Abstract
Post-trial pharmacological activation of the noradrenergic system can facilitate memory consolidation. Because exercise activates the locus coeruleus and increases brain norepinephrine release, we hypothesized that post-trial exercise could function as a natural stimulus to enhance memory consolidation. We investigated this in amnestic mild cognitive impairment (aMCI) and cognitively normal elderly individuals by examining the effects of an acute bout of post-learning, aerobic exercise (6 minutes at 70% VO2 max on a stationary bicycle) on memory for some emotional images. Exercise significantly elevated endogenous norepinephrine (measured via the biomarker, salivary alpha-amylase) in both aMCI patients and controls. Additionally, exercise retrogradely enhanced memory in both aMCI patients and controls. Acute exercise that activates the noradrenergic system may serve as a beneficial, natural, and practical therapeutic intervention for cognitive decline in the aging population.
Keywords: Cognition, exercise, memory, noradrenaline, noradrenergic
INTRODUCTION
An abundance of evidence indicates that emotional material is typically better retained than is neutral material in healthy young animals and humans. Noradrenergic activation that occurs during or immediately after acquisition is critically involved in modulating emotional memory in both animals and humans. Noradrenergic-mediated memory enhancement has been demonstrated in a variety of learning paradigms, such as contextual fear conditioning, object recognition, and extinction of contextual fear conditioning [1–3] via both systemic administration and direct infusions of adrenergic agonists into the basolateral amygdala. In contrast, post-training systemic or intra-basolateral amygdala infusions of adrenergic antagonists, such as propranolol, block memory enhancement produced by systemic injections in a variety of animal learning tasks [3–10], or even impairs memory in animals [11–15].
Pharmacological evidence for the involvement of norepinephrine (NE) in emotional memory enhancement extends to humans, where systemic adrenergic agonist administration significantly enhances emotional memory [16, 17], whereas beta-adrenergic antagonists block this memory enhancement [17–21].
In addition to the pharmacological evidence that the adrenergic system is involved in memory modulation, there is a strong relationship between training-induced endogenous noradrenergic activation and enhanced emotional memory in animals and humans [22, 23]. The amount of increase in NE measured after acquisition of stressful learning tasks strongly correlated with memory performance for those tasks [22, 24]. These findings converge with evidence from pharmacological studies indicating that noradrenergic activation is critically involved in memory modulation for emotional events. Segal and Cahill [23] reported that the amount of increase in endogenous NE (as measured via salivary alpha-amylase, sAA) that occurs while subjects view emotional stimuli are significantly correlated with recall of those stimuli, but not with recall of emotionally neutral stimuli. These findings converge with evidence from pharmacological studies indicating that noradrenergic activation is critically involved in memory modulation for emotional events. While emotional memory enhancement appears to be at least partially preserved in older individuals [25–27], the extent to which noradrenergic activation modulates emotional memory in healthy older men and women has not been explored.
Since pharmacological manipulation of the noradrenergic system could be potentially dangerous in the older population, the use of exercise is a healthy alternative. Microdialysis experiments show that treadmill running rapidly increases NE release in the rodent brain, with the effect sustained through the duration of running [28]. In healthy young adults, exercise significantly enhances sAA levels, a biomarker for NE [29]. sAA significantly increased in response to 20 min of running on a stationary treadmill in young men and women [29, 30]. While the relationship between exercise and endogenous noradrenergic activation has not been examined in older men and women, psychosocial stress has been reported to increase endogenous NE release, as indicated by sAA in older individuals [31], suggesting that sAA is also a reliable biomarker for endogenous noradrenergic activation in older individuals.
Considerable epidemiologic data supports the concept that long-term exercise benefits cerebral and cognitive function in healthy older individuals [32–39]. Clinical trial data are mounting, most in normal healthy elderly (with exercise interventions ranging from 5 weeks to 1 year), which have demonstrated improvements in executive function and memory in response to aerobic exercise [32]. However, none of these experiments examined the influence of an acute bout exercise on memory. Clinical evidence indicates that chronic aerobic exercise may enhance cognition in mild cognitive impairment (MCI). For example, a recent randomized clinical trial of a home exercise program in MCI subjects found a small benefit of exercise on global cognition (measured by the Alzheimer’s Disease Assessment Scale-cognitive subscale) [40]. Similarly, Baker and colleagues [33] assessed effects of 6 months vigorous aerobic exercise at 70% VO2 max (versus stretching controls), and demonstrated cognitive improvements, particularly on measures of executive function. However, as in the case of healthy older individuals, none of these experiments examined the role of an acute bout of exercise on memory.
Although extensive evidence indicates that the noradrenergic system modulates emotional memory in healthy young animals and humans, the relationship between NE and memory modulation in healthy older humans has not, to our knowledge, been examined. In addition, degenerative processes of the locus coeruleus (LC) have been described in patients with MCI or early Alzheimer’s disease (AD) [41], warranting further investigation regarding the role of NE and memory modulation in this clinical population.
A primary goal of the present experiment was to examine the effects of a single bout of acute exercise on the endogenous noradrenergic response (measured via sAA) in healthy older individuals and patients with amnestic MCI (aMCI). Based on evidence that NE can enhance memory, a second goal was to explore whether exercise-induced noradrenergic activation could enhance memory storage in healthy older men and women. A third goal was to determine whether potential memory-enhancing effects of noradrenergic activation were spared in patients with memory deficit (aMCI).
METHODS
Subjects
Thirty-one healthy older adults (8 males and 23 females; 69 ± 2 y), and 23 (14 males and 9 females; 71.4 ± 2.4 y) patients with aMCI participated in this experiment. Participants were recruited from the UCI Alzheimer’s Disease Center (ADRC) and had no clinical signs of cardiovascular, respiratory, psychiatric, or other neurological disease. Patients were excluded if they were taking medication that affects the adrenergic system (such as beta-blockers). All participants were diagnosed with aMCI and no dementia by a local neurologist prior to the start of the study. One healthy control participant was excluded from analysis since he reported that he expected a memory test, and his performance was higher than two standard deviations from the mean.
Study design
All participants were randomly assigned to either the Exercise condition (15 controls, 11 aMCI) or the Sedentary condition (15 controls, 12 aMCI). Participants were tested on two subsequent days. The first day consisted of assessing VO2 max using a stationary bike, so that on the second day we could deliver the same intensity of exercise to each subject (based on a fixed % of their VO2 max). On the second day, after a 15-min acclimation period, a baseline saliva sample was taken. The participant then viewed a series of 20 mild emotionally positive images. Participants were not informed that they would be asked for recall of the images, but in order to ensure that they would attend to the image, participants were asked to rate the intensity of their personal emotional reaction to viewing each slide on a 5 point scale. A second saliva sample was taken immediately after termination of the slide show. Following the image presentation, the participant either exercised for 6 min at 70% VO2 max, or rested quietly (sedentary group). Additional saliva samples were taken immediately after exercise or rest, 10 min, 30 min, and 50 min later. 60 min after the exercise bout, participants underwent a surprise free-recall test, in which they were asked to orally describe each picture and to give as many details as possible. The descriptions were recorded by the experimenter, and a second independent scorer blinded to the experimental conditions determined the total number of images recalled for each participant.
VO2 max procedure
A progressive exercise test on an electronically braked cycle ergometer (Sensor Medics Ergoline 800 S, Yorba Linda, CA) was used to measure physical/cardiorespiratory fitness. Electrodes and a small breathing mask were placed on each participant prior to the test. Electrodes were used to monitor the electrocardiogram response and the mask was used to monitor the gas exchange during exercise for each participant. Each participant began the test with no resistance on the bicycle. The work rate was increased by 10 watts per min (or adjusted according to the subject’s age and fitness level) so that the total exercise time was 12–14 min, each participant exercising to the limit of his or her tolerance. Participants were told prior to beginning the test that they should raise their right hand to stop the test when it became too difficult and they wished to stop. Gas exchange was measured breath-by-breath via Sensor Medics V Max 229 Metabolic cart. VO2 max was calculated via the Fick Equation: VO2 max = Q(CaO2-CvO2), when these values are obtained during an exertion at a maximal effort where Q is the cardiac output of the heart, CaO2 is the arterial oxygen content, and CvO2 is the venous oxygen content.
Image presentation
A set of 20 positive images taken from the International Affective Picture Set (IAPS) were presented on a 15.6 inch monitor located approximately 28 inches in front of the participant. The images ranged from beautiful landscapes, to sports scenes, to baby animals and corresponded with IAPS numbers 1710, 8190, 2150, 8501, 2550, 8470, 7330, 1999, 7230, 5470, 8030, 1590, 8490, 7350, 5629, 8300, 1670, 1720, 4250, and 8033. For each slide, participants were asked to rate how emotionally arousing they found each image, on a scale of 1 to 5 with 1 being the lowest and 5 being the highest ratings, by pressing a button on the keyboard between the numbers 1–5. Each slide was presented for five seconds and each participant had as much time they needed to rate each image; the average length of time was five seconds. As soon as the participant rated the image (by pressing a key between 1 and 5) the slide show advanced to the next image.
Saliva sampling
sAA served as the index of central NE activation, based on the extensive pharmacological evidence supporting sAA as a valid biomarker for NE activity in humans [20, 42]. The enzyme sAA is released into saliva within 20 seconds after NE activation, and has been shown to be a more accurate reflection of central noradrenergeric activation than plasma NE [42]. Saliva was collected in sterile tubes using the “passive drool” method, in which subjects allow saliva to drain from their mouth into the tube, without actively stimulating the saliva [43]. All training and testing were conducted between the hours of 1300 h and 1800 h, to reduce variation due to the circadian rhythm of alpha-amylase. Subjects fasted for one hour prior to the first session of the experiment, and refrained from the consumption of alcohol and caffeine, as well as any participation in cardiovascular exercise for twenty-four hours prior to the study.
Saliva samples were immediately placed on ice and subsequently in a freezer stored at −20°C for a minimum of 24 h to allow mucins to precipitate. They were then thawed and centrifuged at 2,080 × g for 15 min to extract particulates from saliva. Clear supernatant was decanted into separate sterile tubes and centrifuged for an additional 15 min, then transferred to microtubes and stored at −20°C until assayed.
Alpha-amylase measurement
Alpha-amylase levels were measured in saliva samples via Salimetrics (State College, PA) enzyme kinetic assay kits. Substrate was heated to 37°C in the trough provided by Salimetrics in a preheated microtiter plate incubator for 20 min. Samples were diluted (1 : 200) with Salimetrics diluent. Eight microliters of prediluted Salimetrics controls for calibration of the assay, as well as each diluted sample were transferred to a 96 well microtiter plate. Three-hundred and twenty microliters of the preheated substrate were added to each well with a multichannel pipette. The plate was then placed in a microtiter plate mixer and mixed at 500–600 RPM for 1 min. The plate was immediately transferred to a microplate reader and read at 450 nm. The plate was then mixed at 500–600 RPM for 2 min and read immediately at 450 nm. The enzymatic action of alpha-amylase on the maltotriose yields 2-chloro-p-nitrophenol, which can be spectrophototometrically measured at 405 nm. The amount of alpha-amylase is directly proportional to the absorbance at 405 nm. Increase in sAA was considered anything above 3 U/mL, the lowest level of detection according to the Salimetrics assay kit protocol.
Statistical analysis
Recall scores were assessed with analysis of variance (ANOVA) where exercise or rest condition served as independent variables. The relationship between sAA and subsequent memory recall was characterized using regression analysis conducted with Statview Statistical Software (version 5.0.1).
RESULTS
Recall
Comparing the non-exercise groups, cognitively-intact aged controls overall recalled significantly more images than did the aMCI subjects in the (p < 0.0001), as expected, confirming that aMCI subjects possess a partially defective memory. Exercise effects were striking for both aMCI and cognitively-intact aged control groups. Post-trial exercise significantly enhanced recall in the aMCI participants (p < 0.001), as well as the healthy controls (p < 0.01). Further, there appeared to be a greater effect in the aMCI group than in controls, with a nearly 2-fold improvement over sedentary performance (versus an approximate 1.3-fold improvement in controls), an unexpected outcome. See Fig. 1.
Fig. 1.
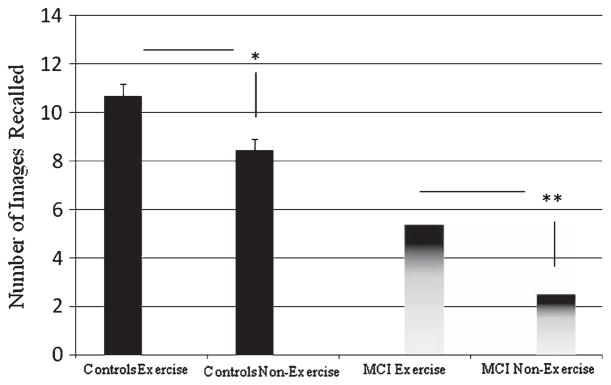
A single bout of post-training acute exercise significantly enhanced memory for aMCI patients (**p < 0.001) and age-matched controls (*p < 0.01).
Salivary alpha-amylase
In parallel with enhanced memory consolidation, exercise significantly increased sAA levels relative to baseline in both cognitively-intact aged and aMCI groups (p < 0.001) (Fig. 2). All participants in the exercise condition displayed a significant increase in sAA immediately after exercise. The sAA increase in the aMCI Exercise participants did not differ significantly from the sAA increase in the controls (p ≫ 0.1). Evaluation of the relationship between recall and sAA levels revealed a significant positive correlation in the MCI subjects (aMCI: r = 0.716, p < 0.001). Interestingly, within the aMCI subjects, the correlation was stronger for males (r = 0.774, p < 0.01) than females (r = 0.578, p < 0.01), although this difference was not statistically significant. Overall, these results suggest that post-trial NE activation may be relatively more dominant mechanisms for exercise effects in males than females, at least for the aMCI population. Gender differences in the cognitively-intact elderly could not be addressed because of the small sample size for males (n = 7), however, there was a positive correlation between sAA and recall in females (r = 0.4266, p < 0.05), supporting the concept that NE activation may be a fundamental mechanism underlying the exercise-enhancement of consolidation.
Fig. 2.
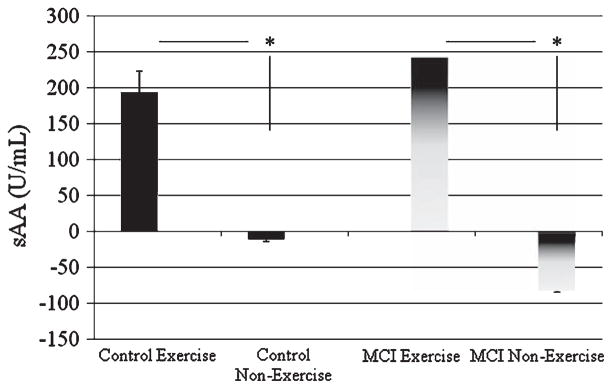
Exercise significantly increased sAA in both the aMCI patients and the age-matched controls (*p < 0.001).
DISCUSSION
These findings, to our knowledge, are the first to indicate that a single acute bout of post-learning exercise both activates the NE system and retrogradely enhances memory in cognitively healthy normal older humans. Although some prior evidence from postmortem studies has indicated that NE concentration decreases with age in healthy older people [44], the present study suggests that the NE system retains a considerable degree of function in healthy older individuals, indeed sufficient to retain at least some of the memory-enhancing qualities it possesses in healthy younger people.
The current study compared the relationship between exercise-induced noradrenergic activation and memory in aMCI patients and healthy age-matched controls. The findings indicate that post-learning, acute exercise both activates the NE system similarly in aMCI patients and controls, and significantly enhances memory in both groups. Given the very practical nature of acute exercise treatments, our findings appear to have considerable therapeutic potential for patients diagnosed with aMCI or perhaps other cognitive disorders.
Our findings also suggest that, while cholinergic pharmacological approaches are accepted as effective (if modest) treatments for dementia-related amnesia, a shift towards the adrenergic system for pharmacological targets may yield effective therapies. While loss of cholinergic neurons is a key contributor to AD pathogenesis, LC neuron loss is prominent in AD neuropathology and contributes to cognitive impairments [44–47]. Reduced concentrations of NE have been reported in both the antemortem and postmortem AD brain [48], and noradrenergic dysfunction in the cortex may be associated with neuronal loss in the LC [49, 50]. In the temporal cortex, noradrenergic markers were significantly reduced in patients who displayed clinical symptoms for less than two years, up to at least 47% of that in controls, suggesting that NA dysfunction occurs early in the onset of the disease [48]. However, in these same patients the ratio of 3-methoxy-4-hydroxyphenylglycol (a potential index of noradrenaline turnover) to NE was elevated, suggesting that there was an increase in noradrenergic activation as a compensatory mechanism. Degeneration of LC neurons in a small group of MCI and early AD patients has been reported in one study [41]. However, it is unclear whether the mechanism underlying the cognitive deficits observed in AD patients is associated with the loss of NE itself, or the loss of other LC cotransmitters, such as galanin, brain-derived neurotrophic factor (BDNF), and other neuropeptides synthesized and released by LC neurons.
While evidence suggests that the NE system is compromised in patients with AD, the amount of NE dysfunction in MCI patients is less clear and warrants further investigation. LC neuron loss has been reported in MCI patients [41], however, these findings pertained to only four MCI patients. The present findings suggest that the NE response to acute exercise is not severely, if at all, compromised in MCI patients, and further, that this NE response can enhance memory storage in aMCI patients.
There are several caveats to consider when examining the current findings. The first is that several hormones are released during and immediately after exercise. It is quite possible that other factors may contribute to the memory enhancing effects that were observed in the current experiment. Glucocorticoids, for example have been reported to enhance memory consolidation in animals and humans [2, 51]. However, previous research suggests that glucocorticoids are not released by an acute cardiovascular exercise regimen such as that used in the current experiment [52]. A more direct test of exercise-induced NE in memory enhancement should involve pharmacological blockade of NE (via beta-blockers such as propranolol) prior to exercise in future studies, however, it is possible that beneficial effects of exercise on memory engage more than one mechanism.
There are non-hormonal growth factors, such as BDNF, that have reportedly had enhancing effects on memory [53–55] and the current study does not address to what extent BDNF may have had an influence on memory. The shortest duration of exercise reported to have a significant effect on increasing serum BDNF in humans was 15 minutes [56]. Further research is needed to understand the relationship between a single bout of acute exercise and BDNF. Furthermore, since BDNF is partially localized to noradrenergic nerve fibers and terminals, it is synthesized and transported by noradrenergic neurons in the LC, and BDNF levels are decreased in AD patients, future studies should examine possible interactions between NE and BDNF in AD and MCI patients.
Since we were interested in the influence of noradrenergic activation on memory in patients specifically with memory deficits, instead of general cognitive impairment, we confined our participants to the subset of MCI patients diagnosed as amnestic (aMCI). However, future experiments should address the role of NE in patients with other sub-categories of MCI, to investigate the clinical implications of NE in terms of attention, executive function, etc.
The present findings have practical clinical implications that warrant further investigation. Most strikingly, they suggest that even a single bout of acute exercise can significantly enhance memory in both healthy and clinically impaired aged participants. While long-term exercise interventions have been suggested to improve cognitive performance in MCI patients and older adults at risk for AD [33, 40], this is the first evidence that exercise can act retrogradely, enhancing consolidation processes specifically.
Future studies can now better delineate the parameter space (amount and type of exercise, duration of memory enhancement, types of memories affected) in which acute exercise enhances memory in healthy older adults and MCI patients. For example, future studies should examine whether increasing the duration of exercise at 70% VO2 max increases NE significantly more than does six minutes of exercise. Greater NE increase could conceivably increase the memory-enhancing effects of the exercise. However, as the relationship between NE activation and memory consolidation enhancement generally follows an “inverted-U” dose-response function, [2, 57], it may be critical for practical purposes to identify in a given patient his/her optimal level of exercise to maximize potential memory-strengthening effects. The current findings suggest a natural, effective, and relatively safe alternative to pharmacological interventions in the older population, where acute exercise results in retrograde memory enhancement.
Acknowledgments
This work was supported by NIMH 575082 to LC and Alzheimer’s Disease Research Center (ADRC) Pilot Grant 2010–2011 AG016573.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1435).
References
- 1.Lalumiere RT, Buen T, McGaugh JL. Post-training intra-basolateral amygdale infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlau D, McGaugh JL. Enhancement of extinction memory consolidation: The role of the GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh JL, Introini-Collison IB. Interaction of GABAergic and beta-adrenergic drugs in the regulation of memory storage. Behav Neural Biol. 1994;61:150–155. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- 5.Introini-Collison IB, Baratti CM. Memory-modulatory effects by centrally acting noradrenergic drugs: Possible involvement of brain cholinergic mechanisms. Behav Neural Biol. 1992;57:248–255. doi: 10.1016/0163-1047(92)90234-u. [DOI] [PubMed] [Google Scholar]
- 6.Introini-Collison IB, Castellano C, McGaugh JL. Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav Brain Res. 1994;61:150–155. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg DB, Isaacs KR, Gold PE, McGaugh JL. Epinephrine facilitation of appetitive learning: Attenuation with adrenergic receptor antagonists. Behav Neural Biol. 1985;44:447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg DB, Korol D, Novack GD, McGaugh JL. Epinephrine-induced memory facilitation: Attenuation by adrenocepto antagonists. Eur J Pharmacol. 1986;129:189–193. doi: 10.1016/0014-2999(86)90353-5. [DOI] [PubMed] [Google Scholar]
- 9.Miranda MI, Lalumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur J Neurosci. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- 10.Miranda MA, Ferry B, Ferreira G. Basolateral amygdala noradrenergic activity is involved in the acquisition of conditioned odor aversion in the rat. Neurobiol Learn Mem. 2003;88:260–263. doi: 10.1016/j.nlm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: Evidence for a specific neurochemical system in the amygdala. Science. 1997;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher M, Knapp BS. Effect of phentolamine administration into the amygdala complex of rats on time-dependent memory processes. Behav Neural Biol. 1981;1:90–95. doi: 10.1016/s0163-1047(81)91130-4. [DOI] [PubMed] [Google Scholar]
- 13.McGaugh JL, Liang KC, Bennet C, Sternberg D. Involvement of the amygdala in memory storage: Interaction of peripheral and central systems. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of Learning and Memory. Guilford; New York: 1984. [Google Scholar]
- 14.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: Interaction with other brain regions. Proc Natl Acad Sci U S A. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockage. Neurobiol Learn Mem. 2000;74:259–266. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- 16.Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 17.O’Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med. 1999;29:1083–1088. doi: 10.1017/s0033291799008703. [DOI] [PubMed] [Google Scholar]
- 18.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 19.Cahill L, vanStegeren A. Sex-related impairment of memory for emotional events with beta-adrenergic blockade. Neurobiol Learn Mem. 2003;79:81–88. doi: 10.1016/s1074-7427(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 20.van Stegeren AH, Everaerd W, Cahill LF, McGaugh JL, Gooren LJG. Memory for emotional events: Differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology. 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- 21.van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer J, Rombouts S. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 23.Segal SK, Cahill L. Endogenous noradrenergic activation and memory for emotional material in men and women. Psychoneuroendocrinology. 2009;34:1263–1271. doi: 10.1016/j.psyneuen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Galvaz R, Mesches M, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 25.Kazui H, Mori E, Hashimoto M, Hirono N. Enhancement of declarative memory by emotional arousal and visual memory function in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15:221–226. doi: 10.1176/jnp.15.2.221. [DOI] [PubMed] [Google Scholar]
- 26.Hamann S, Monarch E, Goldstein F. Memory enhancement for emotional stimuli is impaired in early Alzheimer’s disease. Neuropsychology. 2000;14:82–92. [PubMed] [Google Scholar]
- 27.Kensinger E, Brierley B. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- 28.Pagliari R, Peyrin L. Norepinephrine release in the rat frontal cortex under treadmill exercise: A study with micro-dialysis. J Appl Physiol. 1995;78:2121–2130. doi: 10.1152/jappl.1995.78.6.2121. [DOI] [PubMed] [Google Scholar]
- 29.Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton LD, Fogle EA, Meston CM. The role of testosterone and alpha-amylase in exercise-induced sexual arousal in women. J Sex Med. 2008;4:845–853. doi: 10.1111/j.1743-6109.2007.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strahler J, Berndt C, Kirschbaum C, Rohleder N. Salivary alpha-amylase stress reactivity across different age groups. Psychophysiology. 2010;47:587–595. doi: 10.1111/j.1469-8986.2009.00957.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as preventative or disease-modifying treatment of dementia and brain aging. Mayo Clin Proceed. 2011;363:43–48. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson SG, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchanan AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennet DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 36.Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: Recent findings and future directions. J Mol Neurosci. 2004;224:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- 37.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;1:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 38.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity including waking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch Intern Med. 2011;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 40.Lautenschlager NT, Cod KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 41.Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;3:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Ehlert U, Erni K, Hebisch G, Nater U. Salivary alpha-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab. 2006;91:5130–5133. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- 43.Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: Cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 44.Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer. Br J Psychiatry. 1979;135:216–233. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- 45.Mann DM, Yates PO, Hawkes J. The noradrenergic system in Alzheimer and multi-farct dementias. J Neurosurgic Psychiatry. 1982;45:113–119. doi: 10.1136/jnnp.45.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews KL, Chen CP, Esiri MM. Noradreneric changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51:407–416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- 47.Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Neurology. 1982;32:164–168. doi: 10.1212/wnl.32.2.164. [DOI] [PubMed] [Google Scholar]
- 48.Palmer AM, Francis PT, Bowen DM, Benton JS, Neary D, Mann DM, Snowden JS. Catecholaminergic neurons assessed ante-mortem in Alzheimer’s disease. Brain Res. 1987;414:365–375. doi: 10.1016/0006-8993(87)90018-7. [DOI] [PubMed] [Google Scholar]
- 49.Mann DM, Yates PO, Marcyniuk B. Monoaminergic neurotransmitter systems in presenile Alzheimer’s disease and in senile dementia of the Alzheimer type. Clin Neuropathol. 1984;3:199–205. [PubMed] [Google Scholar]
- 50.Marcyniuk B, Mann DM, Yates PO. The topography of cell loss from locus caeruleus in Alzheimer’s disease. J Neurol Sci. 1986;76:335–345. doi: 10.1016/0022-510x(86)90179-6. [DOI] [PubMed] [Google Scholar]
- 51.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 52.Jacks DE, Sowash J, Anning J, McGloughlin T, Andres F. Effect of exercise at three exercise intensities on salivary cortisol. J Strength Cond Res. 2002;16:286–289. [PubMed] [Google Scholar]
- 53.Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- 54.Yamada K, Toshitaka N. Therapeutic approaches to the treatment of Alzheimer’s disease. Drugs Today (Barc) 2002;38:631–637. doi: 10.1358/dot.2002.38.9.696538. [DOI] [PubMed] [Google Scholar]
- 55.Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short-and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- 56.Tang SW, Chu E, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008;431:62–65. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 57.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]


