular cartilage in Panel A. No positive X-gal staining is observed in the growth plate or the articular cartilage. C: RNA in situ hybridization of a histological section with an aggrecan labeled digoxigenin probe showing the growth plate of an uninjected 2.5 Agc1tm(IRES-creERT2/+); R26R mouse. Aggrecan mRNA is detected in the reserve zone (RZ), the proliferative zone (PZ) and the upper hypertrophic zone (HZ). However, aggrecan mRNA is not detected in a large number of hypertrophic chondrocytes in the lower hypertrophic zone (arrows). D: Histological section of the knee joint from a 2.5 week old Agc1tm(IRES-CreERT2); R26R mouse injected once with tamoxifen at 2 weeks and stained with X-gal. Positive X-gal staining is observed in the resting, proliferative, and hypertrophic zones of the growth plate. E: View of panel D showing positive X-gal staining in all of the chondrocytes of the articular cartilage including the deep layers. F: Histological section of the knee joint from a 4.5 week old Agc1tm(IRES-CreERT2); R26R mouse injected once with tamoxifen at 4 weeks and stained with X-gal. G: View of Panel F focusing on the growth plate showing positive X-gal staining for over 90% of growth plate chondrocytes including all hypertrophic chondrocytes. H: View of Panel F focusing on the articular cartilage showing high number of X-gal positive chondrocytes in the uncalcified cartilage above the tidemark, but very little X-gal positive chondrocytes in the calcified cartilage below the tidemark (labeled as asterisk). Scale bar is 200 microns.
In adult 6-month old Agc1tm(IRES-creERT2)/+; R26R mice, the mRNA expression pattern of aggrecan was investigated by RNA in situ hybridization of histological sections of the knee joint. Aggrecan mRNA was detected in chondrocytes of the growth plate (Fig. 5A) and the chondrocytes of the uncalcified articular cartilage above the tidemark (Fig. 5A), but not in the chondrocytes of the calcified cartilage below the tidemark. The aggrecan mRNA was only detected in the superficial layer of the meniscus.
Figure 5.

Histological sections of 6 month-old Agc1tm(IRES-creERT2/+); R26R knee joints. A: Histological section of the knee joint from a 6-month-old mouse hybridized with an antisense aggrecan digoxigenin labeled RNA probe. The aggrecan mRNA is detected in the growth plate cartilage (GP). In the articular cartilage (AC) overlying the secondary ossification center (SO), the aggrecan mRNA is detected in the chondrocytes of the uncalcified cartilage above the tidemark (see arrow), but not in the chondrocytes of the calcified cartilage below the tidemark. In the meniscus (ME), the aggrecan mRNA is detected only in the outer superficial chondrocytes of the meniscus (white arrowhead). B: The growth plate from a histological section of a 6 month-old Agc1tm(IRES-CreERT2); R26R mouse injected with tamoxifen and stained with X-gal and counterstained with nuclear fast red. Positive X-gal staining is detected in the chondrocytes of the growth plate. C: Histological section of the knee joint from a tamoxifen-injected 6-month-old Agc1tm(IRES-CreERT2); R26R stained with X-gal. Positive X-gal stained chondrocytes are observed in the uncalcified cartilage above the tidemark (see arrow), but not in the chondrocytes of the calcified cartilage below the tidemark. In the meniscus (ME), positive X-gal stained chondrocytes are only detected in the outer superficial chondrocytes of the meniscus (arrowhead). D: Histological section of the knee joint showing both the tibial and femoral articular cartilages from a 6 month-old Agc1tm(IRES-creERT2/+); R26R mouse injected with tamoxifen and stained with X-gal. X-gal positive chondrocytes are seen in the uncalcified cartilage above the tidemark (shown as curved black line), but not detected in the calcified cartilage (shown as asterisk). E: Histological section of the knee joint stained with X-gal from a 6 month-old Agc1tm(IRES-creERT2/+); R26R control mouse injected with vehicle alone (oil). No X-gal staining was observed in the cartilage.
The knee joints from tamoxifen-injected 6-month-old Agc1tm(IRES-creERT2)/+; R26R mice shown in Fig. 3A were sectioned for histology and chondrocytes of the growth plate (Fig. 5B), articular cartilage (Fig. 5C, D), as well as the meniscus (Fig. 5C) were X-gal positive; whereas, oil injected animals were negative for X-gal staining (Fig. 5E). The articular cartilage in the joints of tamoxifen-injected animals exhibits robust X-gal staining in chondrocytes of uncalcified cartilage (Fig. 5C), but not in the region of calcified cartilage. In the meniscus, X-gal staining is restricted to the superficial layers of the fibrocartilage (Fig. 5C). A magnified view of a section showing the tibial and femoral articular cartilage reveals a lack of X-gal stained chondrocytes below the tidemark. Therefore, the X-gal staining pattern of chondrocytes from a 6-month-old Agc1tm(IRES-creERT2)/+; R26R mouse injected with tamoxifen is similar to the mRNA expression pattern of aggrecan, indicating that the failure to activate cre recombinase in the calcified region of the articular cartilage and the deep layers of the meniscus cannot be explained by the lack of penetration of tamoxifen into calcified articular cartilage or the deep layers of the meniscus.
The spines dissected from 6-month-old Agc1tm(IRES-creERT2)/+; R26R mice injected either with oil or tamoxifen for three consecutive days, and sacrificed three days later, were stained with X-gal. A strong X-gal positive signal was observed in the vertebral column from the tamoxifen-injected animal, but not in the oil injected animal (Fig. 6A). Histological sections from the vertebral column of the tamoxifen injected animals revealed strong X-gal staining in the growth plate chondrocytes of the vertebral body, and both the nucleus pulposus and annulus fibrosis of the intervertebral disc (Fig. 6B). A magnified view of the cells embedded in the fibrocartilage rings of the annulus fibrosis and the growth plate chondrocytes of the vertebral body displayed intense X-gal staining (Fig. 6C).
Figure 6.
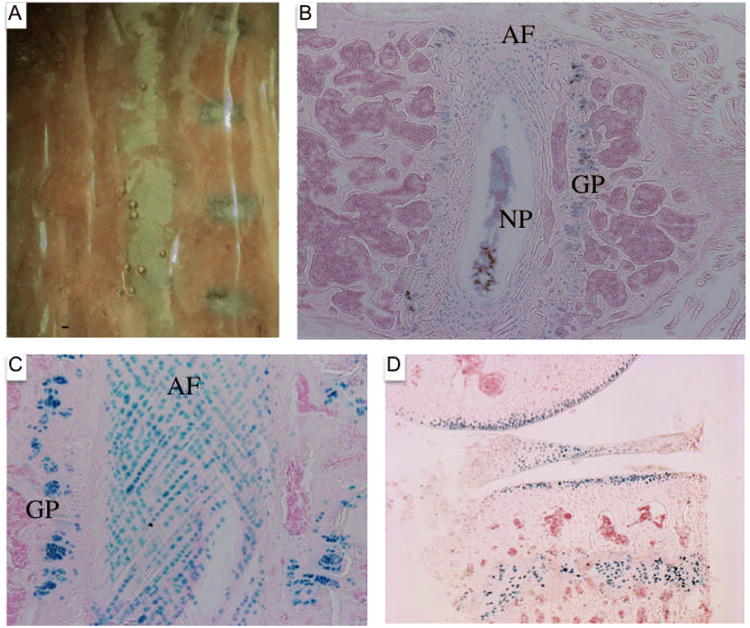
The vertebral columns from tamoxifen and oil injected 6-month-old Agc1tm(IRES-creERT2/+); R26R mice were isolated and stained with X-gal. A: Vertebral column from oil injected mouse to the left and from tamoxifen injected animal to the right. B: Histological section of the vertebral column from the tamoxifen-injected 6-month-old Agc1tm(IRES-CreERT2); R26R mouse stained with X-gal and counterstained with nuclear fast red. Positive X-gal staining is detected in the growth plate (GP) of the vertebral body, the nucleus pulposus (NP), and the rings of the annulus fibrosis (AF). C: Positive X-gal staining of cells contained in the rings of the annulus fibrosis (AF) and the chondrocytes of the growth plate (GP) in the vertebral body. D: Histological section of the knee joint from a 1-year-old Agc1tm(IRES-CreERT2); R26R mouse injected with tamoxifen starting at one year and sacrificed one week later. The X-gal stained joint showed positive signal in the growth plate, articular cartilage, and the meniscus.
Furthermore, histological sections from the knee joint of a 1-year-old Agc1tm(IRES-creERT2)/+; R26R mouse (Fig. 6D) injected with tamoxifen for three consecutive days and sacrificed one week later revealed positive X-gal staining in the growth plate cartilage, articular cartilage, and meniscus in a manner similar to the X-gal stained section from the joint of the 6-month-old Agc1tm(IRES-creERT2)/+; R26R (Fig. 5). This indicates that Cre-loxP mediated gene excision continues to be very active in cartilages of one-year-old mice.
We have generated mice in which Cre recombinase can be activated in chondrocytes at different times after birth by administration of tamoxifen. In these mice, a Cre-ERT2 cassette was introduced by homologous recombination in the 3′ UTR of the aggrecan gene. As determined by X-gal staining of tissues, the excision of the stop cassette flanked by loxP sites in the Rosa 26R reporter locus was highly specific and efficient in postnatal mice at 6 and 12 months of age in articular chondrocytes, in growth plate chondrocytes and in fibrocartilage cells of the meniscus and of the nucleus pulposus and annulus fibrosis of intervertebral disks. The excision was also very efficient at different times during embryonic development and during the earlier postnatal growth period.
Cre activity faithfully reproduced the pattern of expression of the endogenous aggrecan gene in growth plate and articular cartilage as well as in fibrocartilage. As expected CreER was produced only in cells expressing aggrecan RNA and as long as the aggrecan gene was expressed. In two different transgenic mice lines producing CreER under the control of Col2a1 regulatory elements, Cre recombinase activity was progressively lost postnatally (Grover and Roughley, 2006; Nakamura et al., 2006).
At embryo stage 15.5, aggrecan mRNA was detected by RNA in situ hybridization experiments in the proliferating chondrocytes of the ulna growth plate but was not observed in the hypertrophic chondrocytes. Nevertheless, positive X-gal staining was observed in both the proliferating and hypertrophic chondrocytes of the ulna from an E15.5 Agc1tm(IRES-creERT2)/+; R26R embryo harvested from a tamoxifen injected mother. Postnatally, at two and half weeks of age, aggrecan mRNA was detected in the resting, proliferative, and upper hypertrophic zones of the growth plate, but not in the lower hypertrophic zone. The aggrecan mRNA expression pattern closely parallels with the X-gal staining pattern observed in chondrocytes of the growth plate of tamoxifen-injected 2.5-week-old Agc1tm(IRES-creERT2)/+; Rosa26 mice with one exception. The positive X-gal staining observed in the hypertrophic chondrocytes of the ulna from an E15.5 Agc1tm(IRES-creERT2)/+; R26R embryo harvested from the tamoxifen injected mother as well as the lower hypertrophic zone of the tamoxifen-injected 2.5-week-old Agc1tm(IRES-creERT2)/+; Rosa26 mice is due to the stability of the beta-galactosidase protein and the progression of cells, in which the Rosa26 stop cassette is deleted, into the more terminally differentiated chondrocytes. Nevertheless, the Agc1tm(IRES-creERT2)/+ mice can be used for the activation of cre recombinase in the resting, proliferative, and upper hypertrophic regions of the growth plate in mice of the early postnatal period. However, in the articular cartilage of one-month old Agc1tm(IRES-creERT2)/+ mice, the activation of cre recombinase is limited to the uncalcified region of the articular cartilage above the tidemark. We speculate that the lack of X-gal staining in the deeper hypertrophic zone of articular cartilage is due the slower progression of cells from overtly differentiated chondrocytes into more terminally differentiated cells than in the growth plate. Alternatively, the beta-galactosidase could turn over more rapidly in articular than in growth plate chondrocytes.
In several other previous transgenic mice lines inducible cre recombinase activity was detected in cartilage of postnatal animals. In one of these, in which the regulatory elements of a 3 kb promoter and 3 kb first intron of Col2a1 (Zhou et al., 1995) were driving the doxycycline responsive system, inducible cre recombinase activity was observed in articular and growth plate cartilage of one-month old mice but no longer at later timepoints (Grover and Roughley, 2006). In a second transgenic mouse, in which CreERT1 expression was directed by a minimal promoter and an enhancer element of Col2a1 (Zhou et al., 1995), tamoxifen induced cre recombinase activity was detected in articular cartilage of postnatal 3-week old animals but this activity decreased progressively with age and was no longer detected in 3-month old animals (Nakamura et al., 2006). In a third transgenic line (Chen et al., 2007; Zhu et al., 2008a), in which promoter and enhancer elements of Col2a1 were driving expression of CreERT2, efficient tamoxifen-induced cre recombination activity was demonstrated in articular chondrocytes of adult 6 month-old animals, but the Cre recombinase activity was much less efficient in growth plate cartilage (Zhu et al., 2008b). A previous study of mouse knee joints (Chambers et al., 2002) showed that endogenous Col2a1 mRNA was detected in the growth plate by in situ hybridization but not in articular cartilage or in the meniscus of adult mice ranging from 7-10 months of age. Importantly, in the same experiments aggrecan mRNA was detected in the articular, growth plate and meniscus cartilage of these adult mice, indicating that the aggrecan gene continues to be expressed in articular cartilage of adult mice at a time when Col2a1 RNA is no longer detectable. The discrepancy between the expression of Col2a1 mRNA in adult mice and that of the Col2a1- CreERT2 transgene in the third transgenic mice line might be a consequence of the transgene integration site.
Regardless of the differences in the pattern of Cre activity amongst the different Col2a1-CreER transgenic mice, the generation of Aggrecan-CreERT2 mice, in which CreERT2 expression very closely parallels expression of the endogenous aggrecan gene, should provide a very valuable tool to examine the role of specific proteins in the maintenance of growth plate and articular cartilage as well as of intervertebral disks at different timepoints after birth in intact animals. These proteins include regulatory molecules such as key transcription factors, signaling molecules and extracellular matrix proteins. We hypothesize that the characterization of mice harboring the aggrecan-CreERT2 in combination with conditional alleles for these different proteins will provide new rational models for human degenerative cartilage diseases and will help us dissect the in vivo mechanisms that govern the renewal, stability, and integrity of the complex, multicomponent extracellular matrix of different cartilages.
Methods
Generation of Agc1tmIRES-creERT2 mice
The targeting vector plasmid for the Aggrecan knockin mice contains a 2.4 kb 5′ homologous arm, an IRES-CreERT2 cassette followed by a Pgk-neomycin (neo) cassette flanked with FRT sites, a 3.7 kb 3′ homologous arm, and a MC1-thymidine kinase cassette outside of the homology (Indra et al., 1999; Mansour et al., 1988). The homologous arms were generated by PCR amplification using BAC template DNA from a clone isolated by a screening of a RPCI-22 129S6 Mouse BAC library (BAC PAC Resources). The nucleotide sequence of the PCR products were sequenced, and compared to sequences deposited in the mouse genome database using the BLAT algorithm (Kent et al., 2002). In the targeting vector, the neo cassette is in the reverse transcriptional orientation to the IRES-CreERT2 cassette. G4 ES cells (George et al., 2007) were electroporated with 25 micrograms of linearized targeting vector and placed under positive and negative selection according to standard procedures. Correctly targeted ES cell clones were screened by Southern blotting with flanking 5′ and 3′ probes. Chimeras generated by injection of C57BL/6 blastocysts were bred to C57BL/6 mice and all of the analysis was conducted in a mixed 129S6 × C57BL/6 genetic backround. Heterozygous Rosa26 reporter mice (Soriano, 1999b) were bred to heterozygous aggrecan knockin mice (Agc1tm(IRES-creERT2)/+) and tissues from resulting progeny containing one copy of the R26R allele and the Aggrecan knockin allele were stained with X-gal (Gold Bio Technology). We used heterozygous Agc1tm(IRES-creERT2)/+ mice for all experiments, and the Frt-flanked Neo cassette was not removed.
Mouse genotyping
Genomic DNA was extracted using boiling alkaline lysis. The aggrecan-CreERT2 allele was detected with a CRE genotyping assay, and the R26R allele was detected with a LacZ genotyping assay. The CRE and LacZ genotyping assays were performed separately using fluorogenic 5′ exonuclease assays. TaqMan® GeneExpression Master Mix (2X-Applied Biosystems) was used along with primers (CRE For: 5′TGGGCCAGCTAAACATGCTT3′, CRE Rev: 5′AACAGCATTGCTGTCACTTGGT3′, LacZ For: 5′ATCGCCCTTCCCAACAGTT3′, LacZ Rev: 5′GCCGGAAACCAGGCAAA-IDT) and dye-labeled probe (CRE: 6FAM-GTCGGTCCGGGCTGCC-TAMRA, LacZ: 6FAM-CCA TTC GCC ATT CAG GCT GCG-TAMRA-Applied Biosystems) at a final volume of 10 μl. Concentrations of primers for CRE and LacZ were 0.8 μM and probe concentrations were 0.05 μM. The reaction mixture also contained endogenous mouse goosecoid gene control primers and probes (For: 5′CGGCACCGCACCATCT3′, Rev: 5′TCGTCTCCTGGAAGAGGTTCC-IDT and Probe: 5′VIC-CCGATGAGCAGCTCG-3′MGBNFQ-Applied Biosystems) at concentrations of 0.8 μM for primers and 0.1 μM for probe, multiplexed with the gene of interest. The assays were performed on an Applied Biosystems 7900HT Fast Real-Time PCR System and the data analyzed using the 7900HT system software. Genotyping was performed by the University of Texas MD Anderson Cancer Center DNA Analysis Facility.
Administration of 4OH-tamoxifen and tamoxifen
4-hydroxytamoxifen (Sigma, H7904) was dissolved in ethanol, emulsified in sunflower oil (Sigma Catalog, S5007) and then sonicated for 10 minutes at a concentration of 10 mg/ml. For pregnant mothers, 4OH-tamoxifen was used at a final dose of 3 mg per female (mother weighed 30 gms) and intraperitoneal injections were performed 24 hours prior to sacrificing the animals. Embryos were harvested from mothers, and processed for X-gal staining. For all other injections, tamoxifen (Sigma, T5648) was dissolved in ethanol and emulsified in sunflower oil at a concentration of 30 mg/ml. All animals were injected intraperitoneally with 3 mg of tamoxifen per 10 gm of animal body weight. Mice aged 6-months or older were injected with lower doses of 1.5 mg of tamoxifen per 10 gm of animal body weight for three consecutive days.
Histology
All animals were euthanized according to protocols approved by the Institutional Animal Care and Use Committee (IACUC). Postnatal tissue was fixed for 2 hours in a 2.5% formalin solution (diluted from 10% Phosphate Buffered Formalin, Fisher Scientific, SF100-20) and subjected to X-gal staining until appropriate color development and later postfixed overnight in 10% buffered formalin. Tissues containing bones were decalcified for 1-3 weeks in 0.5 M EDTA solution prior to embedding in paraffin (Paraplast Plus, McCormick Scientific). After decalcification, all tissues were dehydrated, immersed in 100% isopropanol, 50% isopropanol/50% molten paraffin, and two series of molten paraffin for either 4 hours or overnight depending upon the age of the tissue. Histological sections were cut at 6 microns, and the histological sections were deparaffinized, rehydrated, counterstained with Nuclear Fast Red (Vector Laboratories, diluted 4 times), dehydrated in ascending concentrations of ethanol, and placed in HistoClear (National Diagnostics) before mounting the slides with Histomount (Invitrogen).
In situ hybridization
A plasmid containing a 474 base pair fragment of the mouse aggrecan mRNA (Glumoff et al., 1994) was used to generate an antisense digoxigenin labeled RNA probe for nonradioactive RNA in situ hybridization of histological sections (protocol adapted from Roche Applied Science)
Acknowledgments
The following work was supported by grants from the National Institutes of Health (NIH) AR053568 to BdC and HD30284 and the Ben F. Love Endowment to RRB. An Arthritis Foundation Fellowship as well as a NIH training grant (T32) sustained SPH. Veterinary resources were supported by the National Institutes of Health (NIH) Cancer Center Support Grant CA16672.
References
- Bruckner G, Grosche J, Hartlage-Rubsamen M, Schmidt S, Schachner M. Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J Chem Neuroanat. 2003;26:37–50. doi: 10.1016/s0891-0618(03)00036-x. [DOI] [PubMed] [Google Scholar]
- Chambers MG, Kuffner T, Cowan SK, Cheah KS, Mason RM. Expression of collagen and aggrecan genes in normal and osteoarthritic murine knee joints. Osteoarthritis Cartilage. 2002;10:51–61. doi: 10.1053/joca.2001.0481. [DOI] [PubMed] [Google Scholar]
- Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O'Keefe RJ, Chen D. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Tortosa R, Domenech A, Vidal E, Pumarola M, Bassols A. Mapping of aggrecan, hyaluronic acid, heparan sulphate proteoglycans and aquaporin 4 in the central nervous system of the mouse. J Chem Neuroanat. 2007;33:111–123. doi: 10.1016/j.jchemneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- George SH, Gertsenstein M, Vintersten K, Korets-Smith E, Murphy J, Stevens ME, Haigh JJ, Nagy A. Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:4455–4460. doi: 10.1073/pnas.0609277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glumoff V, Savontaus M, Vehanen J, Vuorio E. Analysis of aggrecan and tenascin gene expression in mouse skeletal tissues by northern and in situ hybridization using species specific cDNA probes. Biochim Biophys Acta. 1994;1219:613–622. doi: 10.1016/0167-4781(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Grover J, Roughley PJ. Generation of a transgenic mouse in which Cre recombinase is expressed under control of the type II collagen promoter and doxycycline administration. Matrix Biol. 2006;25:158–165. doi: 10.1016/j.matbio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- Lakso M, Sauer B, Mosinger B, Jr, Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22:430–440. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram M, Sprung CN, Gao Q, Lo AW, Reynolds GE, Murnane JP. Telomere position effect and silencing of transgenes near telomeres in the mouse. Mol Cell Biol. 2006;26:1865–1878. doi: 10.1128/MCB.26.5.1865-1878.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999a;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999b;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Williams A, Harker N, Ktistaki E, Veiga-Fernandes H, Roderick K, Tolaini M, Norton T, Williams K, Kioussis D. Position effect variegation and imprinting of transgenes in lymphocytes. Nucleic Acids Res. 2008;36:2320–2329. doi: 10.1093/nar/gkn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun Z, Liu J, Guo X. Advances in susceptibility genetics of intervertebral degenerative disc disease. Int J Biol Sci. 2008;4:283–290. doi: 10.7150/ijbs.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Garofalo S, Mukhopadhyay K, Lefebvre V, Smith CN, Eberspaecher H, de Crombrugghe B. A 182 bp fragment of the mouse pro alpha 1(II) collagen gene is sufficient to direct chondrocyte expression in transgenic mice. J Cell Sci. 1995;108(12):3677–3684. doi: 10.1242/jcs.108.12.3677. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen M, Lichtler AC, O'Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreER(T2) transgenic mice. Osteoarthritis Cartilage. 2008a;16:129–130. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O'Keefe RJ, Chen D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008b;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]



