Abstract
Human neonates have reduced and delayed CD4+ T-cell immunity to certain pathogens compared to older children and adults, but the mechanisms for these developmental differences in immune function remain poorly understood. We investigated the hypothesis that impaired human neonatal CD4+ T-cell immunity was due to reduced signaling by naive CD4+ T cells following engagement of the αβ-TCR/CD3 complex and CD28. Surprisingly, calcium flux following engagement of CD3 was significantly higher in neonatal naive CD4+ T cells from umbilical cord blood compared to naive CD4+ T cells from adult peripheral blood. Enhanced calcium flux was also observed in adult CD4+ recent thymic emigrants. Neonatal naive CD4+ T cells also had higher activation-induced Erk phosphorylation. The microRNA miR-181a, which enhances activation-induced calcium flux in murine thymocytes, was expressed at significantly higher levels in cord blood naive CD4+ T cells compared to adult cells. Overexpression of miR-181a in adult naive CD4+ T cells increased activation-induced calcium flux, implying that the increased miR-181a levels of cord blood naive CD4+ T cells contributed to their enhanced signaling. In contrast, AP-1-dependent transcription, which is downstream of Erk and required for full T-cell activation, was decreased in cord blood naive CD4+ T cells compared to adult cells. Thus, cord blood naive CD4+ T cells have enhanced activation-dependent calcium flux, indicative of the retention of a thymocyte-like phenotype. Enhanced calcium signaling and Erk phosphorylation are decoupled from downstream AP-1-dependent transcription, which is reduced and likely contributes to limitations of human fetal and neonatal CD4+ T-cell immunity.
Introduction
There is substantial evidence that human neonates have a limitation in CD4+ T-cell immunity, particularly for adaptive immune responses mediated by Th1 cells (1). Following primary HSV infection, the HSV-specific Th1 and CD4+ T-cell dependent antibody response are markedly diminished and delayed in appearance in neonates compared to adults (2, 3). Limitations in antigen-specific CD4+ T-cell function also likely contribute to the vulnerability of the neonate and infant to severe infection with M. tuberculosis (4), a pathogen for which Th1 immunity is essential in humans (5). Decreased effector function of naive CD4+ T cells of the neonate in vivo is also suggested by the lower incidence of acute graft-versus-host-disease (GVHD) after cord blood (CB) hematopoietic cell transplants compared to mobilized adult peripheral blood transplants (6, 7). As GVHD requires naive T-cell activation and a Th1 response (8), these clinical observations suggest a cell-autonomous limitation of CB T-cell immunity following allogeneic transplantation.
Consistent with reduced neonatal CD4+ T-cell immunity in vivo, naive CD4+ T cells from CB compared to those of adult peripheral blood (APB) have reduced expression of activation-induced proteins in vitro, including the cell surface molecules CD69 and CD154 (CD40 ligand) and the Th1 cytokine IFN-γ (9). In the case of CD154 and IFN-γ, reduced expression by naive CD4+ T cells of CB is observed after both pharmacologic stimulation using the combination of calcium ionophore and PMA and more physiologic simulation with alloantigen, superantigen, or CD3 and CD28 mAbs ((9–13), and our unpublished observations). Reduced CD154 expression by neonatal CD4+ T cells likely contributes to the impaired Th1 immune response (9), as CD154 is required for generation of antigen-specific Th1 cells (14).
IL-2 production by neonatal naive CD4+ T cells is also significantly reduced compared to adult naive CD4+ T cells after physiological activation with allogeneic dendritic cells (9). These findings are notable, given the observation that toxic shock syndrome toxin (TSST)-1-reactive T cells in infants infected with TSST-1 expressing Staphylococcus aureus strains have reduced proliferation and IL-2 production, both properties of anergic T cells (15, 16). These results suggest that neonatal naive CD4+ T cells may have a tendency to become anergic following antigenic activation due, at least in part, to impaired IL-2 production. The mechanisms responsible for this phenotype remain unclear.
The full activation of naive CD4+ T cells requires the engagement of the αβ-TCR/CD3 complex and CD28 by cognate peptide/MHC and CD80/CD86, respectively, a process that can mimicked by polyclonal treatment with anti-CD3 and anti-CD28 mAbs. This treatment results in activation of the tyrosine kinases Lck, ZAP-70, and phospholipase C (PLC)γ1. Activated PLCγ1, in turn, catalyzes production of the second messengers inositol triphosphate (IP3) and diacylglycerol (DAG). Production of IP3 stimulates calcium release from the endoplasmic reticulum, which initiates an influx of extracellular calcium through the calcium release activated calcium (CRAC) channel of the cell membrane. This increase in the free intracellular concentration of Ca2+ ([Ca2+]i) results in the calcineurin-dependent activation and nuclear translocation of the NFAT family of transcription factors (17).
DAG and other ZAP-70 derived signals activate Ras, which, in turn, activates Erk in a MAPK cascade that results in the generation of AP-1, a heterodimeric transcription factor of Fos and Jun proteins (18). The activation-dependent expression of cytokines, such as IL-2 and IFN-γ, and TNF ligand family members, such as CD154, by T cells requires de novo transcription of their cognate genes by the engagement of NFAT and AP-1 in promoter cis-elements (19–22). There is considerable evidence that the generation of calcium flux, and consequent NFAT activation in the absence of AP-1 activation, results in a program of gene transcription promoting anergy rather than a productive immune response (22–24).
Limitations at many steps in T-cell activation-induced signaling and cytokine gene transcription may contribute to decreased antigen-induced cytokine and CD154 expression by CD4+ T cells of the fetus or neonate. In support of proximal signaling limitations, some studies of CB T cells have found reduced tyrosine phosphorylation of the tyrosine kinases, such as Lck and ZAP-70 (25) or PLCγ1 (26) following CD3 and CD28 mAb stimulation compared to T cells from APB. An important limitation of these studies and many others (reviewed in (1)) was that they did not directly compare naive (CD45RAhigh CD45ROlow) CD4+ T cells of CB with those from APB, but used unfractionated APB T cells instead, which have a substantial number of memory/effector cells. Because memory/effector T cells have distinct, often enhanced, activation-dependent signaling properties compared to naive T cells (27), this approach is not as informative for identifying true developmental limitations in signaling by naive T-cell populations. Limits imposed upon CD4+ T-cell immunity in neonates by developmental changes in activation-dependent signaling in naive CD4+ T cells remain unclear.
Here, we tested the hypothesis that reduced signaling in naive CD4+ T cells following engagement of the αβ-TCR/CD3 complex and CD28 contributes to limitations in CD4+ T-cell immunity in the human neonate. Unexpectedly, we found that TCR-induced increases in [Ca2+]i and Erk phosphorylation were higher in naive CD4+ T cells from CB relative to those from APB. This enhanced calcium response was mediated, at least in part, by increased levels of the microRNA miR-181a in CB naive CD4+ T cells, and reflects the retention by CB CD4+ T cells of a thymocyte-like phenotype. This enhanced signaling was decoupled from downstream activation-dependent AP-1 induction. This reduced AP-1 activity in CB naive CD4+ T cells may account for previous observations of decreased cytokine and CD154 production by these cells (9), as well as a tendency towards CD4+ T-cell anergy rather than a productive immune response in neonates and young infants.
Materials and Methods
Blood mononuclear cell and naive CD4+ T-cell isolation
Blood was collected from healthy adult donors with informed consent in accordance with the requirements of the Stanford University Internal Review Board (IRB). Umbilical cord blood was collected from the placentas of healthy births that were term gestation, normal weight, and delivered vaginally at Lucile Packard Children’s Hospital in accordance with IRB requirements. CB and APB were anticoagulated with preservative-free heparin sodium sulfate (Sigma, St. Louis, MO) and subjected to density gradient centrifugation using Ficoll-Hypaque (GE Healthcare Biosciences, Pittsburgh, PA) to isolate the mononuclear cell (MC) fraction. Unless otherwise indicated, naive CD4+ T cells used in experiments were purified from MCs by magnetic-activated cell sorting after treatment with a Human Naive CD4+ T Cell Negative Selection Kit II and application to paramagnetic LS columns (Miltenyi Biotec, Auburn, CA). Cells for miR-181a measurements were positively selected with a CD4 Selection Kit (Miltenyi), and FACS-sorted on a FACS Aria or modified FACS Vantage (both BD Biosciences, San Jose, CA). Naive CD4+ T cells were sorted and defined as follows: CD3+ (allophycocyanin (APC)-AlexaFluor 750-conjugated, clone OKT3, eBioscience, San Diego, CA, or APC-AlexaFluor750-conjugated clone S4.1, Invitrogen, Carlsbad, CA), CD4+ (APC-conjugated, clone S3.5, Invitrogen), CD45RA+ (FITC-conjugated, clone HI100, BD Biosciences), CD45RO− (PE-conjugated, clone UCHL1, Invitrogen) and 7-aminoactinomycin D− (7AAD, BD Biosciences).
Fluorescent labeling and measurement of calcium flux
Purified naive CD4+ T cells (1x107 cells/ml) were either incubated with 8.0 µg/ml of Alexa488 succinimidyl ester (Molecular Probes/Invitrogen) in PBS (Gibco/Invitrogen) or in PBS alone for 20 min at room temperature. Labeled cells were washed twice with HBSS with calcium and magnesium and without phenol red (Gibco/Invitrogen) with 1% heat-inactivated human AB serum (hAB) (Gemini Bioproducts, West Sacramento, CA) and combined with equivalent numbers of unlabeled naive CD4+ T cells. The combined labeled and unlabeled cells were then stained with APC-conjugated CD19 (clone SJ25-C1, Invitrogen), CD8α (clone 3B5, Invitrogen), and CD45RO (clone UCHL1, BD Biosciences or Invitrogen) mAbs. After washing, cells (1x107/ml) in HBSS with 1.0 % hAB were incubated with indo-1 acetoxymethyl ester (Molecular Probes/Invitrogen) at a final concentration of 500 nM for 30 min at 37°C. After washing, cells were incubated in HBSS with 1.0 % hAB for another 30 min at room temperature and stained with 7AAD. Indo-1 emission at 346 and 330 nm was measured on a ultraviolet laser-equipped modified FACS Vantage or an LSR II instrument (both from BD Biosciences). Samples were equilibrated at 37°C for 10 min prior to data acquisition. A 30 s baseline was collected, followed by addition of 1:1000 (v/v) of OKT3 (CD3ε) mAb-containing ascites (Cocalico Biologicals, Reamstown, PA) at 30 s. After 2 min 45 s, 30 µg of goat anti-mouse Ig (Jackson Immunoresearch, West Grove, PA) or sheep anti-mouse Ig (Millipore, Danvers, MA) was added to cross-link surface bound CD3ε mAb. After 8 min, ionomycin (Sigma) was added at a final concentration of 1.0 µM, and after 10 min 15 s, acquisition was either terminated (for miRNA transfected samples) or EGTA was added at a final concentration of 5.0 mM and acquisition was terminated at 12 min 30 s. The peak and mean 330/346 nm ratios from the CD3 mAb cross-linking period was calculated using FlowJo software (Treestar, Ashland, OR).
Protein tyrosine kinase 7 surface staining of naive CD4+ T cells
Purified APB naive CD4+ T cells were stained with rat IgG monoclonal anti-human protein tyrosine kinase 7 (PTK7) (generated by Genovac, Freiburg, Germany) or, as a negative control, purified rat IgG (Sigma) for 20 min at room temperature. After blocking with 10% hAB serum, cells were incubated with PE-conjugated goat-anti-rat-IgG (Jackson Immunoresearch), followed by additional blocking by incubation with unlabeled rat IgG for 15 min at 4°C. For calcium flux assays, cells were stained with APC-conjugated CD19, CD8α, and CD45RO mAbs, as described above. 7AAD was added after washing, and samples were loaded with indo-1 acetoxymethyl ester and analyzed as described above. For miRNA expression analysis, PTK7+ and PTK7− 7AAD− CD3+ (APC-Alexa750, clone S41, Invitrogen or PE-Cy7 conjugated, clone UCHT1, eBioscience), CD4+ (PerCP-Cy5.5, clone SK3, BD Biosciences; Alexa700, clone OKT4, eBioscience; or APC-Alexa750-conjugated, clone S3.5, BD Biosciences) CD45RA+ (FITC-conjugated ,clone HI100, BD Biosciences), CD45RO− (APC-conjugated, clone UCHL1, Invitrogen) cells were sorted using a BD FACS ARIA II.
Phospho-flow analysis
After isolation of CB or APB MCs by Ficoll-Hypaque gradient centrifugation as described above, cells were stained with CD3-Alexa700 (clone UCHT1, BD Biosciences), CD4-Alexa 488 (clone OKT4, eBioscience), and CD45RA-Qdot 605 (clone MEM-56, Invitrogen). Stained MCs (1x106/well) were incubated in RPMI (Mediatech, Manassas, VA) with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) at 37°C in Maxisorb 96-well plates (Nunc, Thermo Scientific, Rockford, IL) previously coated overnight with 1.0 µg/ml of functional grade CD3 mAb (clone OKT3, eBioscience, San Diego, CA) and 2.5 µg/ml of CD28 mAb (clone CD28.2, eBioscience) in PBS for 10, 15, 30, and 60 min or with 250 nM ionomycin (Sigma) and 50 ng/ml of PMA (Sigma) for 1, 5, and 15 min. Cells were fixed with a final concentration of 1.5% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS with 1.0 mM β-glycerol phosphate (Sigma) and 1.0 mM sodium orthovanadate (Sigma) for 10 min at room temperature. Cells were pelleted and resuspended in ice-cold methanol (Fisher, Waltham, MA), incubated at −20°C for 20 min or −80°C overnight, then washed twice in PBS with 0.5% BSA (HyClone Labs, Logan, UT). Samples were then blocked with 10% (v/v) hAB for 15 min at room temperature. Anti-phospho-Erk1/2 pT202/pT204 (PE-conjugated, clone 20a, BD Biosciences) was added and incubated for 30 min at room temperature. Cells were washed twice in PBS with 0.5% BSA, resuspended in 0.5% paraformaldehyde in PBS with 1% (w/v) BSA, and stored at 4°C in PBS with 0.5% paraformaldehyde and 0.5% BSA until analysis on a BD Biosciences LSR II instrument. Naive CD4+ T cells were defined as CD3+ CD4+ CD45RA+.
AP-1-dependent transcription reporter gene activity
Naive CD4+ T cells were activated with 2.5 µg/ml of PHA (Sigma) for 19.5 h as previously described (28), and then transfected with plasmids (2.0 µg/1x106 cells) using a Neon electroporation system (Invitrogen) set for 2400 V, 12 milliseconds, and 2 pulses. The AP-1 reporter plasmid (generous gift of O. Martinez, Stanford University) has 5 AP-1 binding sites from the metallothionein promoter subcloned into the human IL-2 minimal promoter in a pGL3 plasmid backbone. A β-actin luciferase plasmid (generous gift of G. Crabtree, Stanford University) was used as a control. pEGFP-N1 (Clontech, Mountain View, CA) was used to assess transfection efficiency by flow cytometry. Twenty-four hours after transfection, 2×105 cells were activated with CD3 and CD28 mAb-coated Dynabeads (Invitrogen) at a 1:1 bead to cell ratio, or with 250 nM ionomycin (Sigma) and 50 ng/ml PMA (Sigma) for 4 hours in a total volume of 100 µl. After 4 h, 100 µl of One-Glo reagent (Promega Biosystems, Madison, WI) was added to each well, and after 3 min of incubation lysates were read for 1.0 s on a luminometer (Veritas, Promega). Five transfection replicates were performed for each sample. Transfection efficiency and cell death were assessed by evaluation of the GFP signal measured on an Accuri C6 flow cytometer after staining with 7AAD and CD4-PE (clone S3.5, Invitrogen) and CD45RA-APC (clone MEM-56, Invitrogen) mAbs.
microRNA isolation and quantitative PCR analysis
RNA was isolated using a mirVana isolation kit (Ambion/Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol from purified naive CD4+ T cells. RNA was analyzed by quantitative PCR using a BioRad (Hercules, CA) CFX384 Real-Time System or an ABI 7900 system (ABI/Life Technologies) and TaqMan miRNA assays or gene-specific assays (Ambion). The fold-change and relative expression were calculated using the ΔΔCt method. miRNAs were normalized to RNU48 and mRNAs to GAPDH.
Transfection of primary CD4+ T cells with pre-miRNAs
Purified naive CD4+ T cells (1x106 cells) in RPMI with 10% FBS were transfected with 75 µM of mirVana miR-181a mimic or a control, non-targeting miRNA mimic (all from Ambion), and 5% v/v siPort reagent (Ambion) in Opti-MEM medium (Gibco/Invitrogen) at room temperature, according to the manufacturer’s protocol. Prior to transfection, the miRNAs were labeled with Cy3 using Label IT siRNA Tracker (Mirus Bio, Madison, WI) following the manufacturer’s instructions. Total RNA was isolated for analysis after 24h and 48h of incubation, and calcium flux was measured after 48 h.
Activation of naive CD4+ T cells and total RNA isolation
MACS-purified naive CD4+ T cells were stimulated for 4 hours at 37°C at 2.0x107 cells/mL with 2.0 µg/mL anti-CD3 (clone OKT3, eBioscience) and 10.0 µg/mL anti-CD28 (clone CD28.2, eBioscience), cross-linked with 33 µg/mL goat anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) in RPMI with 10% FBS. Unstimulated cells were incubated in RPMI with 10% FBS under the same conditions. After stimulation, cells were pelleted and lysed with Tri Reagent (Molecular Research Center, Cincinnati, OH). Samples were stored at −80°C prior to RNA isolation, which was performed according to the manufacturer’s protocol.
Statistical analysis
Results were analyzed using GraphPad Prism software (GraphPad, San Diego, CA), with p-values < 0.05 considered significant. Unless otherwise specified, a two-tailed unpaired Student’s t-test was used.
Results
Activation-induced calcium flux is higher in CB naive CD4+ T cells compared to APB naive CD4+ T cells
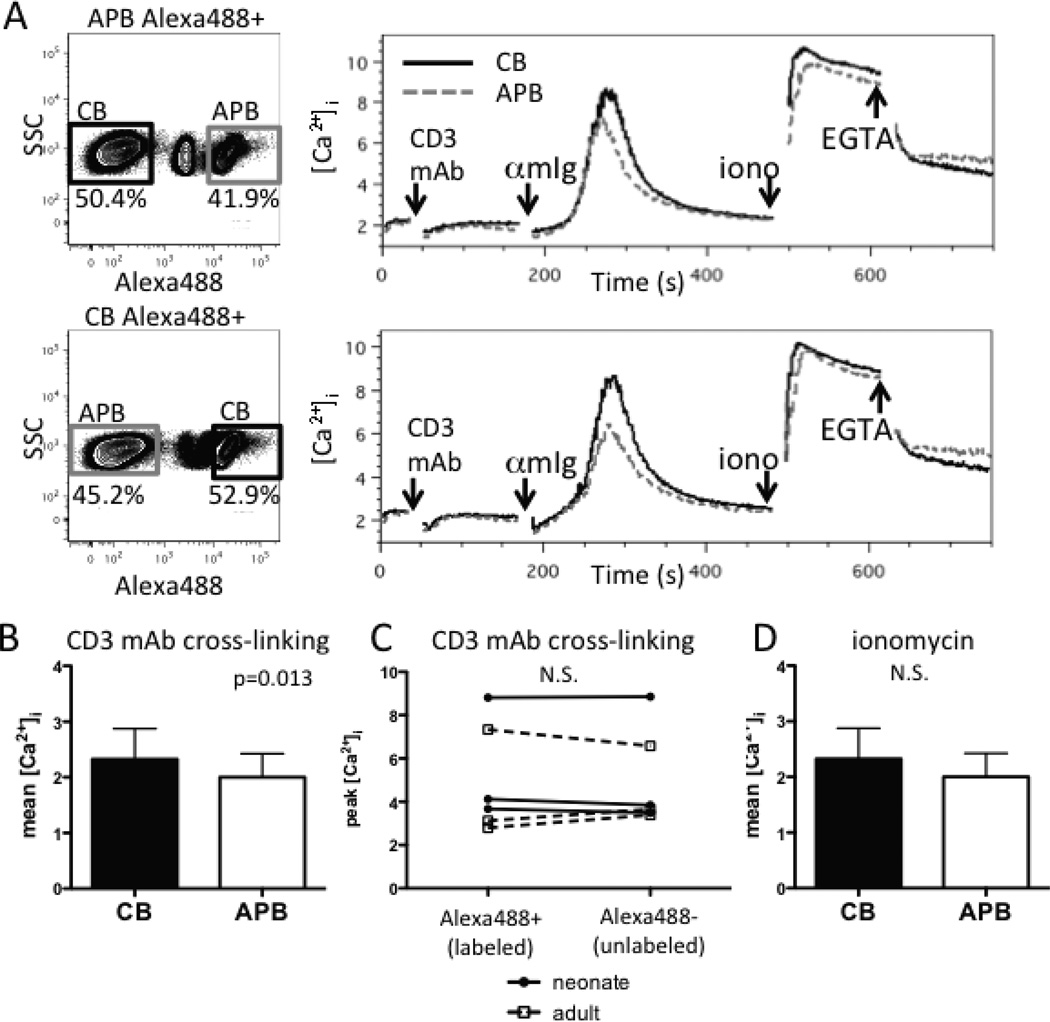
As activation-dependent calcium-dependent signaling is critical for inducing the de novo transcription of genes encoding cytokines and CD154 in CD4+ T cells (19, 29), we first determined if limitations in such signaling in CB CD4+ T cells might result in reduced expression of these gene products. We developed a flow cytometric assay in which naive CD4+ T-cell populations were either fluorescently labeled with Alexa488 succinimidyl ester (barcoded) or left unlabeled (Fig. S1) and then combined, permitting the two cell populations to be simultaneously stimulated and analyzed for calcium flux under the same conditions. The labeling procedure did not significantly affect the calcium flux compared to that of unlabeled cells from the same donor (Fig. S1). Using this approach, naive CD4+ T cells from CB versus APB were directly compared after the cross-linking of surface bound CD3 mAb. Unexpectedly, the peak (p=0.025) and mean (p=0.01) calcium flux following CD3 mAb cross-linking were higher in naive CB CD4+ T cells than in APB naive CD4+ T cells (n=3) (Fig. 1A, B, and data not shown). The labeling did not significantly affect calcium flux (p=0.98, Fig. 1C). In contrast, there was no difference in either the peak (p=0.31) or mean (p=0.21) calcium flux after the addition of the calcium ionophore, ionomycin (Fig. 1D and data not shown), indicating that the capacity for pharmacologically-induced calcium flux in these two CD4+ T-cell types was equivalent.
FIGURE 1.
Naive CD4+ T cells of CB have a higher calcium flux than APB naive CD4+ T cells after CD3 mAb cross-linking using anti-mouse IgG (αmIg). (A) Representative flow cytometry plots of Alexa488 labeled and unlabeled naive CD4+ T-cell cell populations (left panels) and their median indo-1 ratio of Alexa488 labeled CB (solid black line) and unlabeled APB (dashed gray line) naive CD4+ T cells (right top panel) versus unlabeled CB and Alexa488 labeled APB CD4+ T cells (right bottom panel). (B) Mean [Ca2+]i after CD3 mAb cross-linking is significantly higher in CB naive CD4+ T cells compared to those of APB (n=3). (C) Alexa488 cell labeling does not have a significant effect on a peak calcium flux of a cell population (n=6). Individual donors are connected by lines. (D) Mean [Ca2+]i following ionomycin addition in CB and APB naive CD4+ T cells (n=3). p-values were calculated using a paired, two-way ANOVA to account for both labeling and sample source. All samples were gated on live, CD8− CD19− CD45RO− MACS-purified naive CD4+ T cells.
Activated-induced calcium flux is higher in RTEs than in mature naive CD4+ T cells of APB
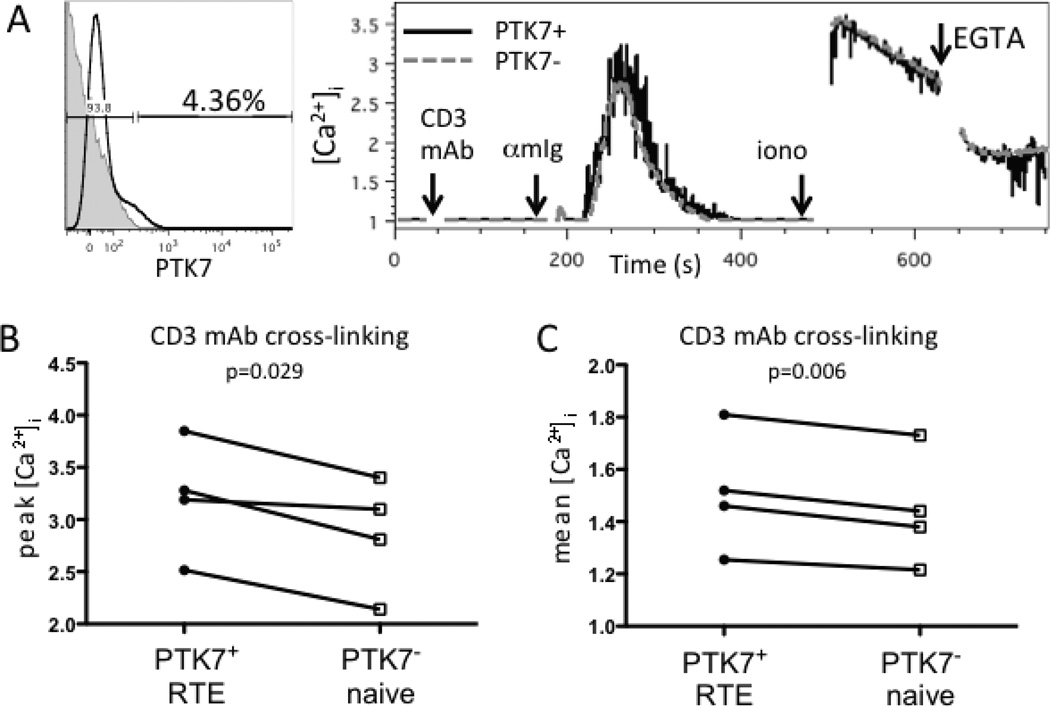
The CB naive CD4+ T-cell compartment appears to be relatively enriched in RTEs, T cells that have recently completed their intrathymic maturation and have emigrated to the periphery, based on relatively high content of signal joint T-cell receptor excision circles (sjTRECs) (30) and high levels of surface expression of PTK7, a human CD4+ RTE marker (31). To determine whether RTEs of APB had similar signaling properties as CB naive CD4+ T cells, we compared calcium flux in PTK7+ and PTK7− APB naive CD4+ T cells (Fig. 2A). Both the peak (Fig. 2B) and the mean (Fig. 2C) calcium flux after anti-CD3 mAb cross-linking were higher in PTK7+ cells than PTK7− from the same donor (n=4) (p=0.029 and p=0.006, respectively). There was also no significant difference in peak calcium flux following addition of ionomycin (p=0.41, data not shown), indicating that RTEs and more mature naive CD4+ T cells had similar capacity to flux calcium after pharmacologic stimulation. Together, these results suggest that the enhanced calcium flux of CB naive CD4+ T cells and APB CD4+ RTEs reflect the retention of a thymocyte-like phenotype.
FIGURE 2.
CD4+ PTK7+ RTEs of APB have higher calcium flux after CD3 mAb cross-linking than more mature naive CD4+ T cells. (A) Representative flow cytometry plot of PTK7 staining (black line) overlaid on an isotype control stain (filled gray histogram), and kinetics plot of calcium flux after CD3 mAb crosslinking, ionomycin addition, and EGTA addition in samples from an individual adult donor. (B) Peak [Ca2+]i and (C) mean [Ca2+]i of PTK7+ naive CD4+ T cells (RTE) and PTK7− naive CD4+ T cells after CD3 mAb cross-linking (n=4). PTK7+ and PTK7− cell populations from the same individual are connected by lines. p-values were calculated using a two-tailed, paired student’s t-test. All samples were gated on live, CD8− CD19− CD45RO− purified naive CD4+ T cells.
Erk phosphorylation is higher in naive CD4+ T cells from CB than APB
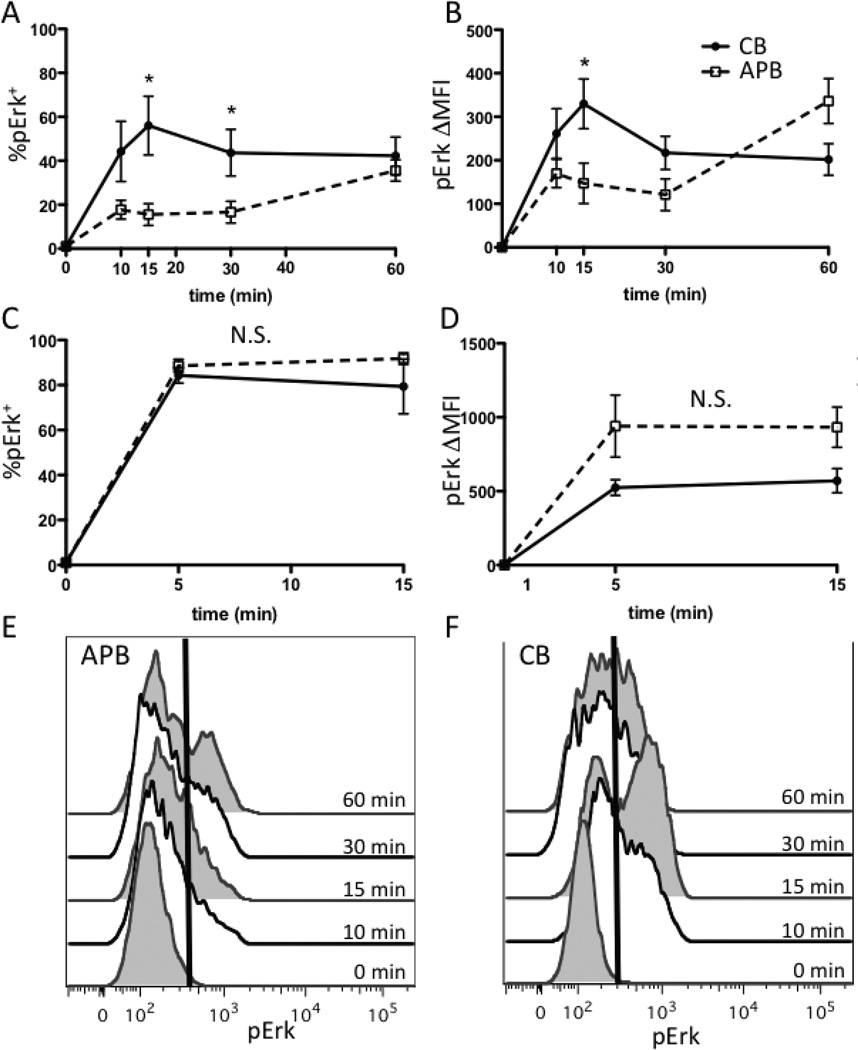
Phosphorylation of Erk1/2 is induced in response to TCR engagement and co-stimulation (32–34) and is critical for the AP-1-dependent transcription of genes involved in T-cell activation, so we next determined whether phosphorylation of Erk1/2 was also enhanced in CB naive CD4+ T cells compared to those of APB. pErk was detectable by 10 minutes after stimulation with plate-bound anti-CD3 and anti-CD28 mAbs, and the percent of cells positive for pErk (%pErk+) was significantly higher in CB after 15 and 30 minutes of stimulation (Fig. 3A), though %pErk+ was similar after 60 minutes of stimulation. The change in mean fluorescent intensity (ΔMFI) was significantly higher (p<0.05) in CB after 15 minutes of stimulation (Fig. 3B). There was no significant difference in the percentage of pErk+ cells or ΔMFI in response to stimulation with ionomycin and PMA at 5 or 15 minutes after stimulation (Fig. 3C, D). Representative histograms for pErk in APB and CB are shown in Fig. 3E and F, respectively. These data indicate that the higher Erk phosphorylation observed in naive CD4+ T cells from CB compared to those of APB applied to physiologic, rather than pharmacologic activation.
FIGURE 3.
Erk phosphorylation is higher in CB naive CD4+ T cells than APB cells. (A) %pErk+ after stimulation with plate-bound anti-CD3 and anti-CD28 is significantly higher in CB naive CD4+ T cells after 15 (p=0.01), and 30 (p=0.03) minutes of stimulation. (B) Change in MFI (ΔMFI) is significantly higher (p=0.04) in CB naive CD4+ T cells after 15 minutes of stimulation with plate-bound anti-CD3 and anti-CD28. (C) %pErk+ is not significantly different between CB and APB naive CD4+ T cells after stimulation with ionomycin and PMA. (D) ΔMFI is not significantly different between CB and APB (p>0.05) after stimulation with ionomycin and PMA. (E) Representative plot of pErk staining in one APB sample stimulated with anti-CD3 and anti-CD28. Time points are alternately filled or white, for clarity. Line indicates position of positive gate. (F) Representative plot of pErk staining in one CB sample stimulated with anti-CD3 and anti-CD28. Cells are gated on CD3+ CD4+ CD45RA+ lymphocytes.
Expression of miR-181a and its positive regulation of activation-induced calcium flux in CD4+ T cells
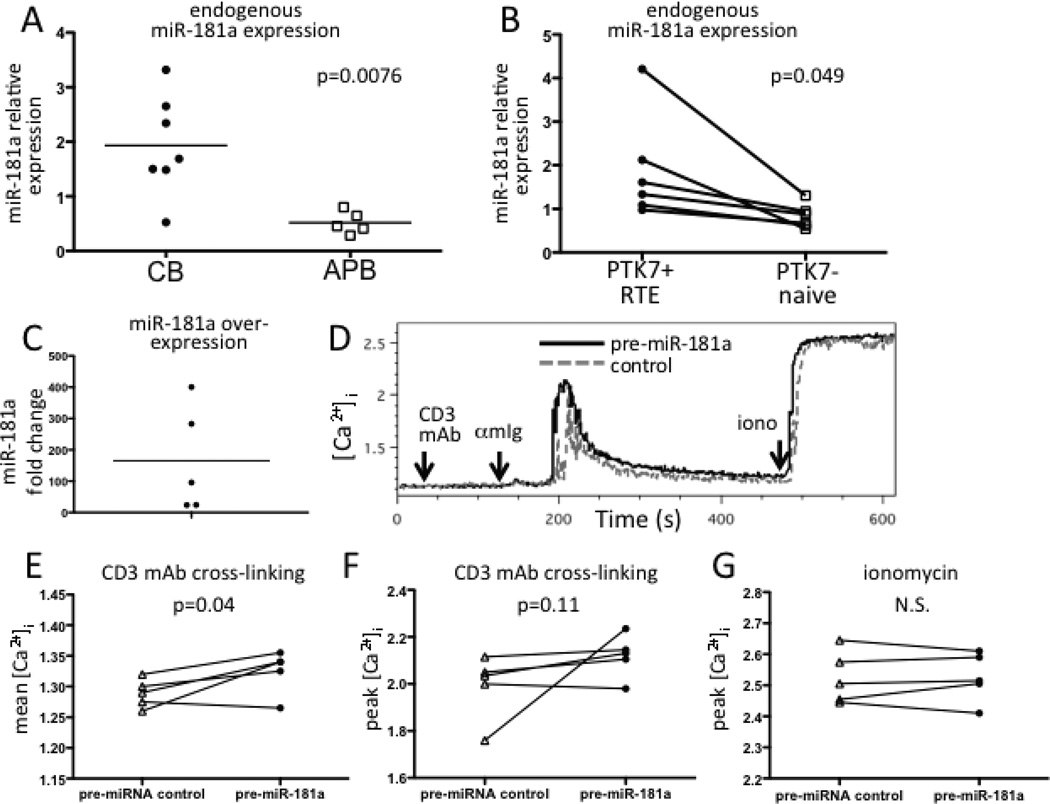
The microRNA miR-181a has been reported to regulate calcium flux and Erk phosphorylation positively in murine CD4+ T lineage cells, including in thymocytes, which highly express miR-181a (35). As CB naive CD4+ T cells have features suggestive of an enrichment in RTEs and retention of a thymocyte-like signaling properties, we next investigated whether miR-181a contributes to increased [Ca2+]i. Expression of miR-181a was significantly higher in naive CD4+ T cells of CB compared to those of APB (Fig. 4A, p=0.0076), as measured by quantitative PCR. Similarly, adult PTK7+ RTEs had significantly higher miR-181a expression (p<0.05) than PTK7− naive CD4+ T cells from the same donor (Fig. 4B), consistent with higher calcium flux in PTK7+ than PTK7− cells. To determine if miR-181a positively regulates calcium flux in peripheral naive CD4+ T cells, we chose to over-express miR-181a in naive CD4+ T cells from APB, rather than inhibiting miR-181a in CB T cells, as others have reported difficulties in knocking down miR-181a (36). Overexpression was achieved by transient transfection with pre-miRNAs because preliminary studies using lentiviral vectors resulted in very low infection rates and required prolonged culture (data not shown). Transfection of APB naive CD4+ T cells with pre-miR-181a resulted in a robust increase in mature miR-181a levels (Fig. 4C) and significantly increased the mean calcium flux (p=0.04) after CD3 mAb crosslinking compared to cells transfected with a non-targeting control (Fig. 4D, E). There was also a trend towards an increase in peak calcium flux for cells transfected with pre-miR-181a (Fig. 4F, p=0.11). We did not observe decreases in mRNAs of predicted miR-181a target phosphatases previously identified using murine T-lineage cells (35) (data not shown). Peak and mean calcium flux after addition of ionomycin were not different between pre-miR-181a and control-transfected cells (Fig. 4G and data not shown, p=0.63 and p=0.86, respectively), indicating that the enhancement of calcium flux by miR-181a was specific for that following αβ-TCR/CD3 activation. miR-181a over-expression did not affect the viability of the cells as measured by 7AAD staining of all cell events or lymphocytes (Fig. S2).
FIGURE 4.
Higher expression of miR-181a in CB naive CD4+ T cells regulates calcium flux. (A) CB naive CD4+ T cells (n=5) have significantly higher levels of miR-181a expression than those of APB (n=7). (B) miR-181a expression is significantly higher in PTK7+ RTEs than in PTK7- naive CD4+ T cells of APB (n=6). Lines indicate samples from individual donors. (C) Transfection with pre-miR-181a RNA results in increased expression of mature miR-181a. (D) Representative [Ca2+]i plot of replicates of pre-miR-181a transfected (solid black line) and control pre-miRNA transfected (dashed gray line) APB naive CD4+ T cells. (E) Mean and (F) peak [Ca2+]i following CD3 mAb cross-linking from all APB donors was higher after transfection with the pre-miRNA control or pre-miR181a. (G) Peak [Ca2+]i in response to addition of ionomycin of CB and APB naive CD4+ cells was not different after transfection with a pre-miRNA control or pre-miR-181a RNA.
AP-1-dependent transcriptional activity is reduced in CB naive CD4+ T cells
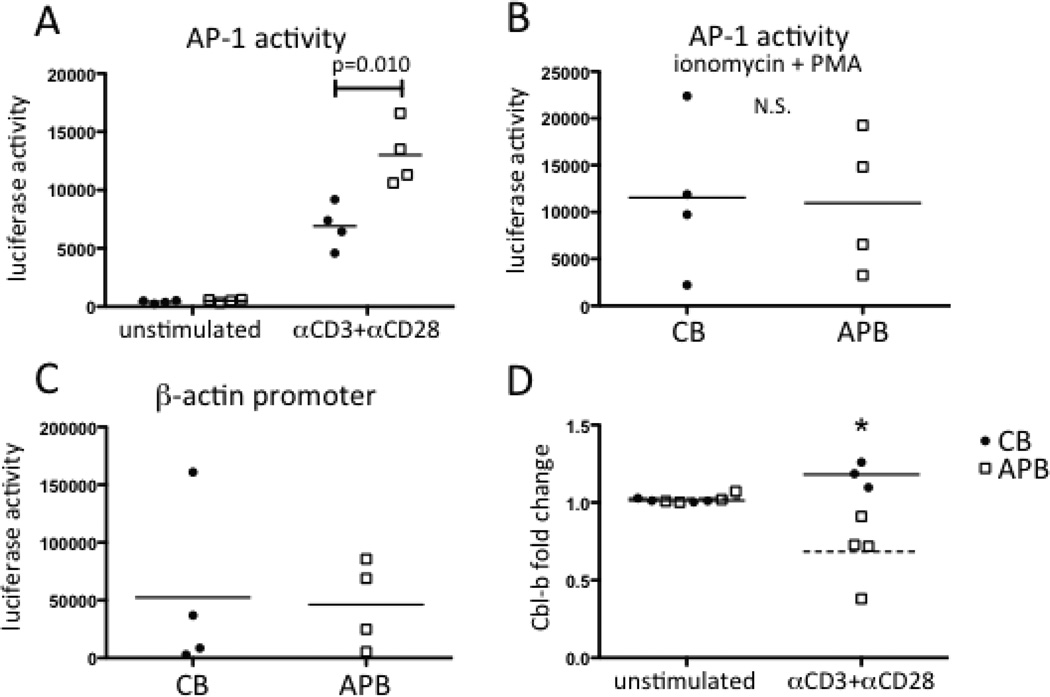
CB and neonatal naive CD4+ T cells have previously been shown to have features of anergic cells, such as reduced IL-2 production and proliferation in certain contexts, such as following exposure to bacterial superantigen (15, 16). As anergy induction can result when intact calcium-dependent activation of NFAT occurs in the absence of CD28 engagement or transcriptional activity of AP-1 (23, 37–39), we evaluated AP-1 activity by performing transient transfection of an AP-1-dependent luciferase reporter gene. We found that CB naive CD4+ T cells had significantly lower levels of AP-1-dependent transcription than APB naive CD4+ T cells after CD3 and CD28 mAb stimulation (Fig. 5A, p=0.010). In contrast, there was no significant difference between these cell types for the low level of AP-1-dependent transcription that was detected in the absence of stimulation (Fig. 5A, p=0.12) or for the high levels of activity that were induced by ionomycin and PMA treatment (Fig. 5B, p=0.92). In addition, there was no significant difference between CB and APB CD4+ T cells for a β-actin-promoter driven luciferase signal (Fig. 5C, p=0.89), indicating that differences in transfection efficiency did not account for these findings. Collectively, these results argued for the impairment of AP-1-dependent transcription in CB naive CD4+ T cells specifically following αβ-TCR/CD3 and CD28 engagement rather than a limitation in the amount or functional competence of AP-1.
FIGURE 5.
AP-1-dependent transcription is reduced in activated CB naive CD4+ T cells compared to those of APB. (A) AP-1 reporter luciferase activity in CB (filled circles, n=4) and APB (open squares, n=4) either unstimulated or after 4 hours of stimulation with CD3 and CD28 mAbs. (B) AP-1 reporter activity after stimulation with ionomycin and PMA for 4 hours was not significantly different. (C) Luciferase activity driven by a β-actin promoter reporter was not significantly different between CB and APB naive CD4+ T cells. (D) Cbl-b mRNA was upregulated in CB naive CD4+ T (n=3) cells and downregulated in APB naive CD4+ T cells (n=4) after 4h stimulation with anti-CD3 and anti-CD28 (p=0.025, by a paired Student’s t-test).
Upregulation of anergy genes in activated CB naïve CD4+ T cells
Because reduced AP-1 induction is implicated in establishment of anergy, we examined the upregulation of genes characteristic of anergy in CB compared to APB naive CD4+ T cells. GRAIL and Cbl-b are E3 ubiquitin ligases induced after activation of cells fated to become anergic (38–40). We measured induction of these two mRNAs after 4 h of stimulation with CD3 and CD28 mAbs. Cbl-b was induced in CB naive CD4+ T cells following activation, while mRNA levels decreased in APB naive CD4+ T cells following activation (Fig. 5D). The difference was statistically significant (p=0.024). GRAIL mRNA was undetectable in all conditions assayed (data not shown). Thus, the induction of Cbl-b in CB naive CD4+ T cells provided more molecular evidence for the tendency of these cells to become anergic following activation.
Discussion
Human neonates have functionally decreased and delayed CD4+ T-cell immunity, including for Th1 responses, which likely contributes to their vulnerability to more severe infection by viruses and certain intracellular bacteria (1). The de novo CD4+ T-cell response to neoantigens requires full activation of naive CD4+ T cells after engagement of the αβ-TCR/CD3 complex and the CD28 co-stimulatory molecule and their proliferation and differentiation into effector and memory cells. In the current study, we investigated whether limitations in naive CD4+ T-cell activation could contribute to impaired neonatal CD4+ T-cell immunity. Unexpectedly, we found that CB naive CD4+ T cells had enhanced rather than decreased calcium responses in response to engagement of CD3, and this phenotype also applied to the RTE subset of adult naive CD4+ T cells. Similarly, phosphorylation of Erk was higher in CB naive CD4+ T cells. In contrast, CD3 and CD28 mAb-induced activation AP-1-mediated transcription, which is Erk dependent, was impaired in CB naive CD4+ T cells compared to those of APB. As expression of CD3, CD4, and CD28 is similar between APB and CB T cells (1), differences in surface receptor or co-receptor expression are unlikely to account for our results. Taken together, these results indicate that CB naive CD4+ T cells have a striking combination of both enhanced and decreased signal transduction events with activation, and that enhanced signaling is consistent with the retention of a thymocyte-like phenotype by CB naive CD4+ T cells and circulating adult CD4+ RTEs.
The enhanced calcium signaling by CB naive CD4+ T cells was accounted for by increased levels of miR-181a; overexpression of miR-181a in APB naive CD4+ T cells resulted in enhanced CD3 mAb-induced calcium flux. In murine T-lineage cells, miR-181a expression is highest in double-negative thymocytes followed by a progressive decline as these cells mature into CD4+ CD8+ thymocytes, CD4+ CD8− thymocytes, peripheral naive CD4+ T cells, and, finally, effector CD4+ T cells. Assuming that this pattern of miR-181a expression applies to human CD4+ T-lineage maturation, our finding of greater calcium flux by adult CD4+ RTEs compared to more mature naive CD4+ T cells of APB suggests a role for miR-181a in the enhanced calcium flux of RTEs, although this remains to be directly shown. Higher anti-CD3 mAb-induced calcium flux has been observed in circulating adult naive CD4+ T cells compared to memory CD4+ T cells (41), which may be explained by higher miR-181a expression in naive CD4+ T cells.
Although the kinetics of the thymic production of and peripheral turnover of CD4+ T cells in the neonate are not known, it is likely that CB peripheral CD4+ T cells are highly enriched in RTEs because of the high activity of the thymus during fetal gestation through 6 months of age (42). In previous studies, CB naive CD4+ T cells and CD4+ RTEs of APB have been shown to share a number of phenotypic features, including the expression of high levels of PTK7, sjTRECs, and CD31, and robust proliferative responses to IL-7 (31, 43). The finding of hyper-stimulable calcium flux by CB naive CD4+ T cells and adult RTEs, which for both cell types is likely mediated, at least in part, by miR-181a, is also consistent with the idea that this signal transduction pattern represents the retention of a functional thymocyte-like phenotype. These results also suggest that, as for PTK7 surface expression (31), a decrease in TCR-induced calcium signaling may be part of the normal post-thymic maturation of the circulating neonatal naive and adult CD4+ T-cell compartments (31, 44, 45). Consistent with our results, Gorozny and colleagues have found that miR-181a expression and Erk phosphorylation are reduced in naive CD4+ T cells isolated from elderly people (46), who have a markedly reduced number of RTEs compared to younger adults.
In mice, miR-181a regulates calcium flux in CD4+ T cells by downregulating a number of phosphatases that dephosphorylate Lck and Erk (35). In an MHC class II-restricted αβ-TCR transgenic murine system, miR-181a has been shown to enhance αβ-TCR/CD3-mediated calcium signaling and Erk phosphorylation resulting in increased positive and negative selection of CD4+ CD8+ (double-positive) thymocytes and increased activation of mature CD4+ T cells for IL-2 production and proliferation (35). Our data support a similar mechanism in humans, as we have found increased Erk phosphorylation in CB naive CD4+ T cells. In addition, the finding that miR-181a is highly expressed in adult CD4+ PTK7+ RTEs is consistent with the pattern of expression of miR-181a in murine T-lineage cells.
Robust initial responses by CB CD4+ T cells, followed by apoptosis of effector cells have previously been reported (47). Our data are consistent with these findings, as CB naive CD4+ T cells have high calcium flux, due, in part, to high expression of miR-181a. Pro-apoptotic effects of miR-181a have been reported in cancer cell lines (48–50), and over-expression of miR-181a sensitizes cell lines to a variety of apoptosis-inducing chemotherapy agents and increases their activation of Caspases 3 and 9 (49, 50). miR-181a has also been shown to target multiple anti-apoptotic Bcl2 family members (51). While some anti-apoptotic effects of miR-181a have been reported (52), the preponderance of published results suggests that high expression of this miRNA may mediate both the enhanced calcium flux and the high frequency of apoptosis of activated CB CD4+ T cells. As shown in Fig. S2, we did not see effects of miR-181a over-expression on survival of APB naive CD4+ T cells, but the effect of miR-181a on modulating post-activation apoptosis remains to be determined.
The observation that CB naive CD4+ T cells have increased αβ-TCR/CD3-induced calcium flux but decreased AP-1-dependent transcription after CD3 and CD28 engagement is consistent with a signaling pattern that induces anergy rather than cell proliferation and production of IL-2 and other cytokines. Anergic murine CD4+ T-cell clones or αβ-TCR transgenic primary CD4+ T cells result from the presence of calcium-dependent activation of transcription by NFAT in the absence of Erk phosphorylation and transcription by AP-1; this signaling profile has been achieved using calcium ionophore treatment alone, or APCs displaying antigenic peptide/MHC complexes but lacking ligands for CD28 (23, 53–56). In support of this hypothesis, we found that Cbl-b, an E3 ubiquitin ligase necessary in early induction of anergy (39), was modestly upregulated in CB naive CD4+ T cells after stimulation with anti-CD3 and anti-CD28, but was downregulated in APB naive CD4+ T cells under the same conditions. The anergy-like signal transduction profile described here may account for the greater tendency of TSST-1-reactive Vβ2+ CB naive CD4+ T cells than APB cells to become anergic in vitro or in vivo following exposure to bacterial superantigen TSST-1 (15, 16). A tendency towards the induction of anergy rather than full effector function may also account for the observation that hematopoietic transplants with umbilical cord blood cells elicit fewer instances of Th1-mediated GVHD than do transplants with adult-derived cells (6, 7).
The observation that αβ-TCR/CD3 engagement simultaneously induces higher levels of calcium flux and Erk phosphorylation, but reduced AP-1 activity, indicates that there is a decoupling of Erk activation from AP-1 activity. Erk is an immediate upstream activator of Fos, a component of the AP-1 heterodimer (57), and Erk activity is negatively regulated by death-associated protein kinase (DAPK), which sequesters phosphorylated Erk (pErk) in the cytoplasm and prevent its translocation to the nucleus where it directly activates Fos (58). As we have found that DAPK mRNA is more highly expressed in CB naive CD4+ T cells compared to those of APB (our unpublished data), it is plausible that DAPK may contribute to decreased AP-1-dependent transcription (57) as well as NF-κB-dependent transcription (59). Thus, these inhibitory effects of DAPK could explain the discrepancy between increased Erk activation and decreased AP-1 activity we have observed.
Following T-cell activation, AP-1 and NFAT proteins cooperate for the transcription of genes encoding CD154 (19, 60), IL-2 (22, 24), IL-4, IFN-γ (21), and many other cytokines. Impaired AP-1 induction in CB naive CD4+ T cells may, therefore, be an important contributor to impaired CD154, IL-2, and IFN-γ production following stimulation with superantigen or allogeneic dendritic cells (9, 15, 16). In addition to the signaling pattern, we have found that Cbl-b is induced in CB naive CD4+ T cells, providing more molecular evidence that these cells may become anergic upon stimulation. Importantly, our results do not exclude other factors in contributing to impaired IFN-γ gene expression, such increased DNA methylation of the IFN-γ gene (61), reduced levels of NFATc2 in unfractionated CB CD4+ T cells (62), and altered antigen-presenting cell function (63, 64).
As we have previously shown that adult CD4+ RTEs also display relatively impaired responses after αβ-TCR/CD3 engagement for IL-2 and IFN-γ production compared to more mature APB naive CD4+ T cells (31), it will be of interest to determine to what extent adult RTEs and CB naive CD4+ T cells share impairments in signal transduction involving the Erk/AP-1 pathway and a tendency for anergy rather than full activation. In the mouse, single-positive thymocytes do not produce IL-2 upon stimulation under conditions in which peripheral CD4+ T cells do (65), suggesting that a decoupling between TCR engagement and effector cytokine may be a characteristic of the late stage of thymocyte development. Thus, including human CD4+ single-positive mature thymocytes (CD4+ CD8− CD3high CD1alow) (1515) in an analysis of signaling in peripheral naive CD4+ T cell populations may also help determine to what extent alterations in signaling reflect the retention by these peripheral CD4+ T-cell populations of a thymocyte phenotype.
In summary, we have observed a distinct activation-induced signal transduction profile in CB naive CD4+ T cells, with an increased calcium response, Erk phosphorylation, and expression of the Cbl-b anergy-promoting gene, but decreased AP-1-dependent transcription. The increase in calcium flux is mediated, at least in part, by increased expression of the microRNA miR-181a, which regulates calcium flux in murine CD4+ T cells, and, as we have shown here, in primary human CD4+ T cells and, based on studies by others, may also contribute to CB naive CD4+ T cells being prone to undergoing apoptosis. These features of increased activation-induced calcium flux and mR-181a levels are also found in APB naive CD4+ RTEs, which is consistent with likely high proportion of CB naive CD4+ T cells that are RTEs. Together, these results indicate that CB naive CD4+ T cells have a unique signaling pattern that may contribute to their impaired IFN-γ secretion capacity, as well as their tendency to become anergic in response to stimulation with superantigen. CB naive CD4+ T cells are not broadly hyporesponsive, despite impaired effector cell function. These findings also highlight, and provide evidence for, the need for peripheral maturation and regulation of TCR sensitivity during the late stages of intrathymic and early stages of post-thymic T-lineage cell development.
Supplementary Material
Acknowledgements
The authors are grateful to members of the laboratory, including Kira Dionis and David Hong for helpful discussions and technical advice, Lan Liu for assistance with processing blood samples, and Stephen Chmura for his expert critique of the manuscript. We would like to Olivia Martinez, Gerald Crabtree, and other members of the Stanford Immunology Program, for assistance with reagents and protocols. We also wish to thank the staff of Labor and Delivery at Lucille Packard Children’s Hospital for sample collection, all of our adult blood donors, and the Stanford Shared FACS Facility for advice and training.
This work was supported by the Lucille P. Markey Charitable Trust and Stanford Graduate Fellowships (to A.C.P.), National Institutes of Health Grants T32 HD-007249 (to A.C.P.), R56 AI-083757 (to D.B.L.), R01 AI-83757 (to D.B.L.), R01 AI-100121 (to D.B.L.), and the Jeffrey Modell Foundation (to D.B.L).
Abbreviations used in this article
- AAD
aminoactinomycin
- APB
adult peripheral blood
- APC
allophycocyanin
- CB
cord blood
- DAG
diacyl glycerol
- GVHD
graft-versus-host disease
- hAB
human AB serum
- IP3
inositol triphosphate
- MC
mononuclear cell
- MFI
mean fluorescent intensity
- miR
microRNA
- PLC
phospholipase C
- PTK7
protein tyrosine kinase 7
- sjTRECs
signal joint T-cell receptor excision circles
- TSST
toxic shock syndrome toxin
References
- 1.Lewis DB, Wilson CB. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Klein JO, Maldonado Y, Nizet V, Remington JS, Wilson CB, editors. Infectious Diseases of the Fetus and Newborn. 7th ed. Philadelphia: Elsevier Saunders; 2011. pp. 80–191. [Google Scholar]
- 2.Burchett SK, Corey L, Mohan KM, Westall J, Ashley R, Wilson CB. Diminished interferon-gamma production and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J. Infect. Dis. 1992;165:813–818. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 3.Sullender WM, Miller JL, Yasukawa LL, Bradley JS, Black SB, Yeager AS, Arvin AM. Humoral and cell-mediated immunity in neonates with herpes simplex virus infection. J. Infect.Dis. 1987;155:28–37. doi: 10.1093/infdis/155.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Smith S, Jacobs R, Wilson C. Immunobiology of childhood tuberculosis: A window on the ontogeny of cellular immunity. J Pediatr. 1997;131:16–26. doi: 10.1016/s0022-3476(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 5.Alcais A, Fieschi C, Abel L, Casanova J-L. Tuberculosis in children and adults: two distinct genetic diseases. J. Exp. Med. 2005;202:1617–1621. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha V, Wagner JE, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N. Eng. J. Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 7.Ballen KK, Spitzer TR. The great debate: haploidentical or cord blood transplant. Bone Marrow Transplant. 2010;46:323–329. doi: 10.1038/bmt.2010.260. [DOI] [PubMed] [Google Scholar]
- 8.Wolf D, von Lilienfeld-Toal M, Wolf AM, Schleuning M, von Bergwelt-Baildon M, Held SAE, Brossart P. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119:16–25. doi: 10.1182/blood-2011-08-339465. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol. Blood Marrow Transplant. 2006;12:160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Nonoyama S, Penix LA, Edwards CP, Lewis DB, Ito S, Aruffo A, Wilson CB, Ochs HD. Diminished expression of CD40 ligand by activated neonatal T cells. J. Clin. Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jullien P, Cron RQ, Dabbagh K, Cleary A, Chen L, Tran P, Stepick-Biek P, Lewis DB. Decreased CD154 expression by neonatal CD4+ T cells is due to limitations in both proximal and distal events of T cell activation. Int. Immunol. 2003;15:1461–1472. doi: 10.1093/intimm/dxg145. [DOI] [PubMed] [Google Scholar]
- 12.Cantó E, Rodríguez-Sánchez JL, Vidal S. Naive CD4+ cells from cord blood can generate competent Th effector cells. Transplantation. 2005;80:850–858. doi: 10.1097/01.tp.0000174135.32068.65. [DOI] [PubMed] [Google Scholar]
- 13.Gans HA, Yasukawa LL, Zhang CZ, Wakim RH, Rinki M, Dehovitz R, Arvin AM. Effects of interleukin-12 and interleukin-15 on measles-specific T-cell responses in vaccinated infants. Viral Immunol. 2008;21:163–172. doi: 10.1089/vim.2007.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain A, Atkinson TP, Lipsky PE, Slater JE, Nelson DL, Strober W. Defects of T-cell effector function and post-thymic maturation in X-linked hyper-IgM syndrome. J. Clin. Invest. 1999;103:1151–1158. doi: 10.1172/JCI5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi N, Imanishi K, Nishida H, Uchiyama T. Evidence for immunologic immaturity of cord blood T cells. Cord blood T cells are susceptible to tolerance induction to in vitro stimulation with a superantigen. J. Immunol. 1995;155:5213–5219. [PubMed] [Google Scholar]
- 16.Takahashi N, Kato H, Imanishi K, Miwa K, Yamanami S, Nishida H, Uchiyama T. Immunopathophysiological aspects of an emerging neonatal infectious disease induced by a bacterial superantigen. J. Clin. Invest. 2000;106:1409–1415. doi: 10.1172/JCI10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Ann. Rev. Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 18.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Ann. Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert LA, King G, Cron RQ, Lewis DB, Aruffo A, Hollenbaugh D. The human gp39 promoter. Two distinct nuclear factors of activated T cell protein-binding elements contribute independently to transcriptional activation. J. Biol. Chem. 1995;270:29624–29627. doi: 10.1074/jbc.270.50.29624. [DOI] [PubMed] [Google Scholar]
- 20.Tsytsykova AV, Tsitsikov EN, Geha RS. The CD40L promoter contains nuclear factor of activated T cells-binding motifs which require AP-1 binding for activation of transcription. J. Biol.Chem. 1996;271:3763–3770. doi: 10.1074/jbc.271.7.3763. [DOI] [PubMed] [Google Scholar]
- 21.Penix LA, Sweetser MT, Weaver WM, Hoeffler JP, Kerppola TK, Wilson CB. The proximal regulatory element of the interferon-gamma promoter mediates selective expression in T cells. J. Biol. Chem. 1996;271:31964–31972. doi: 10.1074/jbc.271.50.31964. [DOI] [PubMed] [Google Scholar]
- 22.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 23.Macián F, García-Cózar F, Im S-H, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 24.Macián F, García-Rodríguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Nagayama H, Takahashi TA. Aberrant CD3- and CD28-mediated signaling events in cord blood T cells are associated with dysfunctional regulation of Fas ligand-mediated cytotoxicity. J. Immunol. 1999;162:4464–4471. [PubMed] [Google Scholar]
- 26.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana RA, Vitale M, Di Valerio V, Manzoli FA. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J. Immunol. 1999;163:2416–2424. [PubMed] [Google Scholar]
- 27.Chandok MR, Farber DL. Signaling control of memory T cell generation and function. Sem. Immunol. 2004;16:285–293. doi: 10.1016/j.smim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Cron RQ, Schubert LA, Lewis DB, Hughes CC. Consistent transient transfection of DNA into non-transformed human and murine T-lymphocytes. J. Immunol. Meth. 1997;205:145–150. doi: 10.1016/s0022-1759(97)00065-3. [DOI] [PubMed] [Google Scholar]
- 29.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr. Opin. Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcolino I, Przybylski GK, Koschella M, Schmidt CA, Voehringer D, Schlesier M, Pircher H. Frequent expression of the natural killer cell receptor KLRG1 in human cord blood T cells: correlation with replicative history. Eur. J. Immunol. 2004;34:2672–2680. doi: 10.1002/eji.200425282. [DOI] [PubMed] [Google Scholar]
- 31.Haines CJ, Giffon TD, Lu L-S, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J. Exp. Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atherfold PA, Norris MS, Robinson PJ, Gelfand EW, Franklin RA. Calcium-induced ERK activation in human T lymphocytes. Mol. Immunol. 1999;36:543–549. doi: 10.1016/s0161-5890(99)00076-0. [DOI] [PubMed] [Google Scholar]
- 33.Franklin RA, Atherfold PA, McCubrey JA. Calcium-induced ERK activation in human T lymphocytes occurs via p56(Lck) and CaM-kinase. Mol. Immunol. 2000;37:675–683. doi: 10.1016/s0161-5890(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 34.Nunès JA, Collette Y, Truneh A, Olive D, Cantrell DA. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7-1, activates p21ras. J. Exp. Med. 1994;180:1067–1076. doi: 10.1084/jem.180.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q-J, Chau J, Ebert PJR, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen C-Z. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Cuesta R, Martinez-Sanchez A, Gebauer F. miR-181a Regulates Cap-Dependent Translation of p27kip1 mRNA in Myeloid Cells. Mol. Cell. Biol. 2009;29:2841–2851. doi: 10.1128/MCB.01971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandyopadhyay S, Duré M, Paroder M, Soto-Nieves N, Puga I, Macián F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 39.Jeon M-S, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu Y-C, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Bandyopadhyay S, Soto-Nieves N, Macián F. Transcriptional regulation of T cell tolerance. Semin. Immunol. 2007;19:180–187. doi: 10.1016/j.smim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roederer M, Bigos M, Nozaki T, Stovel RT, Parks DR, Herzenberg LA. Heterogeneous calcium flux in peripheral T cell subsets revealed by five-color flow cytometry using log-ratio circuitry. Cytometry. 1995;21:187–196. doi: 10.1002/cyto.990210211. [DOI] [PubMed] [Google Scholar]
- 42.Weerkamp F, de Haas EF, Naber BA, Comans-Bitter WM, Bogers AJ, van Dongen JJ, Staal FJ. Age-related changes in the cellular composition of the thymus in children. J. Allergy Clin. Immunol. 2005;115:834–840. doi: 10.1016/j.jaci.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 43.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 44.Hassan J, Reen DJ. IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur. J. Immunol. 1998;28:3057–3065. doi: 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Webb LM, Foxwell BM, Feldmann M. Interleukin-7 activates human naive CD4+ cells and primes for interleukin-4 production. Eur. J. Immunol. 1997;27:633–640. doi: 10.1002/eji.1830270309. [DOI] [PubMed] [Google Scholar]
- 46.Li G, Yu M, Lee W-W, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thornton CA, Upham JW, Wikström ME, Holt BJ, White GP, Sharp MJ, Sly PD, Holt PG. Functional maturation of CD4+CD25+CTLA4+CD45RA+T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J. Immunol. 2004;173:3084–3092. doi: 10.4049/jimmunol.173.5.3084. [DOI] [PubMed] [Google Scholar]
- 48.Fei J, Li Y, Zhu X, Luo X. miR-181a Post-Transcriptionally Downregulates Oncogenic RalA and Contributes to Growth Inhibition and Apoptosis in Chronic Myelogenous Leukemia (CML) PLoS One. 2012;7:e32834. doi: 10.1371/journal.pone.0032834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, Servant N, Paccard C, Hupé P, Robert T, Ripoche H, Lazar V, Harel-Bellan A, Dessen P, Barillot E, Kroemer G. miR-181a and miR-630 Regulate Cisplatin-Induced Cancer Cell Death. Cancer Res. 2010;70:1793–1803. doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- 50.Bai H, Cao Z, Deng C, Zhou L, Wang C. miR-181a sensitizes resistant leukaemia HL-60/Ara-C cells to Ara-C by inducing apoptosis. J. Cancer Res. Clin. Oncol. 2012;138:595–602. doi: 10.1007/s00432-011-1137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang Y-B, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lwin T, Lin J, Choi YS, Zhang X, Moscinski LC, Wright KL, Sotomayor EM, Dalton WS, Tao J. Follicular dendritic cell-dependent drug resistance of non-Hodgkin lymphoma involves cell adhesion-mediated Bim down-regulation through induction of microRNA-181a. Blood. 2010;116:5228–5236. doi: 10.1182/blood-2010-03-275925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gajewski TF, Qian D, Fields P, Fitch FW. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 1994;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morton AM, McManus B, Garside P, Mowat AM, Harnett MM. Inverse Rap1 and phospho-ERK expression discriminate the maintenance phase of tolerance and priming of antigen-specific CD4+ T cells in vitro and in vivo. J. Immunol. 2007;179:8026–8034. doi: 10.4049/jimmunol.179.12.8026. [DOI] [PubMed] [Google Scholar]
- 55.Doherty M, Osborne DG, Browning DL, Parker DC, Wetzel SA. Anergic CD4+ T cells form mature immunological synapses with enhanced accumulation of c-Cbl and Cbl-b. J. Immunol. 2010;184:3598–3608. doi: 10.4049/jimmunol.0902285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J. Immunol. 2006;176:2279–2291. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- 57.Kamal M, Pawlak A, BenMohamed F, Valanciuté A, Dahan K, Candelier M, Lang P, Guellaën G, Sahali D. C-mip interacts with the p85 subunit of PI3 kinase and exerts a dual effect on ERK signaling via the recruitment of Dip1 and DAP kinase. FEBS Let. 2010;584:500–506. doi: 10.1016/j.febslet.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Chen C-H, Wang W-J, Kuo J-C, Tsai H-C, Lin J-R, Chang Z-F, Chen R-H. Bidirectional signals transduced by DAPK-ERK interaction promote the apoptotic effect of DAPK. EMBO J. 2005;24:294–304. doi: 10.1038/sj.emboj.7600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuang Y-T, Fang L-W, Lin-Feng M-H, Chen R-H, Lai M-Z. The tumor suppressor death-associated protein kinase targets to TCR-stimulated NF-kappa B activation. J. Immunol. 2008;180:3238–3249. doi: 10.4049/jimmunol.180.5.3238. [DOI] [PubMed] [Google Scholar]
- 60.Noel G, Brinster C, Semana G, Bruniquel D. Modulation of the TCR stimulation strength can render human activated CD4+ T cells suppressive. Int. Immunol. 2009;21:1025–1036. doi: 10.1093/intimm/dxp068. [DOI] [PubMed] [Google Scholar]
- 61.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J. Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 62.Kadereit S, Junge GR, Kleen T, Kozik MM, Kaminski BA, Daum-Woods K, Fu P, Tary-Lehmann M, Laughlin MJ. Deficient IFN-gamma expression in umbilical cord blood (UCB) T cells can be rescued by IFN-gamma-mediated increase in NFATc2 expression. J. Clin. Immunol. 2003;23:485–497. doi: 10.1023/b:joci.0000010425.30967.0f. [DOI] [PubMed] [Google Scholar]
- 63.Naderi N, Pourfathollah AA, Alimoghaddam K, Moazzeni SM. Cord blood dendritic cells prevent the differentiation of naïve T-helper cells towards Th1 irrespective of their subtype. Clin. Exp. Med. 2009;9:29–36. doi: 10.1007/s10238-008-0020-2. [DOI] [PubMed] [Google Scholar]
- 64.Trivedi HN, HayGlass KT, Gangur V, Allardice JG, Embree JE, Plummer FA. Analysis of neonatal T cell and antigen presenting cell functions. Hum. Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 65.Chang JF, Thomas CA, Kung JT. Induction of high level IL-2 production in CD4+8-T helper lymphocytes requires post-thymic development. J Immunol. 1991;147:851–859. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.