Abstract
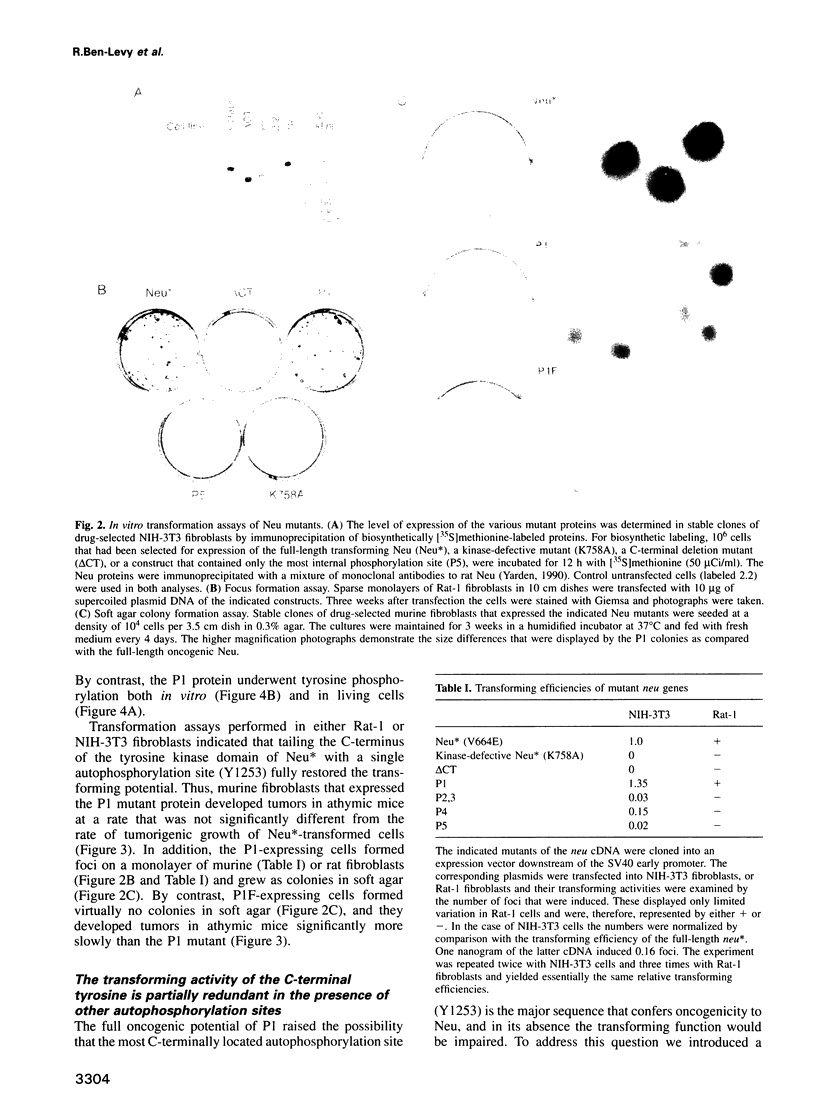
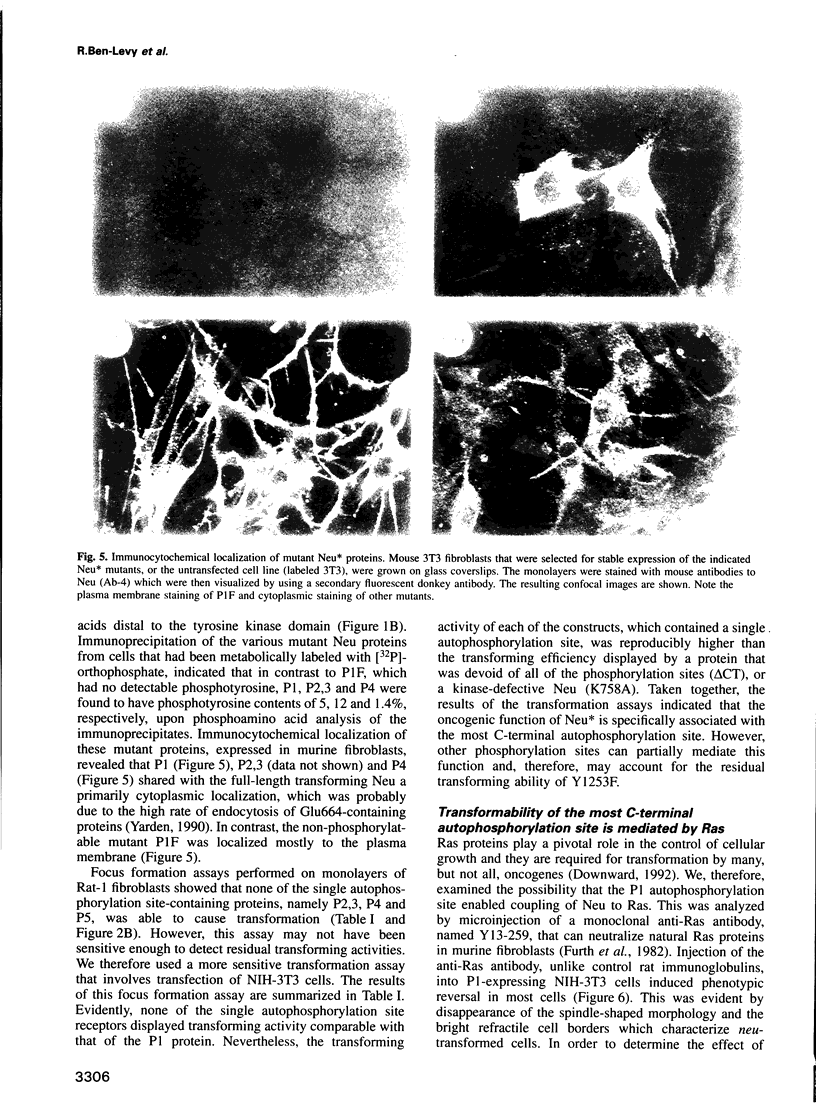
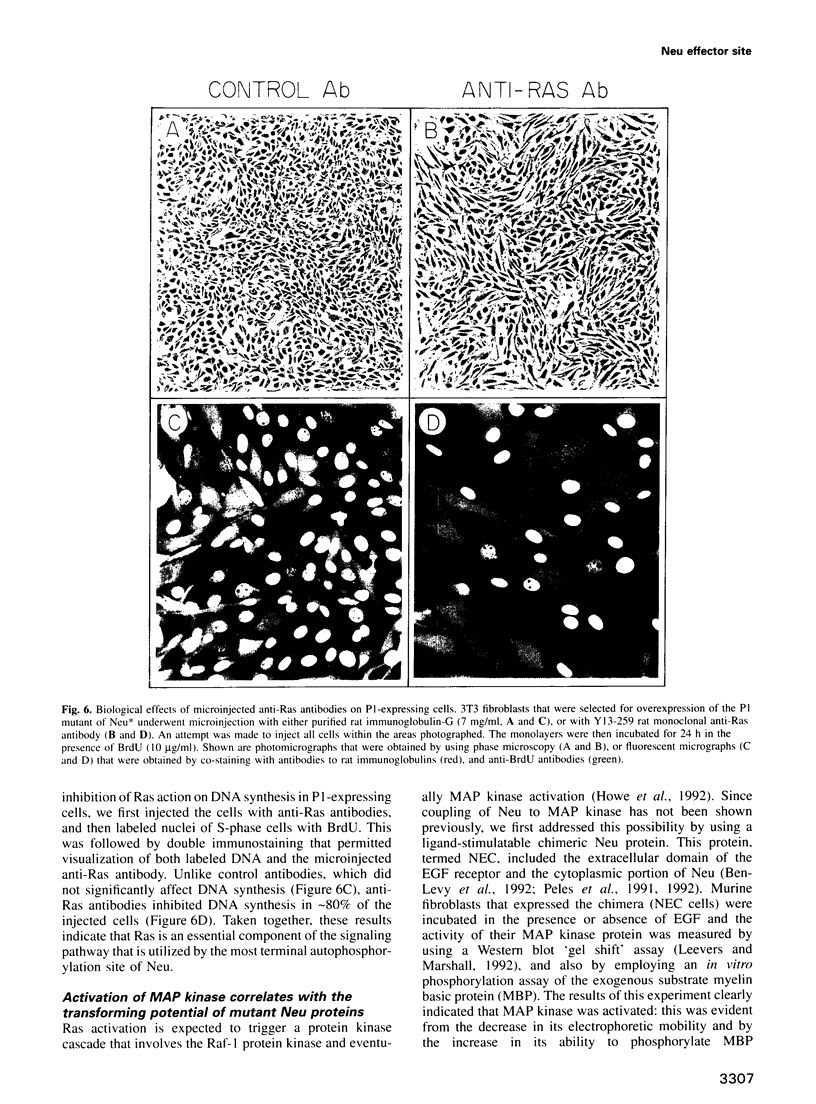
The transforming potential of the Neu/ErbB-2 receptor tyrosine kinase undergoes inactivation by deletion of the non-catalytic C-terminal tail, which contains five autophosphorylation sites. To determine which site is essential for oncogenicity, we tailed the C-terminally-deleted mutant with individual autophosphorylation sites. Complete restoration of the transforming action in vitro and in vivo was conferred by a stretch of 12 amino acids that contained the most C-terminal tyrosine autophosphorylation site (Y1253). Reconstitution of transformation was specific to this amino acid sequence because none of the other autophosphorylation sites, when grafted individually, caused transformation, and replacement of the tyrosine with a phenylalanine residue significantly reduced the oncogenic potential of both the full-length and the tailed proteins. When present alone the most C-terminal sequence enabled coupling to a biochemical pathway that includes Ras, MAP kinase and transactivation of Jun. These results indicate that the multiplicity of autophosphorylation sites on a receptor tyrosine kinase is not essential for transformability, and implicate the MAP kinase pathway in transduction of the oncogenic signal of Neu/ErbB-2.
Full text
PDF



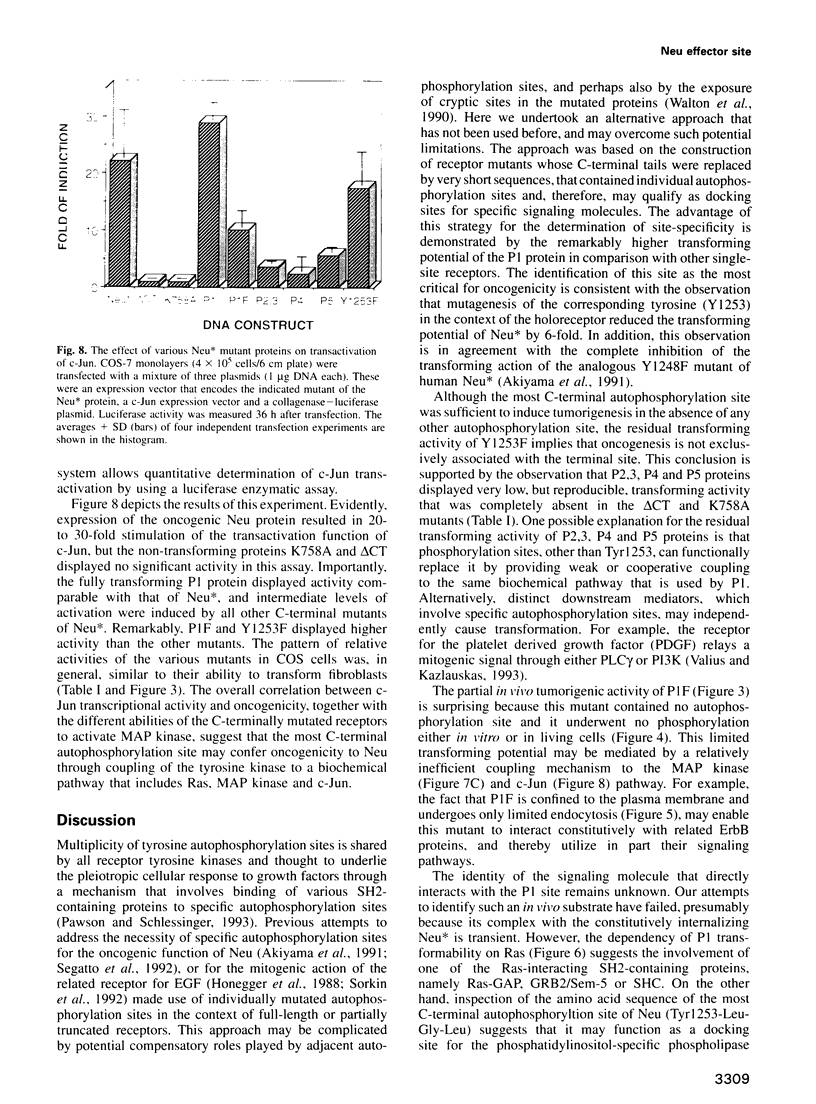


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Matsuda S., Namba Y., Saito T., Toyoshima K., Yamamoto T. The transforming potential of the c-erbB-2 protein is regulated by its autophosphorylation at the carboxyl-terminal domain. Mol Cell Biol. 1991 Feb;11(2):833–842. doi: 10.1128/mcb.11.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Levy R., Peles E., Goldman-Michael R., Yarden Y. An oncogenic point mutation confers high affinity ligand binding to the neu receptor. Implications for the generation of site heterogeneity. J Biol Chem. 1992 Aug 25;267(24):17304–17313. [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. Regulatory mechanisms for ras proteins. Bioessays. 1992 Mar;14(3):177–184. doi: 10.1002/bies.950140308. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F., Kim U. H., Rhee S. G., Molloy C. J., Segatto O., Di Fiore P. P. The erbB-2 mitogenic signaling pathway: tyrosine phosphorylation of phospholipase C-gamma and GTPase-activating protein does not correlate with erbB-2 mitogenic potency. Mol Cell Biol. 1991 Apr;11(4):2040–2048. doi: 10.1128/mcb.11.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Hazan R., Margolis B., Dombalagian M., Ullrich A., Zilberstein A., Schlessinger J. Identification of autophosphorylation sites of HER2/neu. Cell Growth Differ. 1990 Jan;1(1):3–7. [PubMed] [Google Scholar]
- Honegger A., Dull T. J., Bellot F., Van Obberghen E., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Biological activities of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988 Oct;7(10):3045–3052. doi: 10.1002/j.1460-2075.1988.tb03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
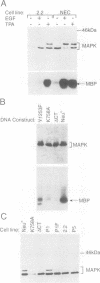
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., Dionne C. A., Li W., Li N., Spivak T., Honegger A. M., Jaye M., Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992 Aug 20;358(6388):681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Peles E., Lamprecht R., Ben-Levy R., Tzahar E., Yarden Y. Regulated coupling of the Neu receptor to phosphatidylinositol 3'-kinase and its release by oncogenic activation. J Biol Chem. 1992 Jun 15;267(17):12266–12274. [PubMed] [Google Scholar]
- Peles E., Levy R. B., Or E., Ullrich A., Yarden Y. Oncogenic forms of the neu/HER2 tyrosine kinase are permanently coupled to phospholipase C gamma. EMBO J. 1991 Aug;10(8):2077–2086. doi: 10.1002/j.1460-2075.1991.tb07739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E., Yarden Y. Neu and its ligands: from an oncogene to neural factors. Bioessays. 1993 Dec;15(12):815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- Peters K. G., Marie J., Wilson E., Ives H. E., Escobedo J., Del Rosario M., Mirda D., Williams L. T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992 Aug 20;358(6388):678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Rotin D., Margolis B., Mohammadi M., Daly R. J., Daum G., Li N., Fischer E. H., Burgess W. H., Ullrich A., Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992 Feb;11(2):559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segatto O., Lonardo F., Helin K., Wexler D., Fazioli F., Rhee S. G., Di Fiore P. P. erbB-2 autophosphorylation is required for mitogenic action and high-affinity substrate coupling. Oncogene. 1992 Jul;7(7):1339–1346. [PubMed] [Google Scholar]
- Segatto O., Lonardo F., Pierce J. H., Bottaro D. P., Di Fiore P. P. The role of autophosphorylation in modulation of erbB-2 transforming function. New Biol. 1990 Feb;2(2):187–195. [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Helin K., Waters C. M., Carpenter G., Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. Contrasting significance of tyrosine 992 in the native and truncated receptors. J Biol Chem. 1992 Apr 25;267(12):8672–8678. [PubMed] [Google Scholar]
- Stancovski I., Peles E., Ben Levy R., Lemprecht R., Kelman Z., Goldman-Michael R., Hurwitz E., Bacus S., Sela M., Yarden Y. Signal transduction by the neu/erbB-2 receptor: a potential target for anti-tumor therapy. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):95–103. doi: 10.1016/0960-0760(92)90192-l. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. Neu receptor dimerization. Nature. 1989 Jun 22;339(6226):587–587. doi: 10.1038/339587a0. [DOI] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Valius M., Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell. 1993 Apr 23;73(2):321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Chen W. S., Rosenfeld M. G., Gill G. N. Analysis of deletions of the carboxyl terminus of the epidermal growth factor receptor reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substrates. J Biol Chem. 1990 Jan 25;265(3):1750–1754. [PubMed] [Google Scholar]
- Weiner D. B., Liu J., Cohen J. A., Williams W. V., Greene M. I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989 May 18;339(6221):230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Agonistic antibodies stimulate the kinase encoded by the neu protooncogene in living cells but the oncogenic mutant is constitutively active. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2569–2573. doi: 10.1073/pnas.87.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]