Abstract
Francisella tularensis is a highly virulent intracellular bacterium causing the zoonotic disease tularemia. It recurrently causes human and animal outbreaks in northern Europe, including Finland. Although F. tularensis infects several mammal species, only rodents and lagomorphs seem to have importance in its ecology. Peak densities of rodent populations may trigger tularemia outbreaks in humans; however, it is still unclear to which extent rodents or other small mammals maintain F. tularensis in nature. The main objective of this study was to obtain information about the occurrence of F. tularensis in small mammals in Finland. We snap-trapped 547 wild small mammals representing 11 species at 14 locations around Finland during 6 years and screened them for the presence of F. tularensis DNA using PCR analysis. High copy number of F. tularensis-specific DNA was detected in tissue samples of five field voles (Microtus agrestis) originating from one location and 2 years. According to DNA sequences of the bacterial 23S ribosomal RNA gene amplified from F. tularensis–infected voles, the infecting agent belongs to the subspecies holarctica. To find out the optimal tissue for tularemia screening in voles, we compared the amounts of F. tularensis DNA in lungs, liver, spleen, and kidney of the infected animals. F. tularensis DNA was detectable in high levels in all four organs except for one animal, whose kidney was F. tularensis DNA-negative. Thus, at least liver, lung, and spleen seem suitable for F. tularensis screening in voles. Thus, liver, lung, and spleen all seem suitable for F. tularensis screening in voles. In conclusion, field voles can be heavily infected with F. tularensis subsp. holarctica and thus potentially serve as the source of infection in humans and other mammals.
Key Words: : Distribution, Francisella tularensis, PCR, Tularemia, Voles
Introduction
Francisella tularensis, a highly virulent facultative intracellular bacterium causing the zoonotic disease tularemia, is endemic in many regions of the Northern Hemisphere (Ellis et al. 2002, Oyston 2008). Four subspecies with different geographical distributions and virulence are currently recognized (Ellis et al. 2002, Keim et al. 2007). The disease tularemia is caused primarily by two subspecies: F. tularensis subsp. tularensis (type A), which is almost completely restricted to North America, and F. tularensis subsp. holarctica (type B), which occurs in the entire holarctic region, including Finland (Sjöstedt 2007).
This pathogen has a wide range of animal hosts, such as rabbits, hares, voles, and other rodents, but its life cycle in nature is not well understood. In addition to mammals, it has also been isolated from surface water, mud, and mosquito larvae collected in endemic areas (Broman et al. 2011, Lundström et al. 2011), which supports the hypothesis that F. tularensis possibly could persist in natural waters, possibly in aquatic amoebae. Yet, there is also evidence that rodents play an important role in the ecology of tularemia because there seems to be a correlation of tularemia outbreaks with preceding peaks of vole cycles, at least in Finland, Sweden (Tärnvik et al. 1996), and Hungary (Gyuranecz et al. 2012). Furthermore, outbreak investigations suggest that high rodent population densities may trigger tularemia outbreaks in humans (Reintjes et al. 2002, Allue et al. 2008, Grunow et al. 2012). Exposure to rodents or their droppings has been suspected to be the source of infection in a large tularemia outbreak in Sweden in 1966–1967 (Dahlstrand et al. 1971) and in Kosovo in 1999–2000 (Reintjes et al. 2002, Grunow et al. 2012). Some studies on F. tularensis occurrence in wild rodents have been carried out earlier and the results have been slightly conflicting. Relatively high prevalences of F. tularensis infections in wild rodents have been detected at human tularemia outbreak sites in Germany (Kaysser et al. 2008), China (Zhang et al. 2006), Slovakia, and Austria (Gurycova et al. 2001). During interepizootic periods, however, the pathogen is found in rodents only rarely (Vest et al. 1965, Arata et al. 1973, Broman et al. 2011, Gyuranecz et al. 2011). Despite relatively high periodical incidence in humans, no systematic investigations on possible reservoirs of F. tularensis have been carried out in Finland so far, and only few previous studies have been done in Fennoscandia (Mörner et al. 1988, Broman et al. 2011).
The clinical manifestations of F. tularensis infection in humans depend mainly on the route of infection (Oyston 2008). Humans can get infected through arthropod bites, direct contact with infected animals, inhalation of infective aerosols, or ingestion of contaminated food or water. In Sweden, the disease seems to be mainly transmitted by mosquito bites (Eliasson et al. 2002, Rydén et al. 2012), whereas in Norway human tularemia is most commonly associated with contaminated water (Larssen et al. 2011).
In Finland, tularemia is a notifiable disease, and clinical microbiology laboratories report all confirmed tularemia cases to the National Infectious Disease Register kept by the National Institute for Health and Welfare (THL). Data are available since 1995, and notifications include information on date of sample collection, date of birth, sex, and place of treatment. During 1995–2012, the National Infectious Diseases Register received 5072 notifications of laboratory-confirmed tularemia cases. The annual number of cases ranged from 29 to 926 and the incidence rates show marked geographical and temporal differences. The provinces of Central Finland and North and South Bothnia clearly dominate in human incidence, with fewer cases in other districts. The cumulative incidence in Finland in 1996–2004 was more than 37 cases/100,000 inhabitants (Splettstoesser et al. 2009). The geographical distribution of tularemia is typically uneven, and most cases are reported from relatively small foci (Tärnvik et al. 2004, Svensson et al. 2009, Splettstoesser et al. 2009). Finland and Sweden reported the highest incidence of human tularemia of all European Union member states (Splettstoesser et al. 2009). Here we studied if, how commonly, and where F. tularensis occurs in wild small mammals in Finland. The screened species represent the small mammal fauna in Finland.
Materials and Methods
Sample collection
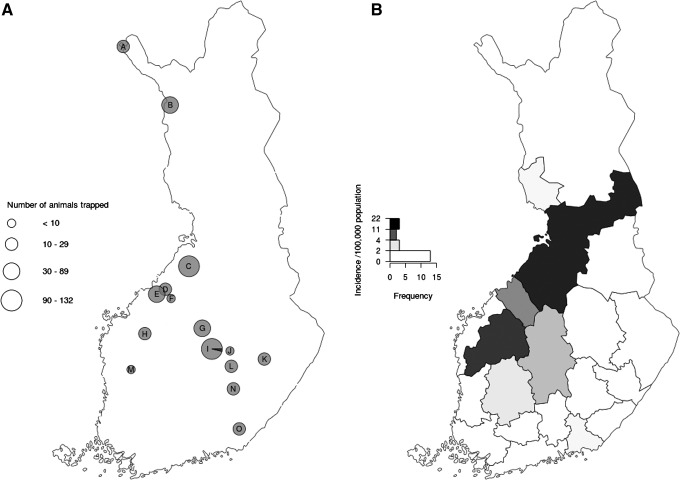
A total of 547 small mammals representing 11 species (Table 1) were collected between 2006 and 2011 from 15 locations in Finland (Fig. 1A) in connection with national monitoring of vole fluctuations by the Finnish Forest Research Institute. The locations included both districts where tularemia clusters in humans are recurrent and those with low incidence. The incidence of human tularemia by health care district is shown in Figure 1B. Small mammals were snap-trapped, frozen immediately on dry ice, and dissected later at the laboratory. At dissection, samples from lung, liver, spleen, and kidney were collected and stored at −20°C. Furthermore, species, sex, and age of the animals were determined.
Table 1.
Detection Of F. tularensis in Small Mammals by Species and Geographic Origin, Finland 2006–2011
| No. PCRa-positive/ no. captured | ||||||||
|---|---|---|---|---|---|---|---|---|
| Small mammal species | ||||||||
| Trapping location, trapping year(s) | Arvicola amphibius, water vole | Lemmus lemmus, Norway lemming | Microtus agrestis, field vole | Myodes glareolus, bank vole | Myopus schisticolor, wood lemming | Sorex araneus, common shrew | Other speciesb | Total no. |
| Kilpisjärvi (A), 2010 | 0/17 | 0/2 | 0/1 | 0/2 | 0/22 | |||
| Muonio (B), 2010 | 0/40 | 0/8 | 0/1 | 0/4 | 0/3 | 0/56 | ||
| Vihanti (C), 2011 | 0/6 | 0/67 | 0/15 | 0/6 | 0/94 | |||
| Kannus (D), 2008 | 0/15 | 0/15 | ||||||
| Kokkola (E), 2008–2009 | 0/1 | 0/36 | 0/3 | 0/40 | ||||
| Toholampi (F), 2009 | 0/4 | 0/4 | ||||||
| Viitasaari (G), 2008–2009 | 0/62 | 0/62 | ||||||
| Lapua (H), 2006 | 0/20 | 0/20 | ||||||
| Konnevesi (I), 2007–2009c | 5/55 | 0/77 | 5/132 | |||||
| Suonenjoki (J), 2009 | 0/2 | 0/2 | ||||||
| Heinävesi (K), 2008–2009 | 0/2 | 0/18 | 0/20 | |||||
| Pieksämäki (L), 2008 | 0/20 | 0/20 | ||||||
| Karvia (M), 2009 | 0/6 | 0/6 | ||||||
| Mikkeli (N), 2008–2009 | 0/25 | 0/25 | ||||||
| Luumäki (O), 2008 | 0/29 | 0/29 | ||||||
| Total PCR-positive/captured | 0/20 | 0/57 | 5/237 | 0/182 | 0/22 | 0/18 | 0/11 | 5/547 |
Denominators represent the total amount of screened animals.
Real-time PCR targeting the bacterial 23-kDa gene of F. tularensis (Skottman et al. 2007).
Species (no.): Micromys minutus, Eurasian harvest mouse (1), Myodes rufocanus, grey-sided vole (2), Myodes rutilus, red vole (3), Sicista betulina, northern birch mouse (2), Sorex minutus, pygmy shrew (3).
Year (PCR-positive/captured) 2007 (0/17), 2008 (1/63), 2009 (4/52).
FIG. 1.
(A) Small mammal trapping locations across Finland. The size of the pies represents the total number of samples; the black area is the number of F. tularensis PCR-positive samples, respectively. Accurate trapping locations (municipality, sample number): A (Kilpisjärvi, 22), B (Muonio, 56), C (Vihanti, 94), D (Kannus 15), E (Kokkola, 40), F (Toholampi, 4), G (Viitasaari, 62), H (Lapua, 20), I (Konnevesi, 132), J (Suonenjoki, 2), K (Heinävesi, 20), L (Pieksämäki, 20), M (Karvia, 6), N (Mikkeli, 25), O (Luumäki, 29). (B) Cumulative incidence of tularemia infection in human population by healthcare district in Finland 2006–2011 (National Infectious Disease Registry).
Ethics statement
According to the Finnish Act on the Use of Animals for Experimental Purposes (62/2006) and a further decision by the Finnish Animal Experiment Board (May 16, 2007), snap-trapping is not considered an animal experiment and therefore no ethical permit was needed. Permits (23/571/2001 and 4/571/2007) for capturing protected species (in this study Sorex spp., Micromys minutus, Lemmus lemmus, Myopus schisticolor, Myodes rufocenus, and Myodes rutilus were granted by the Finnish Ministry of the Environment. Other species captured in this study are not protected in Finland, and none of the captured species are included in the Red List of Finnish Species.
DNA extraction and PCR analyses
DNA was extracted from about 20 mg of liver tissue with the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI), according to the manufacturer's protocol for animal tissue. Each sample batch (2–12 samples) contained purified water as a negative control. The concentration and purity of the extracted DNA were determined with the Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE).
The liver DNA samples were screened using a real-time PCR assay targeting specifically the 23-kDa gene of F. tularensis (Skottman et al. 2007). All PCRs were run in duplicate with ABI 7300/7500 instruments (Applied Biosystems, Foster City, CA), and were analyzed in series of three 10-fold (undiluted, 1:10, 1:100) dilutions. Every PCR run included several negative and one positive DNA control (F. tularensis LVS control strain) as well as inhibition controls.
All samples positive in the initial screening were further analyzed from freshly extracted DNA using a semiquantitative real-time PCR assay (qPCR) developed from the PCR used in screening (Skottman et al. 2007). The assay includes internal controls for controlling of inhibition. In this assay, the number of F. tularensis bacterial cells in liver samples was estimated using the genomic equivalent (GE) (Tomaso et al. 2010). Molins and co-workers (2009) have previously determined that each Francisella genome copy amounts to 2 fg. We used stringent criteria when interpreting the results: Only samples repeatedly containing F. tularensis DNA analogous to at least 1 GE (one bacterium) per 1000 animal cells were interpreted as positive. The positivity of the samples was further confirmed with the Genesig Real-Time PCR Detection Kit for Francisella tularensis (PrimerDesign Ltd, Southampton, UK) using the Oasig Lyophilised 2×qPCR Mastermix (PrimerDesign Ltd, Southampton, UK) according to the manufacturer's instructions. In addition to liver samples, DNA samples extracted similarly from kidney, lung, and spleen tissues of the positive animals were analyzed with the 23-kDa qPCR and the Genesig kit to roughly compare their F. tularensis loads and to determine the optimal tissue for screening of F. tularensis. For comparison, these tissues from PCR-negative and borderline animals were tested with the 23-kDa qPCR assay and the Genesig kit as well.
Sequence analysis
To further confirm and decipher the positive F. tularensis–specific PCR findings, we amplified and sequenced a part of the bacterial 23S ribosomal RNA gene from three samples (Kotilainen et al. 1998). PCR products were cloned to Escherichia coli vectors using the TOPO TA cloning kit according to the manufacturer's instructions (Invitrogen Corporation, Carlsbad, CA). Sequencing and manual editing of the sequences was performed with the automated ABI 3130XL DNA sequencer (ABI) using the BigDye chemistry (ABI) and the Sequencer 4.0 program (Gene Codes Corporation, Ann Arbor, MI), respectively. The BLAST search tool (www.ncbi.nml.nih.gov/BLAST/) was used to compare our sequences to reference sequences published in GenBank.
Results
Of the 547 small mammals studied representing 11 species (Table 1), F. tularensis DNA was unequivocally detected in liver samples of five field voles (Microtus agrestis; Table 2). All negative controls remained negative. In addition to the five definite PCR-positive animals, we detected traces of F. tularensis DNA (less than 1 GE per 1000 animal cells) in livers but not in other organs of three more field voles and six bank voles (Myodes glareolus). Results from both quantitative PCR methods used were wholly congruent, and no significant inhibition was noticed. We interpreted the F. tularensis status of these nine rodents as inconclusive.
Table 2.
Comparison of F. Tularensis DNA Loads in Four Organs of PCR-Positive Voles
| F. tularensis load | |||||||
|---|---|---|---|---|---|---|---|
| Species | Trapping year | Trapping location | Gender | Kidney | Liver | Lungs | Spleen |
| Microtus agrestis | 2009 | Konnevesi | Male | ++ | ++ | ++ | ++ |
| Microtus agrestis | 2009 | Konnevesi | Male | ++ | ++ | ++ | ++ |
| Microtus agrestis | 2009 | Konnevesi | Female | + | + | + | + |
| Microtus agrestis | 2009 | Konnevesi | Male | ++ | ++ | ++ | ++ |
| Microtus agrestis | 2008 | Konnevesi | Male | − | + | ++ | + |
+, The number of F. tularensis less than 100 bacterial cells per 1000 animal cells.
++, The number of F. tularensis more than 100 bacterial cells per 1000 animal cells.
−, Negative for F. tularensis.
All PCR-positive animals originated from Konnevesi, Central Finland (Fig. 1). Four out of five infected animals were males. One infected field vole was collected in fall 2008, whereas the rest of them were from spring 2009 (Table 2).
Partial sequencing of the 23S rRNA gene from three positive samples revealed 100% similarity (843/843 bp) with several previously published sequences representing F. tularensis ssp. holarctica, e.g., ATCC 29684F (GU073998). The accession numbers of the new sequences are KC740496, KC740497, and KC740498.
Comparison of the F. tularensis load in four organs of the five F. tularensis DNA-positive animals showed that F. tularensis DNA can be detected in liver, lung, and spleen tissue, but the kidney is not always positive (Table 2). The voles negative in the PCR screening using liver DNA as template remained wholly PCR negative in the tissue comparison.
Discussion
We screened hundreds of small mammals using F. tularensis–specific PCR analyses and demonstrated the presence of F. tularensis in voles. With our stringent criteria for positive results, we found definite F. tularensis infections in field voles only, even though we screened almost as many bank voles from the same sampling location. However, in addition to the five definite PCR-positive field voles, we detected low amounts of F. tularensis DNA in nine additional animals, including bank voles. The interspecies difference in the occurrence of infection may suggest that voles of different species and genera may differ in their susceptibility and importance as reservoir or secondary hosts in the natural cycle of F. tularensis. The possibility of latent infections adds one more potential aspect to the life cycle and maintenance of tularemia, but these findings should be verified with experimental studies.
The trapped small mammal species (Table 1) represent well the actual species prevalence in Finland. As in other holarctic northern regions, voles dominate the small mammal fauna here, the most widespread and abundant species being field voles and bank voles. Three murine species inhabit Finland, in addition, but all of them are restricted to the southern half of the country, and they usually occur at very low densities. Voles, instead, are widespread and periodically extremely abundant, even the most numerous mammal species of Finland. They play a major keystone role in northern ecosystems, acting both as prey for numerous predators and significant grazers, as well as carriers of zoonotic diseases (Cornulier et al. 2013, Korpela et al. 2013). Of holarctic northern mammal species, voles are therefore plausible hosts or even reservoirs for F. tularensis. This is supported, but not proven, by our PCR findings in voles but not in other small mammal species.
Most of our study sites and years showed no presence of acute F. tularensis infections, yet we found a relatively high prevalence in 2009 at one location in central Finland. In northern Fennoscandia, arvicoline rodents show regular multiannual cyclic fluctuations in population densities (Stenseth 1999, Hanski et al. 2001). Fall 2008 was the peak phase of the 3-year vole cycle in central Finland, and the spring of 2009, showing the highest F. tularensis prevalence in voles of our study, was the decline phase (Korpela et al. 2013). However, during these years no human epidemics were reported from central Finland; yet 2009 was an epidemic year elsewhere in Finland, with about 400 cases reported mainly from northern and southern Bothnia (National Infectious Diseases Register). The latest outbreak affecting over 200 patients occurred in central Finland in 2003, when altogether more than 800 cases were reported in Finland. Unfortunately, no rodents were available for this study from this epidemic region and year. Interestingly, similar to the epidemic year 2009, the year 2003 was also the decline phase of the vole cycle after the peak in 2002 (Korpela et al. 2013).
At the focal site in central Finland, the prevalence of F. tularensis infections in field voles was as high as 12% in spring 2009. The overall prevalence of F. tularensis in field voles was 2%. In Sweden and Hungary, researchers have detected F. tularensis in 0–5% of the local rodent population (Broman et al. 2011, Gyuranecz et al. 2011), which is in line with our results. A German study, however, has revealed higher prevalences: 10% in field voles and 4.4% in bank voles, and the highest prevalence (15%) in water voles (Arvicola amphibius) (Kaysser et al. 2008), but these results came from sites with recent human outbreaks. Investigation of an outbreak in Kosovo (Grunow et al. 2012) indicated that specimens collected during the tularemia outbreak had a similar prevalence of F. tularensis in rodent and hare tissues and their feces. When comparing our results with other European studies, it should be emphasized, however, that our data were from samples obtained from a national rodent fluctuation monitoring scheme, designed to facilitate understanding of the spatiotemporal population dynamics of voles and especially their risk of occurrence as pests to forestry (Huitu et al. 2009). The monitoring sites were evenly distributed across the country, being randomly located with respect to tularemia occurrence (Korpela et al. 2013). In particular, none of our sampling sites was a tularemia outbreak focus with a recent/ongoing human epidemic, as has usually been the case with tularemia rodent sampling elsewhere, resulting mainly in higher prevalences. The area where F. tularensis was detected was the one with the highest sample number. Indeed, the biggest limitation of our study is that the number of trapped animals was not evenly distributed over the locations. As expected, taxonomic analysis of the 23S rRNA gene revealed that F. tularensis strains detected in voles in this study belonged to the subspecies holartica.
The five PCR-positive animals had all very high bacterial loads in almost all organs, indicating acute infections. Splenomegaly was noticed at dissection in three out of five individuals, but systematic necropsies were not carried out. Screening of various organs showed that there were no significant differences in bacterial concentration among organs except kidney, which remained negative in one vole exhibiting otherwise positive organ results. None of the organ DNA samples showed significant PCR inhibition. For these reasons, lung, liver, and spleen seem all suitable for tularemia screening in voles. For practical reasons, we favor liver: It is voluminous and easy to homogenize.
In summary, we studied the occurrence of F. tularensis in various wild small mammal species to find out if and how commonly this pathogen occurs in the most prevalent species of our region. Our results indicate that field voles clearly can carry F. tularensis with high bacterial loads, even outside epidemics. Thus, they may have a role in the ecological cycle of this pathogen. The focal geographical occurrence of F. tularensis in voles could possibly be due to unequal sample sizes. However, human tularemia is also unevenly distributed in Finland. Many factors can affect pathogen occurrence in rodents, e.g., species, season, population age structure, and breeding status, and therefore longitudinal monitoring including serological assays for F. tularensis is needed to reveal pathogen dynamics in rodent populations.
Acknowledgments
We thank Marja Isomursu, Finnish Food Safety Authority (Evira), for providing a positive control tissue sample, and Mats Forsman, Swedish Defence Agency, for providing positive control DNA. Salla Toikkanen, National Institute for Health and Welfare, is thanked for helping with figure preparation.
This study was partially funded by European Union grant FP7-261504EDENext and is catalogued by the EDENext Steering Committee as EDENext080 (www.edenext.eu). This study was also financially supported by the Hospital District of Helsinki and Uusimaa (EVO-TYH2011305) and Academy of Finland (grant no. 133495).
Author Disclosure Statement
No competing financial interests exist.
References
- Allue M, Sopena CR, Gallardo MT, Mateos L, et al. Tularaemia outbreak in Castilla y Leon, Spain, 2007: An update. Euro Surveill 2008; 13:18948. [PubMed] [Google Scholar]
- Arata A, Chamsa M, Farhang-Azadi A, Mescerjakova I, et al. First detection of tularaemia in domestic and wild mammals in Iran. Bull World Health Organ 1973; 49:597–603 [PMC free article] [PubMed] [Google Scholar]
- Broman T, Thelaus J, Andersson AC, Bäckman P, et al. Molecular detection of persistent Francisella tularensis subspecies holarctica in natural waters. Int J Microbiol 2011; 2011:851946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornulier T, Yocco NG, Bretagnolle V, Brommer JE, et al. Europe-wide dampening of population cycles in keystone herbivores. Science 2013; 340:63–66 [DOI] [PubMed] [Google Scholar]
- Dahlstrand S, Ringertz O, Zetterberg B. Airborne tularemia in Sweden. Scand J Infect Dis 1971; 3:7–16 [DOI] [PubMed] [Google Scholar]
- Eliasson H, Lindback J, Nuorti JP, Arneborn M, et al. The 2000 tularemia outbreak: A case-control study of risk factors in disease-endemic and emergent areas, Sweden. Emerg Infect Dis 2002; 8:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev 2002; 15: 631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunow R, Kalaveshi A, Kühn A, Mulliqi-Osmani G, et al. Surveillance of tularaemia in Kosovo, 2001 to 2010. Euro Surveill 2012; 17:20217. [DOI] [PubMed] [Google Scholar]
- Gurycová D, Výrosteková V, Khanakah G, Kocianová E, et al. Importance of surveillance of tularemia natural foci in the known endemic area of Central Europe, 1991–1997. Wien Klin Wochenschr 2001; 113:433–438 [PubMed] [Google Scholar]
- Gyuranecz M, Rigo K, Dan A, Földvári G, et al. Investigation of the ecology of Francisella tularensis during an inter-epizootic period. Vector Borne Zoonotic Dis 2011; 11:1031–1035 [DOI] [PubMed] [Google Scholar]
- Gyuranecz M, Reiczigel J, Krisztalovics K, Monse L, et al. Factors influencing emergence of tularemia, 1984–2010, Hungary. Emerg Infect Dis 2012; 18:1379–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski I, Henttonen H, Korpimäki E, Oksanen L, et al. Small rodent dynamics and predation. Ecology 2001; 82:1505–1520 [Google Scholar]
- Huitu O, Kiljunen N, Korpimäki E, Koskela E, et al. Density-dependent vole damage in silviculture and associated economic losses at a nationwide scale. Forest Ecol Manag 2009; 258:1219–1224 [Google Scholar]
- Kaysser P, Seibold E, Matz-Rensing K, Pfeffer M, et al. Re-emergence of tularemia in Germany: Presence of Francisella tularensis in different rodent species in endemic areas. BMC Infect Dis 2008; 8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim P, Johansson A, Wagner DM. Molecular epidemiology, evolution, and ecology of Francisella. Ann NY Acad Sci 2007; 1105:30–66 [DOI] [PubMed] [Google Scholar]
- Korpela K, Delgado M, Henttonen H, Korpimäki E, et al. Nonlinear effects of climate on boreal rodent dynamics: mild winters do not negate high-amplitude cycles. Global Change Biol 201319:697–710 [DOI] [PubMed] [Google Scholar]
- Kotilainen P, Jalava J, Meurman O, Lehtonen OP, et al. Diagnosis of meningococcal meningitis by broad-range bacterial PCR with cerebrospinal fluid. J Clin Microbiol 1998; 36: 2205–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larssen KW, Afset JE, Heier BT, Krogh T, et al. Outbreak of tularaemia in central Norway, January to March 2011. Euro Surveill 2011;16:19828. [PubMed] [Google Scholar]
- Lundström JO, Andersson AC, Backman S, Schäfer ML, et al. Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerg Infect Dis 2011; 17:794–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molins CR, Carlson JK, Coombs J, Petersen JM. Identification of Francisella tularensis subsp. tularensis A1 and A2 infections by real-time polymerase chain reaction. Diagn Microbiol Infect Dis 2009; 64:6–12 [DOI] [PubMed] [Google Scholar]
- Mörner T, Sandström G, Mattson R, Nilsson PO. Infections with Francisella tularensis biovar palaearctica in hares (Lepus timidus, Lepus Europaeus) from Sweden. J Wildl Dis 1988; 24:422–433 [DOI] [PubMed] [Google Scholar]
- Oyston PCF. Francisella tularensis: Unravelling the secrets of an intracellular pathogen. J Med Microbiol 2008; 57:921–930 [DOI] [PubMed] [Google Scholar]
- Reintjes R, Dedushaj I, Gjini A, Rikke T, et al. Tularemia outbreak investigation in Kosovo: Case control and environmental studies. Emerg Infect Dis 2002; 8:69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén P, Björk R, Schäfer ML, Lundström JO, et al. Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis 2012; 205:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt A. Tularemia: History, epidemiology, pathogen physiology, and clinical manifestations. Ann NY Acad Sci 2007; 1105:1–29 [DOI] [PubMed] [Google Scholar]
- Skottman T, Piiparinen H, Hyytiainen H, Myllys V, et al. Simultaneous real-time PCR detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis. Eur J Clin Microbiol Infect Dis 2007; 26:207–211 [DOI] [PubMed] [Google Scholar]
- Splettstoesser WD, Piechotowski I, Buckendahl A, Frangoulidid P, et al. Tularemia in Germany: The tip of the iceberg? Epidemiol Infect 2009; 137:736–743 [DOI] [PubMed] [Google Scholar]
- Stenseth NC. Population cycles in voles and lemmings: Density dependence and phase dependence in a stochastic world. Oikos 1999; 87:427–461 [Google Scholar]
- Svensson K, Back E, Eliasson H, Berglund L, et al. Landscape epidemiology of tularemia outbreaks in Sweden. Emerg Infect Dis 2009; 15:1937–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tärnvik A, Sandström G, Sjöstedt A. Epidemiological analysis of tularemia in Sweden 1931–1993. FEMS Immunol Med Microbiol 1996; 13:201–204 [DOI] [PubMed] [Google Scholar]
- Tärnvik A, Priebe HS, Grunow R. Tularaemia in Europe: An epidemiological overview. Scand J Infect Dis 2004; 36:350–355 [DOI] [PubMed] [Google Scholar]
- Tomaso H, Kattar M, Eickhoff M, Wernery U, et al. Comparison of commercial DNA preparation kits for the detection of Brucellae in tissue using quantitative real-time PCR. BMC Infect Dis 2010; 10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest ED, Lundgren DL, Parker DD, Johnson DE, et al. Results of a five-year survey for certain enzootic diseases in the fauna of Western Utah. Am J Trop Med Hyg 1965;14:124–135 [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu W, Chu MC, He J, et al. Francisella tularensis in Rodents, China. Emerg Infect Dis 2006; 12:994–996 [DOI] [PMC free article] [PubMed] [Google Scholar]