Abstract
Epigenetic mechanisms are fundamental to understanding the regulatory networks of gene expression that govern stem cell maintenance and differentiation. Methylated histone H3 lysine 4 (H3K4) has emerged as a key epigenetic signal for gene transcription; it is dynamically modulated by several specific H3K4 methyltransferases and demethylases. Recent studies have described new epigenetic mechanisms by which H3K4 methylation modifiers control self-renewal and lineage commitments of stem cells. Such advances in stem cell biology would have a high impact on the research fields of cancer stem cell and regenerative medicine. In this review, we discuss the recent progress in understanding the roles of H3K4 methylation modifiers in regulating embryonic and adult stem cells’ fates.
Keywords: Histone methylation, H3K4, Methyltransferase, Demethylase, Stem cell, Self-renewal, Differentiation
Introduction
Stem cells have long-term self-renewing activity and can commit to multiple cell types upon differentiation signals. Since Yamanaka and colleagues demonstrated that the four DNA-binding transcription factors Oct4, Sox2, c-Myc, and Klf4 transform fibroblasts into a type of pluripotent cells known as induced pluripotent stem cells, the importance of transcription factors in cellular reprogramming has been more recognized [1]. However, because the reprogramming efficiency of these four factors is low, it is evident that additional layers of co-regulatory mechanisms exist besides transcription factor-driven regulation [2]. In fact, a recent study demonstrated that the histone modification and DNA methylation profiles differ in one-third of the genome between human embryonic stem (ES) cells and primary fibroblasts [3], indicating that such remarkable epigenetic difference may serve as a major molecular mechanism in determining cellular characteristics of these two cell types. Notably, the functions of epigenetic modifiers in stem cell fate decision have been intensively studied.
Histone lysine methylation has been widely accepted as a key epigenetic modification. Unlike acetylation, the methylation does not change the charge of lysine residues and thus has a minimal direct effect on DNA-histone association. Rather, the different methylation status of specific histone lysines can serve as a unique platform for recruiting methylation “reader” proteins that activate or repress genes’ transcriptional activity. In general, histone H3 lysine 4 (H3K4), H3K36, and H3K79 methylation are gene activation marks, whereas H3K9, H3K27, and H4K20 methylation are gene-repressive modifications [4].
Histone lysine methylation is generated by a battery of histone methyltransferases (HMTs) that transfer the methyl group from S-adenosylmethionine to specific lysine residues. For example, H3K4 methylation is mediated by several SET [Su(var)3-9, Enhancer of zeste, Trithorax] domain-containing methyltransferases, including mixed lineage leukemia 1–5 (MLL1−5), SET1A/B, SET7/9, SET and MYND domain-containing protein 1–3 (SMYD1−3), Absent, Small, or Homeotic 1-like (ASH1L), SET domain and Mariner transposase fusion gene (SETMAR), and PR domain zinc finger protein 9 (PRDM9) [5-24]. Methylated lysines exist in three forms: mono-, di- and tri-methylation (me1, me2, and me3).
Similar to other histone modifications, histone methylation can be reversed by histone demethylases (HDMs). The first identified lysine-specific demethylase 1 [LSD1; also known as FAD-binding protein BRAF35-HDAC complex, 110 kDa subunit (BHC110) and Lysine-specific demethylase 1A (KDM1A)], together with LSD2, belongs to the polyamine oxidase family. LSD1 and LSD2 remove methyl groups from di- and monomethylated H3K4 but are unable to demethylate trimethylated H3K4 [25-28]. LSD1 was reported to also have H3K9 demethylation activity [29]. Subsequently, many Jumonji (JmjC) domain-containing histone demethylases have been discovered. In particular, the JARID1 family of histone demethylases (JARID1A−D) can erase H3K4me3 and H3K4me2 [30-35].
In this review, we summarize the recent progress in understanding the functions of H3K4 methyltransferases and demethylases in modulating stem cells’ fates.
H3K4 methylation
H3K4me3 occupies as many as 75% of all human gene promoters in several cell types (e.g., ES cells), indicating that it plays a critical role in mammalian gene expression [36,37]. In fact, H3K4me3 is required to induce critical developmental genes in animals, including Drosophila and several mammals, and is important for animal embryonic development [38]. H3K4me3 levels are positively correlated with gene expression levels [39,40] (Figure 1A).
Figure 1.
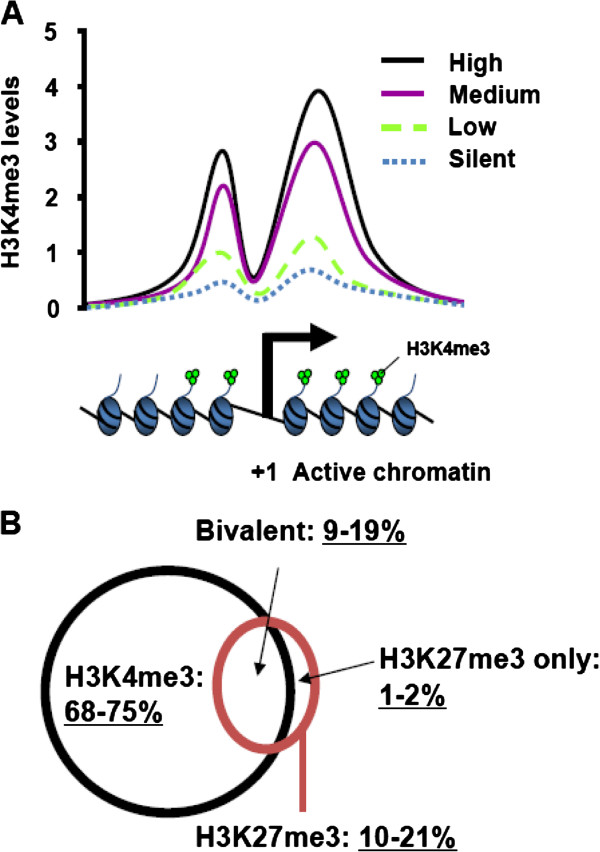
H3K4me3 marks actively transcribed and poised gene promoters in mammals. (A) The genome-wide correlation of mRNA expression levels (High, Medium, Low, and Silent) with H3K4me3 levels at human gene promoters. Note that a dip of H3K4me3 levels may be associated with the nucleosome-free region around the transcriptional start site (TSS). Adapted from [39]. (B) The Venn diagram showing the percentage of genes that have H3K4me3 and/or H3K27me3 in their promoters in mouse and human ES cells. All percentages are based on about total 18,000 genes. The “bivalent” denotes the promoters that contain both H3K4me3 and H3K27me3 marks. Adapted from [36,37,43].
Although H3K4me3 is clearly associated with actively transcribed genes, however, studies have demonstrated that H3K4me3 is localized around the transcription initiation sites of numerous unexpressed genes in human ES cells, primary hepatocytes, and several other cell types [36,37,41]. In particular, it frequently co-resides with the repressive mark H3K27me3 in the promoters of critical differentiation-specific genes [e.g., Homeobox (HOX) gene clusters] that are transcriptionally inactive in ES cells [36,37,42,43] (Figure 1B). It has been proposed that the “bivalent” domains, composed of H3K4me3 and H3K27me3, may maintain differentiation-specific gene promoters in a repressive status in self-renewing stem cells but be poised for prompt gene activation upon differentiation stimuli [42]. Consistent with this, many bivalent genes have increased H3K4me3 levels and decreased H3K27me3 levels while being transcriptionally activated during differentiation. Interestingly, recent studies demonstrated that most bivalent domains are occupied by LSD1 [44,45], indicating that it plays a role in maintaining low levels of dimethylated H3K4 (H3K4me2) that are often co-localized with H3K4me3. For these reasons, H3K4me3 is classified as a chromatin landmark for transcriptionally active or poised genes in ES cells [41].
Compared with mouse thymocytes, mouse ES cells contain higher levels of total genomic H3K4me3 and have higher H3K4me3 occupancy at the promoter of the pluripotent gene Oct4[46]. In agreement with this, global decreases in H3K4me3 levels occur during retinoic acid (RA)-induced differentiation of mouse ES cells [47]. In addition, there are dynamic changes in H3K4me3 profiles at specific sets of genes during ES cell differentiation. Such global and local changes in H3K4me3 profiles are partly because levels of H3K4me3-regulatory factors [e.g., WD repeat-containing protein 5 (WDR5), MLL1 and MLL3] are modulated [47]. It is believed that higher H3K4me3 levels allow the ES cell genome to be more open and transcriptionally permissive by recruiting chromatin-modifying factors. Therefore, unique H3K4me3 profiles at pluripotent and differentiation-specific genes may be key determinants of cellular identity.
Most H3K4me3-containing promoters are also occupied by H3K9/H3K14 acetylation [41]. In transcriptionally active genes, H3K36me3 and H3K79me2 are significantly enriched downstream of H3K4me3-containing promoters: H3K36me3 peaks toward the 3′ end of genes in gene bodies, whereas H3K79me2 is located toward the 5′ end [41]. Therefore, H3K4me3 likely cooperates with other histone marks for gene activation. The combinatorial arrangement of H3K4me3 and other histone marks may support, at least in part, the “histone code” hypothesis [48].
H3K4me2 decorates genomic regions independently of H3K4me3, although most of it overlaps with H3K4me3 near the transcription start sites [49]. H3K4me2 may have an antagonistic effect on DNA methylation [50]. Monomethylated H3K4 (H3K4me1) also co-occupies regions near the start sites with H3K4me3. Apart from the transcription start sites, H3K4me1, together with H3K27 acetylation, specifies enhancer regions [51,52]. In summary, H3K4me1, H3K4me2 and H3K4me3 have a commonality for gene activation, although their subsets play distinct roles in modulating chromatin function.
H3K4 methyltransferases
Some H3K4 methyltransferases are well conserved in different species. In yeast, the Set1 complex, also called Complex of Proteins Associated with Set1 (COMPASS), catalyzes the mono-, di- and trimethylation of H3K4 [5,8]. The protein complex is composed of the catalytic component of Set1 and seven other regulatory subunits (Cps60, Cps50, Cps40, Cps35, Cps30, Cps25, and Cps15) that are essential for full enzyme activity [38] (Table 1). In Drosophila, there are three Set1 homologs: dSet1, Trithorax (Trx), and Trithorax-related (Trr). The deletion of any of their genes results in lethality in flies, indicating that their target genes may not be redundant. In particular, loss of dSet1, but not Trx or Trr, leads to a global reduction of H3K4me2/3, suggesting that Trx and Trr have more specialized functions [38]. Human SET1A, SET1B, and MLL1−4 are yeast Set1 homologs and are related to dSet1 (the counterpart of SET1A and SET1B), Trx (the counterpart of MLL1 and MLL2), and Trr (the counterpart of MLL3 and MLL4) in Drosophila. Other SET domain-containing histone methyltransferases that methylate H3K4 but are not closely related to yeast Set1/COMPASS have also been identified and include MLL5, SET7 (also called SET9), SMYD1-3, SETMAR, and PRDM9 [6,15,24].
Table 1.
Subunit composition of H3K4 methyltransferase complexes in yeast and human
| Yeast SET1 | Human SET1A | Human SET1B | Human MLL1 | Human MLL2 | Human MLL3 | Human MLL4 | Human MLL5* |
|---|---|---|---|---|---|---|---|
| SET1 |
SET1A |
SET1B |
MLL1 |
MLL2 |
MLL3 |
MLL4 |
Mll5 |
| Cps60/Bre2 |
ASH2L |
ASH2L |
ASH2L |
ASH2L |
ASH2L |
ASH2L |
HCF1 |
| Cps50/Swd1 |
RBBP5 |
RBBP5 |
RBBP5 |
RBBP5 |
RBBP5 |
RBBP5 |
OGT |
| Cps30/Swd3 |
WDR5 |
WDR5 |
WDR5 |
WDR5 |
WDR5 |
WDR5 |
STK38 |
| Cpd25/Sdc1 |
DPY-30 |
DPY-30 |
DPY-30 |
DPY-30 |
DPY-30 |
DPY-30 |
PPP1CA |
| Cps40/Spp1 |
CFP1 |
CFP1 |
|
|
|
|
PPP1CB |
| Cps35/Swd2 |
WDR82 |
WDR82 |
|
|
|
|
PPP1CC |
| Cps15/Shg1 |
|
BOD1/BOD1L |
|
|
|
|
ACTB |
| |
HCF1/2 |
HCF1/2 |
HCF1/2 |
HCF1/2 |
NCOA6 |
NCOA6 |
|
| |
|
|
MENIN |
MENIN |
UTX |
UTX |
|
| |
|
|
|
PSIP1 |
PTIP |
PTIP |
|
| PA1 | PA1 |
* The subunits of MLL5 are not related to those of SET1, SET1A/B, and MLL1−4.
SET1A/1B and MLL1−4 are present in multi-protein complexes and share common core subunits, such as WDR5, Retinoblastoma-binding protein 5 (RBBP5), ASH2L, and Dumpy-30 (DPY-30), which are also highly conserved in yeast and flies [38] (Table 1). Several studies have demonstrated that these core subunits are indispensable for the enzyme activity of methyltransferases and biological functions [53-55]. In addition to common core subunits, there are unique subunits in the individual H3K4 methyltransferase complexes: WDR82 and CXXC finger protein 1 (CFP1) in the SET1 complex; Multiple endocrine neoplasia type 1 (MENIN) and PC4 and SFRS1-interacting protein 1 (PSIP1) in MLL1 and 2 complex; Host cell factor 1/2 (HCF1/2) in SET1, MLL1, and MLL2 complexes; and PAX transcription activation domain interacting protein 1 (PTIP), PTIP-associated protein 1 (PA1), Nuclear receptor coactivator 6 (NCOA6), and Ubiquitously transcribed X chromosome tetratricopeptide repeat protein (UTX) in the MLL3 and MLL4 complexes [12,16,19,22,56-63] (Table 1). These subunits may play important roles in recruiting H3K4 methyltransferases to specific genes and integrating additional histone-modifying capacities (see below).
MLL1 and MLL2
MLL1 (also known as MLL and KMT2A) was initially cloned from acute myeloid and lymphoid leukemia that contain frequent MLL1 chromosomal fusions and translocations [64-66]. The MLL1 gene encodes a protein of 3,972 amino acids; this protein contains several highly conserved functional domains, including the N-terminal AT-hook DNA binding domains, Plant homeo domains (PHD), a Bromo domain, and the catalytic SET domain (Figure 2). Inside cells, MLL1 protein is cleaved into MLL-N (320 kDa) and MLL-C (180 kDa) by Taspase I; these two large fragments dimerize through FY-rich motifs to form the functional MLL complex in vivo[67,68].
Figure 2.
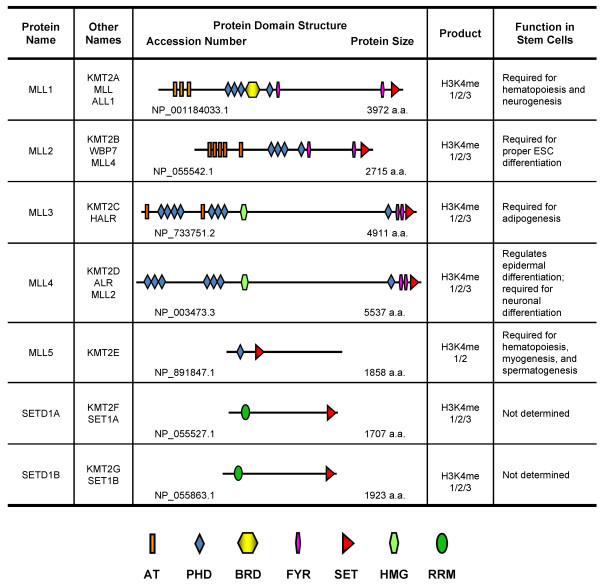
Protein domain architectures and stem cell function of MLL/SET1 H3K4 methyltransferases. AT: AT-hook DNA binding domain; PHD: Plant Homeo Domain; BRD: Bromodomain; FYR: FY-rich domain; SET: Su(var)3-9, Enhancer of zeste, Trithorax domain; HMG: High Mobility Group domain; RRM: RNA Recognition Motif.
Homozygous deletion of Mll1 is embryonic lethal; Mll1+/− mice display retarded growth and hematopoietic defects [69,70]. Specifically, expression of the key developmental genes, including Hoxa7 and Hoxc9, were shifted from the anterior boundaries toward the posterior regions in Mll1+/− embryos and were lost in Mll1−/− mice [69]. In addition, recent studies using a tissue-specific knockout mouse model revealed that Mll1 is essential for sustaining adult hematopoiesis [71,72]. Mll1 is not required for survival, proliferation, and differentiation of subventricular zone neural stem cells but plays an essential role in neurogenesis in the postnatal mouse brain [73]. Mechanistically, Mll1 directly occupies the promoter of Distal-less homeobox 2 (Dlx2), a critical regulator of neurogenesis, and is required to resolve the poised bivalent state to the actively transcribed status with predominant H3K4me3 during neurogenesis of neural stem cells [73].
MLL2 (also called MLL4 and KMT2B) has a similar protein domain structure to that of MLL1 and was found to be the MLL1 paralog [74]. Like Mll1, Mll2 is widely expressed during development and in adult tissues. Mll2-null mice die before embryonic day E11.5, with drastically reduced expression of Hoxb2 and Hoxb5[75]. However, Mll2 may be only required briefly for development, because it appears to be dispensable for mouse development after E11.5 [76]. Mll2−/− ES cells maintain pluripotency, have increased apoptotic activity, and undergo skewed cellular differentiation along three germ layers [77]. Therefore, Mll1 and Mll2 are unlikely redundant for gene regulation during early embryonic development. In support with this notion, the phenotypes of Mll1 and Mll2 knockout mice are different in adult tissues. For example, hematopoietic-specific loss of Mll1 showed defects in hematopoiesis [71,72], whereas Mll2 loss did not show any aberrant blood profiles and notable pathology [76].
MLL3 and MLL4
MLL3 (also called HALR/KMT2C) and MLL4 (alias ALR/KMT2D) are mammalian counterparts of Drosophila Trr and were co-purified as transcriptional coactivator complexes [14,78-80]. MLL3 and MLL4 associate with nuclear hormone receptors in both Drosophila and mammals. For example, the MLL3/MLL4 complex is recruited to HOXC6 gene and activates its transcription in an estrogen receptor-dependent manner [79]. Frequent somatic loss-of-function mutations have been identified in MLL3 and MLL4 genes in human cancers, including colorectal cancer, non-Hodgkin B-cell lymphoma, and medulloblastoma [81-85]. Consistently, a recent study reported that trr gene product suppresses cell growth in Drosophila eye imaginal discs. Of interest, trr mutation markedly reduced H3K4 monomethylation levels without significantly changing H3K4 di- and trimethylation levels [86], in agreement with earlier findings that Trr is a major H3K4 mono-methyltransferase for Drosophila enhancers [87]. Mll3 homozygous mutant mice, which have an in-frame deletion of a 61-aa catalytic core of the SET domain, exhibited reduced white adipose tissue, stunted growth, and slow cellular doubling rate [88,89]. During epidermal differentiation, the MLL4 complex is recruited to differentiation-related genes via the transcription factor GRHL3/GET1 and collaboratively activates the epidermal progenitor differentiation program [90].
Recently, we found that MLL4 is essential for the neuronal differentiation of human NT2/D1 stem cells [91]. Mechanistically, the neuron-specific gene NESTIN and key developmental genes HOXA1–3 are activated by MLL4 during RA-induced differentiation. Intriguingly, the tandem PHD4-6 of seven PHD motifs in MLL4 (Figure 2) specifically recognized unmethylated or asymmetrically dimethylated histone H4 Arg 3 (H4R3me0 or H4R3me2a) and is required for MLL4′s nucleosomal methyltransferase activity and MLL4-mediated differentiation. H4R3 symmetric dimethylation (H4R3me2s), a gene-repressive mark, blocks the binding activity of MLL4′s PHD4-6. Consistent with this, knockdown of the protein arginine methyltransferase 7, which is involved in generation of H4R3me2s, increases MLL4 occupancy and H3K4me3 levels at the MLL4 target gene promoters and enhances the MLL4-dependent neural differentiation program. Therefore, these results revealed that the trans-tail regulation of MLL4-catalyzed H3K4me3 by protein arginine methyltransferase 7-controlled H4R3me2s serves as a novel epigenetic mechanism underlying neuronal differentiation of human stem cells.
MLL5
Independent studies have demonstrated that MLL5 is required for hematopoiesis [92-94]. Moreover, MLL5 promotes myogenic differentiation by controlling expression of cell cycle genes (e.g., Cyclin A2) and myogentic regulator genes (e.g., Myogenin) [95]. Mll5 knockout male mice are sterile, at least in part because of deregulated expression of genes that are required for terminal differentiation during spermatogenesis [96]. Of interest, although MLL5 was reported to be inactive [92,95], GlcNAcylation of MLL5 greatly increased MLL5′s enzymatic activity towards H3K4me1/2 and facilitated RA-induced granulopoiesis in human HL60 promyelocytes [24].
SET1A and SET1B
Human SET1A and SET1B have an N-terminal RNA recognition motif and a C-terminal enzymatic SET domain (Figure 2). The SET1A complex was purified as a multi-protein complex that associates with CFP1 [19]. CFP1 is required for stem cell differentiation and interacts with unmethylated CpGs via its zinc finger domain CXXC [97]. Interestingly, Cfp1−/− ES cells displayed aberrant H3K4me3 peaks at numerous ectopic sites (i.e., distinct regions outside annotated CpG islands), suggesting that CFP1 recruits the SET1 complex to CpG island-containing promoters and consequently prevents it from generating H3K4me3 to inappropriate chromatin locations [19,98,99].
A protein sequence analysis revealed that SET1A shares 39% identity with a SET domain protein named SET1B [22]. Although both proteins associate with a similar set of non-catalytic subunits, a confocal microscopy analysis revealed that SET1A and SET1B exhibit distinct subnuclear localizations in euchromatin regions; thus, this suggests that each protein regulates a unique group of target genes [22].
ASH1L
ASH1L (also called Ash1) is the human homolog of Ash1, a Drosophila Trithorax group protein that is essential for expression of several HOX genes. Some reports have indicated that ASH1L primarily acts as a H3K4 methyltransferase [13,100,101], whereas others have reported that human ASH1L specifically mono- and dimethylates H3K36 [102-104]. ASH1L cooperates with MLL1 in HOX gene activation and is required for the myelomonocytic lineage differentiation of hematopoietic stem cells [105]. Of interest, a mutation of the SET domain of ASH1L did not decrease HOX gene expression, suggesting that ASH1L’s catalytic activity is dispensable for hematopoietic stem cell differentiation [105].
SET7/9
SET7 (or called SET9) is an H3K4 mono- and di-methytransferase [6,106-108]. SET7 expression is upregulated during myoblast differentiation [109]. Specifically, SET7 interacts with Myoblast determination protein 1 (MyoD), a central transcriptional factor for myogenic gene expression, and is indispensable for MyoD-mediated muscle differentiation. Knockdown of SET7 impaired the association of MyoD with the promoter and enhancer regions of the myogenic genes (e.g., Myogenin) and reduced gene expression by decreasing H3K4me1 levels at its target genes. Intriguingly, SET7 antagonizes Suv39h1-mediated H3-K9 methylation at the myogenic differentiation gene promoters [109].
SMYD1−3
Smyd1 (also called Bop) is essential for mouse cardiac differentiation [110]. Consistently, knockdown of Smyd1 in zebrafish embryos results in defective skeletal and cardiac muscle differentiation; this cannot be rescued by the Smyd1 catalytic mutant, which lacks H3K4 methyltransferase activity [21]. SMYD2 methylates H3K4 and H3K36, as well as tumor-suppressor proteins such as p53 and Retinoblastoma protein (pRB) [23,111-113]. Specifically, SMYD2-mediated monomethylation of p53 K370 attenuates the interaction of p53 with p53 target promoters and consequently antagonizes p53-dependent transcriptional regulation [112]. Unlike SMYD1, cardiac-specific knockout of Smyd2 has no phenotype during mouse heart development [114]. SMYD3 is a methyltransferase for both H3K4 and H4K5 [15,115]. It is overexpressed in colorectal and hepatocellular cancers and promotes cell proliferation [15]. During zebrafish embryogenesis, SMYD3 appears to be important for cardiac and skeletal muscle development [116].
SETMAR
SETMAR (also called METNASE) encodes a chimeric protein that contains an N-terminal SET domain and a C-terminal mariner transposase domain [117] (Figure 3). The function of SETMAR in stem cells remains unknown. However, SETMAR-catalyzed methylation of H3K4 and H3K36 may lead to an open chromatin structure, which may facilitate its transposase-dependent processes, such as foreign DNA integration and DNA double-strand break repair [20].
Figure 3.
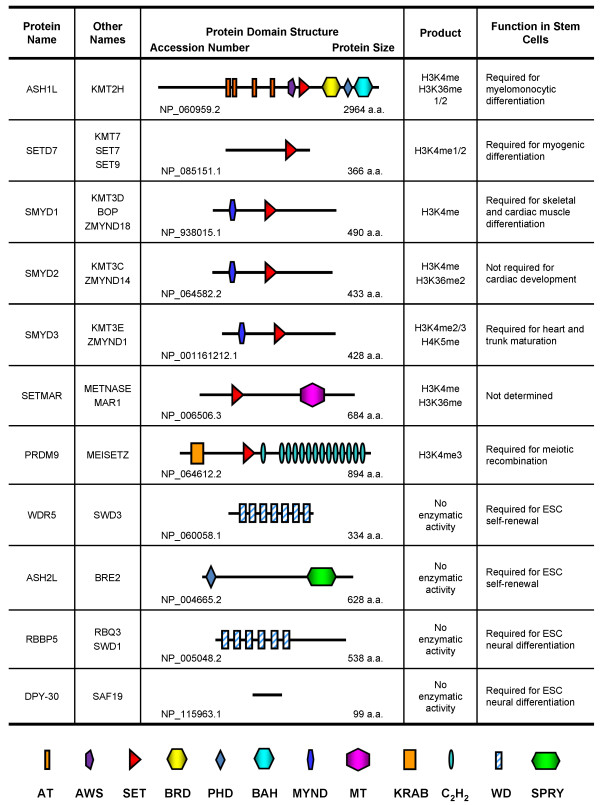
Protein domain architectures and stem cell function of other H3K4 methyltransferases and core subunits. AT: AT-hook DNA binding domain; AWS: Associated With SET domain; SET: Su(var)3-9, Enhancer of zeste, Trithorax domain; BRD: Bromodomain; PHD: Plant Homeo Domain; BAH: Bromo Adjacent Homology domain; MYND: Myeloid, Nervy, and DEAF-1 domain; MT: Mariner Transposase domain; KRAB: Krüppel Associated Box domain; C2H2: C2H2-type zinc finger; WD: WD40 repeat; SPRY: SplA and Ryanodine domain.
PRDM9
PRDM9 (also called MEISETZ) is a PR/SET domain-dependent histone methyltransferase that is required for meiotic prophase progression [18]. Deletion of the Prdm9 gene attenuates H3K4me3 levels, resulting in defective chromosome pairing, impaired sex body formation, damaged meiotic progression, and sterility in both sexes of mice [18]. Mechanistically, Prdm9 binds to 13-base pair DNA elements via its C2H2 zinc fingers. During early meiosis, this binding event may link Prdm9-catalyzed H3K4me3 to mammalian meiotic recombination hotspots that contain the 13-nucleotide DNA elements [118-120].
Subunits of H3K4 methyltransferases
WDR5, a core subunit of the SET1 and MLL1−4 complexes, plays an important role in ES cell self-renewal and somatic cell reprogramming [47]. WDR5 is highly expressed in ES cells and downregulated upon differentiation. Knockdown of WDR5 resulted in loss of ES cell self-renewal and decreased the generation of induced pluripotent stem cells [47]. WDR5 interacts with OCT4 and activates transcription of the self-renewal factors, such as OCT4 and NANOG, in ES cells. Moreover, WDR5, together with OCT4, NANOG and SOX2, regulates the self-renewal-regulatory network [47]. Similarly, ASH2L is required for the pluripotency of mouse ES cells. ASH2L knockdown resulted in elevated expression of mesodermal lineage differentiation genes [121].
DPY-30 and RBBP5 are other core components of the SET1/MLL methyltransferases. In contrast to ASH2L and WDR5, DPY-30 and RBBP5 were not required for ES cell self-renewal [53]. DPY-30 or RBBP5 knockdown reduces global and neuronal gene-specific H3K4me3 levels, resulting in inefficient RA-induced neural differentiation of mouse ES cells.
Differing biological outcomes for ASH2L and WDR5 from DPY-30 and RBBP5 are surprising because these four proteins are core components of the same SET1/MLL1−4 methyltransferases. These unexpected findings might be explained by the following possibilities. Besides the known SET1/MLL1−4 complexes, some of these subunits may be present in other complexes in the same cells so that they may exert different biological functions from SET1/MLL1−4 complexes. In fact, gel filtration analysis of ES cell nuclear extracts showed that elution profiles of WDR5/OCT4 did not overlap with those of WDR5/ASH2L/RBBP5, suggesting that WDR5 also belongs to another new complex containing OCT4 [47]. Another possible scenario is that cellular levels of some core subunits and H3K4 methyltransferases may be dynamically changed between ES cells and differentiated cells. Such changes might allow certain H3K4 methyltransferase complexes to be dominant over the others or lead to formation of new functional complexes, subsequently affecting expression of stemness genes and differentiation-specific genes. In support with this, during ES cell differentiation, ASH2L and WDR5 levels are down-regulated whereas MLL1 and MLL3 are up-regulated [47,121]. In addition, some H3K4 methyltransferase complexes may have non-redundant cellular function by regulating their unique target genes in a cell type-specific manner, as mentioned earlier. Future studies are required to further understand the distinct roles of the SET1/MLL complexes.
H3K4 demethylases
The reversibility of histone methylation was not clear until the discovery of the first histone demethylase LSD1 in 2004 [25]. Subsequently, a new class of JmjC-domain-containing proteins was identified that can demethylate methylated lysine residues in histones. The F-box and leucine-rich repeat protein (FBXL11, also known as KDM2A) is the first identified JmjC domain-containing demethylase that removes methyl groups from H3K36me2/1 [122]. The catalytic JmjC domain requires iron and α-ketoglutarate as cofactors to hydroxylate methyl groups [123]. Among this class of demethylases, JARID1A−D (or KDM5A−D) proteins specifically remove the methyl group from H3K4me2/3. NO66, a bifunctional lysine-specific demethylase and histidyl-hydroxylase, can demethylate H3K4me/ H3K36me and hydroxylate a histidyl group of the non-histone protein Rpl8 [124,125]. Not surprisingly, the LSD family (LSD1 and LSD2) and JARID1 family of H3K4 demethylases play important roles in gene transcription in stem cell homeostasis.
LSD1 and LSD2
LSD1 protein contains an N-terminal SWIRM domain and a long C-terminal FAD-dependent amine oxidase domain (AOD). The AOD is divided by an insertion known as the tower domain (Figure 4). LSD1 alone demethylates H3K4me2/1 on histones but not nucleosomes, while the association of Co-REST with LSD1 allows LSD1 to demethylate nucleosomal H3K4 [26,27,126].
Figure 4.
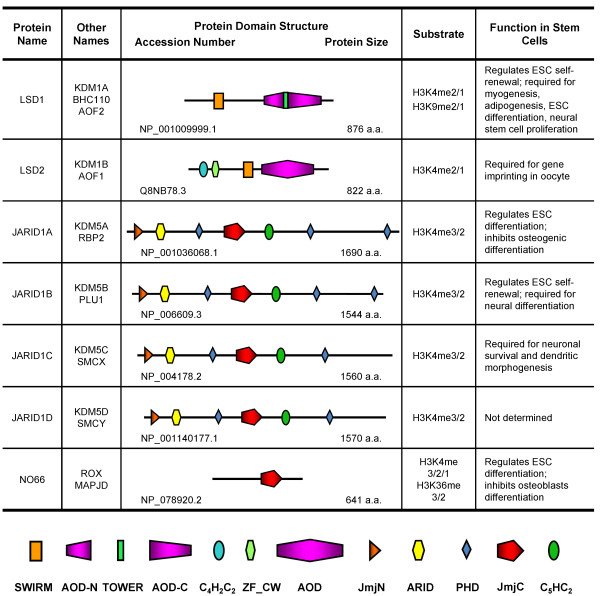
Protein domain architectures and stem cell function of H3K4 demethylases. SWIRM: SWI3, RSC8 and MOIRA domain; AOD-N: Amine Oxidase Domain-N terminal; TOWER: LSD1 tower domain; AOD-C: Amine Oxidase Domain-C terminal; C4H2C2: C4H2C2-type zinc finger; ZF_CW: CW-type zinc finger; AOD: Amine Oxidase Domain; JmjN: Jumonji N domain; ARID: AT-rich interactive domain; PHD: Plant Homeo Domain; JmjC: Jumonji C domain; C5HC2: C5HC2-type zinc finger.
Numerous studies in ES cells and neural stem cells strongly suggest that LSD1 is a key histone methylation modifier in transcriptional regulation for stem cell fate determination. Lsd1-null mice are embryonic lethal around E6.5, and Lsd1-deficient mouse ES cells demonstrate increased cell death and impaired differentiation, such as embryoid body formation defects [127-129]. Similar to mouse ES cells, LSD1 is required for neural stem cell proliferation; it is recruited by the nuclear receptor TLX to repress negative cell cycle regulators, including p21, in neural stem cells [130]. Interestingly, LSD1 is indispensable for differentiation of several cell types, including skeletal muscles and adipocytes [131,132]. In mouse ES cells, LSD1 demethylates and stabilizes DNA methyltransferase 1 (DNMT1), and Lsd1 deletion results in progressive loss of DNA methylation [128]. Moreover, LSD1 and its associated nucleosome remodeling and histone deacetylase (NuRD) complex are recruited to Oct4-occupied enhancers at active stemness genes in ES cells, but the repression activities of LSD1-NuRD may be antagonized by histone acetyltransferases (e.g., p300). During mouse ES cell differentiation, Oct4 and acetyltransferase levels are down-regulated, and LSD1-NuRD decommissions active enhancers by removing H3K4me1 while promoting cellular differentiation [45]. In contrast to the above stem cell studies, seemingly conflicting results regarding the role of LSD1 in ES cells have been reported. Knockdown of LSD1 induces differentiation in human ES cells, which is correlated with de-repression of developmental genes with elevated H3K4me2/3 levels [44]. In addition, Lsd1−/− ES cells had a strong potential to generate extraembryonic tissues from the embryoid body [133].
LSD2 (AOF1 or KDM1B) was recently identified as a homolog of LSD1; it demethylates H3K4me2/1 like LSD1 [28,134-136]. Interestingly, unlike LSD1, LSD2 has no tower domain in the AOD region, but contains unique N-terminal zinc fingers, including C4H2C2 and CW-type zinc fingers, which are required for demethylase activity [136,137] (Figure 4). A genome-wide mapping analysis revealed that LSD2 primarily resides in the intragenic regions of actively expressed genes [28]. LSD2 may activate its target genes, possibly via its association with transcriptional elongation factors [28]. Lsd2 is not essential for mouse development. However, the DNA methylation of several imprinted genes is lost in oocytes from lsd2-deleted females [135]. Consequently, the embryos derived from these oocytes exhibited biallelic expression or silencing (i.e., loss of monoallelic expression) of the affected imprinted genes and died before mid-gestation [135]. The molecular mechanism underlying the functional link between H3K4 demethylation and DNA methylation for expression of imprinted genes remains to be investigated.
JARID1A
JARID1A (RBP2 or KDM5A) was identified as a binding partner of pRB protein in early 1990 [138]. RBP2 contains a highly conserved JmjC domain and was found as a specific H3K4me3/2 demethylase [30,139] (Figure 4). Rbp2−/− mice are viable and display mild phenotypic defects in expansion of hematopoietic stem cells and myeloid progenitors. The weak phenotype of Rbp2−/− mice suggests that other JARID1 family proteins may compensate the loss of Rbp2[139].
During ES cell differentiation, RBP2 is dissociated from HOX genes, resulting in increased H3K4me3 levels and gene activation [30]. Consistently, Pasini et al. reported that RBP2 associates with the important Polycomb repressive complex 2 (PRC2), which enzymatically generates the repressive mark H3K27me3 for silencing of many differentiation-specific genes in ES cells [140]. A genome-wide chromatin immunoprecipitation (ChIP)-on-chip analysis revealed that RBP2 colocalizes on a subset of PRC2 target gene promoters in mouse ES cells. However, the interaction of RBP2 with PRC2 may not be strong, because the mass spectrometric analysis revealed that affinity eluates of the PRC2 component EED, which were purified from ES cell extracts, did not contain RBP2 [141]. Beshiri et al. recently demonstrated that RBP2 augments the repressive effects of the pRB-related protein p130 and E2F4 on cell cycle genes during stem cell differentiation via H3K4me3 demethylation [142]. Interestingly, RBP2 inhibits osteogenic differentiation of human adipose-derived stroma cells [143]. RBP2 interacts with Runt-related transcription factor 2 (RUNX2), a transcriptional factor that is required for osteogenic differentiation. Subsequently, RBP2 represses RUNX2 target genes, including Alkaline phosphatase, Osteocalcin, and Osterix[143].
JARID1B
JARID1B (PLU1 or KDM5B) was shown to be overexpressed in breast cancer cell lines [144]. As a member of the JARID1 family, PLU1 catalyzes the demethylation of H3K4me2/3. Its full activity requires JmjN, ARID, PHD1, and C5HC2 zinc finger in addition to the catalytic domain JmjC [30,34] (Figure 4). Consistent with the result of earlier studies, knockdown of PLU1 reduced MCF7 breast cancer cell proliferation and concomitantly upregulated expression of the Breast cancer1, early onset (BRCA1), Caveolin 1 (CAV1), and HOXA5 genes as a result of increased H3K4me3 levels on their promoters [34]. However, PLU1′s role in ES cell self-renewal and differentiation is controversial. Xie et al. reported that PLU1 is a downstream target of the pluripotent factor Nanog and is required for ES cell self-renewal [145]. PLU1 interacts with the chromodomain protein MRG15 and is recruited to H3K36me3-containing sites within gene bodies of self-renewal-associated genes via MRG15. Knockdown of PLU1 or MRG15 increased intragenic H3K4me3 that produces cryptic intragenic transcription and inhibited the transcriptional elongation [145]. Another study showed that constitutive overexpression of PLU1 blocked neural terminal differentiation [146]. On the contrary, Schmitz et al. has provided evidence that PLU1 is required for the neural differentiation of ES cells but is dispensable for self-renewal [147]. Using a genome-wide ChIP-sequencing analysis, they found that PLU1 predominantly localizes on the transcription start sites of target genes, over 50% of which are also occupied by Polycomb group proteins. PLU1-depleted ES cells fail to differentiate into the neural lineage, which correlates with the inappropriate depression of stem and germ cell genes [147]. These findings are further supported by their recent research in Plu1 knockout mice, which have the phenotype of neonatal lethality and neural defects [148]. The discrepancies in these studies regarding the role of PLU1 in ES cell homeostasis are not entirely clear. However, Schmitz et al. indicated that their PLU1 localization data were obtained using a better PLU1 antibody and that the unimportance of PLU1 in ES cell self-renewal was confirmed by both a lentiviral shRNA knockdown method and a genetic deletion approach.
JARID1C and JARID1D
Compared with RBP2 and PLU1, much less is known about the biological function of JARID1C (SMCX or KDM5C) and JARID1D (SMCY or KDM5D). Both demethylases have similar domain structures and contain a conserved and functional JmjC domain that is responsible for demethylating H3K4me2/3 [30-32]. SMCX is an X-chromosome gene that escapes from X inactivation [149] and is often mutated in renal tumors and X-linked mental retardation (XLMR), suggesting that it has important functions in the human kidneys and brain [150,151]. Indeed, SMCX is highly expressed in brain during zebrafish development and is required for neuron survival [31]. Moreover, SMCX knockdown reduces dendritic length of rat primary neurons, which cannot be rescued by its XLMR-patient mutants with reduced demethylase activity [31]. Therefore, SMCX may play an important role in neuronal development. In addition, Outchkourov et al. reported that SMCX may interact with the transcriptional factors c-MYC and ELK1 to regulate gene expression in mouse ES cells [152].
JARID1D requires multiple domains, including ARID, JmjC, and C5HC2 zinc finger, for its full demethylase activity towards H3K4me3/2 [32] (Figure 4). JARID1D interacts with RING6A/MBLR, a polycomb-like protein with homology to Mel18 and Bmi1 proteins [153]. This interaction stimulates JARID1D’s enzyme activity in vitro; the protein complex mediates H3K4me3 demethylation at the Engrailed 2 gene promoter and is required for Engrailed 2 gene repression [32]. However, JARID1D’s biological role in stem cells is largely unknown. Given its localization on the Y-chromosome, it will be interesting to determine whether JARID1D plays a role in male-specific gene expression in vivo.
NO66
NO66 has been reported to demethylate H3K4me3/2/1 and H3K36me3/2 [124] and to catalyze histidyl hydroxylation of the 60S ribosomal protein Rpl8 [125]. This enzyme inhibits osteoblast differentiation [124]. Specifically, it directly interacts with Osterix, an osteoblast-specific transcription factor, and represses Osterix target gene expression [124]. In addition, NO66 plays a role in mouse ES cell differentiation [154]. During this process, it is recruited to stemness genes (e.g., Oct4 and Nanog) via the PHD finger protein 19 (PHF19), which interacts with the H3K27 methyltransferase complex PRC2; NO66-PHF19-PRC2 represses gene expression by reducing H3K36me3 and increasing H3K27me3 [154].
Conclusions
Stem cells are indistinguishable from somatic cells at the genomic level. In contrast, there are remarkable differences in epigenomes that may be represented by covalent and noncovalent modifications of histones and DNA. As reviewed herein, specific epigenetic modifiers, such as H3K4 methylation modifiers, may play fundamental roles in orchestrating cellular epigenomes whose genomic sequences are identical. Consistent with this, many H3K4 methylation modifiers and their components are required for ES cell self-renewal or differentiation. In addition, some of them cooperate with transcription factors for efficient somatic cell reprogramming. For example, WDR5 is required for the efficient generation of pluripotent stem cells that were induced by Oct4, Sox2, c-Myc, and Klf4 [47]. Therefore, the epigenetic modifiers, with the transcription factor network, may establish epigenomes in a coordinate manner.
Recently, small molecule inhibitors against specific histone methyltransferases, including LSD1 inhibitors, have been developed by several pharmaceutical companies, although their specificities and efficacies require improvement [155]. Certain inhibitors, alone or combined, may increase somatic reprogramming efficiency or drive somatic reprogramming, perhaps providing new avenues for personalized therapeutic interventions using stem cells. With regard to the roles of histone modifiers in stem cell maintenance and differentiation, many more new exciting findings are expected. We predict that our current and future knowledge about stem cell self-renewal and lineage commitment will be highly relevant to cancer stem cell studies, because stem cells and cancer stem cells share several characteristics, such as high degrees of self-renewal and differentiation [156]. We believe that a new era of stem cell epigenetics has begun.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BG prepared the initial draft of the paper. MGL initiated and modified the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Bingnan Gu, Email: bgu@mdanderson.org.
Min Gyu Lee, Email: mglee@mdanderson.org.
Acknowledgements
We sincerely apologize for not citing and reviewing many relevant articles due to space limitation. We are grateful to our laboratory members for their helpful suggestions and comments and Ms. Ann Sutton for the manuscript editing. This work was supported by grants from the NIH (R01 GM095659, R01 CA157919, and CCSG 5 P30 CA0166672 35), Cancer Prevention and Research Institute of Texas (RP110183), and the Center for Cancer Epigenetics at the University of Texas MD Anderson Cancer Center.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;3:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;3:1079–1088. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S. et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;3:479–491. doi: 10.1016/j.stem.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ 3rd, Nishioka K, Reinberg D. Histone lysine methylation: a signature for chromatin function. Trends Genet. 2003;3:629–639. doi: 10.1016/j.tig.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Miller T, Krogan NJ, Dover J, Erdjument-Bromage H, Tempst P, Johnston M, Greenblatt JF, Shilatifard A. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc Natl Acad Sci USA. 2001;3:12902–12907. doi: 10.1073/pnas.231473398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, Zhang Y. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol Cell. 2001;3:1207–1217. doi: 10.1016/s1097-2765(01)00405-1. [DOI] [PubMed] [Google Scholar]
- Briggs SD, Bryk M, Strahl BD, Cheung WL, Davie JK, Dent SY, Winston F, Allis CD. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 2001;3:3286–3295. doi: 10.1101/gad.940201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev A, Schaft D, Shevchenko A, Pijnappel WW, Wilm M, Aasland R, Stewart AF. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 2001;3:7137–7148. doi: 10.1093/emboj/20.24.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk M, Briggs SD, Strahl BD, Curcio MJ, Allis CD, Winston F. Evidence that Set1, a factor required for methylation of histone H3, regulates rDNA silencing in S. cerevisiae by a Sir2-independent mechanism. Curr Biol. 2002;3:165–170. doi: 10.1016/s0960-9822(01)00652-2. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Dover J, Khorrami S, Greenblatt JF, Schneider J, Johnston M, Shilatifard A. COMPASS, a histone H3 (Lysine 4) methyltransferase required for telomeric silencing of gene expression. J Biol Chem. 2002;3:10753–10755. doi: 10.1074/jbc.C200023200. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, Canaani E. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;3:1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;3:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd KN, Shearn A. ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3. Proc Natl Acad Sci USA. 2003;3:11535–11540. doi: 10.1073/pnas.1933593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo YH, Sohn YC, Kim DH, Kim SW, Kang MJ, Jung DJ, Kwak E, Barlev NA, Berger SL, Chow VT. et al. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol Cell Biol. 2003;3:140–149. doi: 10.1128/MCB.23.1.140-149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, Yagyu R, Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;3:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;3:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;3:873–885. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;3:374–378. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- Lee JH, Skalnik DG. CpG-binding protein (CXXC finger protein 1) is a component of the mammalian Set1 histone H3-Lys4 methyltransferase complex, the analogue of the yeast Set1/COMPASS complex. J Biol Chem. 2005;3:41725–41731. doi: 10.1074/jbc.M508312200. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oshige M, Durant ST, Rasila KK, Williamson EA, Ramsey H, Kwan L, Nickoloff JA, Hromas R. The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc Natl Acad Sci USA. 2005;3:18075–18080. doi: 10.1073/pnas.0503676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Rotllant J, Li H, De Deyne P, Du SJ. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci USA. 2006;3:2713–2718. doi: 10.1073/pnas.0509503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Tate CM, You JS, Skalnik DG. Identification and characterization of the human Set1B histone H3-Lys4 methyltransferase complex. J Biol Chem. 2007;3:13419–13428. doi: 10.1074/jbc.M609809200. [DOI] [PubMed] [Google Scholar]
- Abu-Farha M, Lambert JP, Al-Madhoun AS, Elisma F, Skerjanc IS, Figeys D. The tale of two domains: proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Mol Cell Proteomics. 2008;3:560–572. doi: 10.1074/mcp.M700271-MCP200. [DOI] [PubMed] [Google Scholar]
- Fujiki R, Chikanishi T, Hashiba W, Ito H, Takada I, Roeder RG, Kitagawa H, Kato S. GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature. 2009;3:455–459. doi: 10.1038/nature07954. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;3:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;3:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;3:857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Fang R, Barbera AJ, Xu Y, Rutenberg M, Leonor T, Bi Q, Lan F, Mei P, Yuan GC, Lian C. et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol Cell. 2010;3:222–233. doi: 10.1016/j.molcel.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;3:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;3:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, De la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;3:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Lee MG, Norman J, Shilatifard A, Shiekhattar R. Physical and functional association of a trimethyl H3K4 demethylase and Ring6a/MBLR, a polycomb-like protein. Cell. 2007;3:877–887. doi: 10.1016/j.cell.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, Li J, Rao A, Shi Y. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;3:601–605. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;3:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;3:537–551. doi: 10.1101/gad.1523007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;3:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Zhao XD, Han X, Chew JL, Liu J, Chiu KP, Choo A, Orlov YL, Sung WK, Shahab A, Kuznetsov VA. et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;3:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu Rev Biochem. 2012;3:65–95. doi: 10.1146/annurev-biochem-051710-134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;3:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E. et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;3:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;3:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;3:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;3:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, Izpisua Belmonte JC. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;3:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- Whyte WA, Bilodeau S, Orlando DA, Hoke HA, Frampton GM, Foster CT, Cowley SM, Young RA. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;3:221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Tada M, Nakatsuji N, Tada T. Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol. 2004;3:5710–5720. doi: 10.1128/MCB.24.13.5710-5720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B. et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;3:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;3:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ 3rd, Gingeras TR. et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;3:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;3:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F. et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;3:854–858. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW. et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;3:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Shukla A, Wang X, Chen WY, Bernstein BE, Roeder RG. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell. 2011;3:513–525. doi: 10.1016/j.cell.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MM, Lee JS, O'Donovan A, Wyatt M, Bernstein BE, Shilatifard A. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;3:852–854. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;3:713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- Cho YW, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M, Ge K. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem. 2007;3:20395–20406. doi: 10.1074/jbc.M701574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA. et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;3:587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- Issaeva I, Zonis Y, Rozovskaia T, Orlovsky K, Croce CM, Nakamura T, Mazo A, Eisenbach L, Canaani E. Knockdown of ALR (MLL2) reveals ALR target genes and leads to alterations in cell adhesion and growth. Mol Cell Biol. 2007;3:1889–1903. doi: 10.1128/MCB.01506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Skalnik DG. Wdr82 is a C-terminal domain-binding protein that recruits the Setd1A Histone H3-Lys4 methyltransferase complex to transcription start sites of transcribed human genes. Mol Cell Biol. 2008;3:609–618. doi: 10.1128/MCB.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007;3:447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- Mohan M, Herz HM, Smith ER, Zhang Y, Jackson J, Washburn MP, Florens L, Eissenberg JC, Shilatifard A. The COMPASS family of H3K4 methylases in Drosophila. Mol Cell Biol. 2011;3:4310–4318. doi: 10.1128/MCB.06092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Kim D, Levitan I, Dressler GR. The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev Cell. 2007;3:580–592. doi: 10.1016/j.devcel.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nuland R, Smits AH, Pallaki P, Jansen PW, Vermeulen M, Timmers HT. Quantitative Dissection and Stoichiometry Determination of the Human SET1/MLL Histone Methyltransferase Complexes. Mol Cell Biol. 2013;3:2067–2077. doi: 10.1128/MCB.01742-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;3:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce CM, Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;3:701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- Djabali M, Selleri L, Parry P, Bower M, Young BD, Evans GA. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992;3:113–118. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Kitabayashi I, Ayton PM, Cleary ML, Ohki M. Leukemia proto-oncoprotein MLL is proteolytically processed into 2 fragments with opposite transcriptional properties. Blood. 2002;3:3710–3718. doi: 10.1182/blood-2002-04-1015. [DOI] [PubMed] [Google Scholar]
- Hsieh JJ, Cheng EH, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell. 2003;3:293–303. doi: 10.1016/s0092-8674(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;3:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- Ernst P, Mabon M, Davidson AJ, Zon LI, Korsmeyer SJ. An Mll-dependent Hox program drives hematopoietic progenitor expansion. Curr Biol. 2004;3:2063–2069. doi: 10.1016/j.cub.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Gan T, Jude CD, Zaffuto K, Ernst P. Developmentally induced Mll1 loss reveals defects in postnatal haematopoiesis. Leukemia. 2010;3:1732–1741. doi: 10.1038/leu.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon KA, Hiew SY, Hadjur S, Veiga-Fernandes H, Menzel U, Price AJ, Kioussis D, Williams O, Brady HJ. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;3:338–345. doi: 10.1016/j.stem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;3:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald KT, Diaz MO. MLL2: A new mammalian member of the trx/MLL family of genes. Genomics. 1999;3:187–192. doi: 10.1006/geno.1999.5860. [DOI] [PubMed] [Google Scholar]
- Glaser S, Schaft J, Lubitz S, Vintersten K, Van der Hoeven F, Tufteland KR, Aasland R, Anastassiadis K, Ang SL, Stewart AF. Multiple epigenetic maintenance factors implicated by the loss of Mll2 in mouse development. Development. 2006;3:1423–1432. doi: 10.1242/dev.02302. [DOI] [PubMed] [Google Scholar]
- Glaser S, Lubitz S, Loveland KL, Ohbo K, Robb L, Schwenk F, Seibler J, Roellig D, Kranz A, Anastassiadis K, Stewart AF. The histone 3 lysine 4 methyltransferase, Mll2, is only required briefly in development and spermatogenesis. Epigenetics Chromatin. 2009;3:5. doi: 10.1186/1756-8935-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz S, Glaser S, Schaft J, Stewart AF, Anastassiadis K. Increased apoptosis and skewed differentiation in mouse embryonic stem cells lacking the histone methyltransferase Mll2. Mol Biol Cell. 2007;3:2356–2366. doi: 10.1091/mbc.E06-11-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedkov Y, Cho E, Petruk S, Cherbas L, Smith ST, Jones RS, Cherbas P, Canaani E, Jaynes JB, Mazo A. Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila. Nature. 2003;3:78–83. doi: 10.1038/nature02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari KI, Hussain I, Shrestha B, Kasiri S, Mandal SS. HOXC6 Is transcriptionally regulated via coordination of MLL histone methylase and estrogen receptor in an estrogen environment. J Mol Biol. 2011;3:334–349. doi: 10.1016/j.jmb.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim DH, Goo YH, Lee YC, Lee SK, Lee JW. Crucial roles for interactions between MLL3/4 and INI1 in nuclear receptor transactivation. Mol Endocrinol. 2009;3:610–619. doi: 10.1210/me.2008-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N. et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;3:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;3:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C. et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;3:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stutz AM. et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;3:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter SL, Cibulskis K, Erlich RL. et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;3:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Nguyen A, Chen L, Okano H, Hariharan IK. The Drosophila ortholog of MLL3 and MLL4, trithorax related, functions as a negative regulator of tissue growth. Mol Cell Biol. 2013;3:1702–1710. doi: 10.1128/MCB.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Mohan M, Garruss AS, Liang K, Takahashi YH, Mickey K, Voets O, Verrijzer CP, Shilatifard A. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;3:2604–2620. doi: 10.1101/gad.201327.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee DK, Dou Y, Lee J, Lee B, Kwak E, Kong YY, Lee SK, Roeder RG, Lee JW. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc Natl Acad Sci USA. 2006;3:15392–15397. doi: 10.1073/pnas.0607313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Saha PK, Yang QH, Lee S, Park JY, Suh Y, Lee SK, Chan L, Roeder RG, Lee JW. Targeted inactivation of MLL3 histone H3-Lys-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc Natl Acad Sci USA. 2008;3:19229–19234. doi: 10.1073/pnas.0810100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkin AS, Gordon W, Klein RH, Espitia F, Daily K, Zeller M, Baldi P, Andersen B. GRHL3/GET1 and trithorax group members collaborate to activate the epidermal progenitor differentiation program. PLoS Genet. 2012;3:e1002829. doi: 10.1371/journal.pgen.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar SS, Lee SH, Kan PY, Voigt P, Ma L, Shi X, Reinberg D, Lee MG. Trans-tail regulation of MLL4-catalyzed H3K4 methylation by H4R3 symmetric dimethylation is mediated by a tandem PHD of MLL4. Genes Dev. 2012;3:2749–2762. doi: 10.1101/gad.203356.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V, Madan B, Brykczynska U, Zilbermann F, Hogeveen K, Dohner K, Dohner H, Weber O, Blum C, Rodewald HR. et al. Impaired function of primitive hematopoietic cells in mice lacking the Mixed-Lineage-Leukemia homolog MLL5. Blood. 2009;3:1444–1454. doi: 10.1182/blood-2008-02-142638. [DOI] [PubMed] [Google Scholar]
- Heuser M, Yap DB, Leung M, De Algara TR, Tafech A, McKinney S, Dixon J, Thresher R, Colledge B, Carlton M. et al. Loss of MLL5 results in pleiotropic hematopoietic defects, reduced neutrophil immune function, and extreme sensitivity to DNA demethylation. Blood. 2009;3:1432–1443. doi: 10.1182/blood-2008-06-162263. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wong J, Klinger M, Tran MT, Shannon KM, Killeen N. MLL5 contributes to hematopoietic stem cell fitness and homeostasis. Blood. 2009;3:1455–1463. doi: 10.1182/blood-2008-05-159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian S, Sreenivas P, Sambasivan R, Cheedipudi S, Kandalla P, Pavlath GK, Dhawan J. MLL5, a trithorax homolog, indirectly regulates H3K4 methylation, represses cyclin A2 expression, and promotes myogenic differentiation. Proc Natl Acad Sci USA. 2009;3:4719–4724. doi: 10.1073/pnas.0807136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap DB, Walker DC, Prentice LM, McKinney S, Turashvili G, Mooslehner-Allen K, De Algara TR, Fee J, De Tassigny X, Colledge WH, Aparicio S. Mll5 is required for normal spermatogenesis. PLoS One. 2011;3:e27127. doi: 10.1371/journal.pone.0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlone DL, Lee JH, Young SR, Dobrota E, Butler JS, Ruiz J, Skalnik DG. Reduced genomic cytosine methylation and defective cellular differentiation in embryonic stem cells lacking CpG binding protein. Mol Cell Biol. 2005;3:4881–4891. doi: 10.1128/MCB.25.12.4881-4891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CM, Lee JH, Skalnik DG. CXXC finger protein 1 restricts the Setd1A histone H3K4 methyltransferase complex to euchromatin. FEBS J. 2010;3:210–223. doi: 10.1111/j.1742-4658.2009.07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouaire T, Webb S, Skene P, Illingworth R, Kerr A, Andrews R, Lee JH, Skalnik D, Bird A. Cfp1 integrates both CpG content and gene activity for accurate H3K4me3 deposition in embryonic stem cells. Genes Dev. 2012;3:1714–1728. doi: 10.1101/gad.194209.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel C, Imhof A, Greene J, Kremmer E, Sauer F. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature. 2002;3:857–862. doi: 10.1038/nature01126. [DOI] [PubMed] [Google Scholar]
- Gregory GD, Vakoc CR, Rozovskaia T, Zheng X, Patel S, Nakamura T, Canaani E, Blobel GA. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol. 2007;3:8466–8479. doi: 10.1128/MCB.00993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Katagiri Z, Kawahashi K, Kioussis D, Kitajima S. Trithorax-group protein ASH1 methylates histone H3 lysine 36. Gene. 2007;3:161–168. doi: 10.1016/j.gene.2007.04.027. [DOI] [PubMed] [Google Scholar]
- An S, Yeo KJ, Jeon YH, Song JJ. Crystal structure of the human histone methyltransferase ASH1L catalytic domain and its implications for the regulatory mechanism. J Biol Chem. 2011;3:8369–8374. doi: 10.1074/jbc.M110.203380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J Biol Chem. 2011;3:7983–7989. doi: 10.1074/jbc.M110.194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kawahashi K, Katagiri Z, Nakayama Y, Mahajan M, Kioussis D. Dual function of histone H3 lysine 36 methyltransferase ASH1 in regulation of Hox gene expression. PLoS One. 2011;3:e28171. doi: 10.1371/journal.pone.0028171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003;3:652–656. doi: 10.1038/nature01378. [DOI] [PubMed] [Google Scholar]
- Kwon T, Chang JH, Kwak E, Lee CW, Joachimiak A, Kim YC, Lee J, Cho Y. Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9-AdoMet. EMBO J. 2003;3:292–303. doi: 10.1093/emboj/cdg025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Chuikov S, Sarma K, Erdjument-Bromage H, Allis CD, Tempst P, Reinberg D. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 2002;3:479–489. doi: 10.1101/gad.967202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Neppl RL, Huang ZP, Chen J, Tang RH, Cao R, Zhang Y, Jin SW, Wang DZ. The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J Cell Biol. 2011;3:551–565. doi: 10.1083/jcb.201010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PD, Pierce SA, Sims RJ, Yamagishi H, Weihe EK, Harriss JV, Maika SD, Kuziel WA, King HL, Olson EN. et al. Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat Genet. 2002;3:25–32. doi: 10.1038/ng866. [DOI] [PubMed] [Google Scholar]
- Brown MA, Sims RJ 3rd, Gottlieb PD, Tucker PW. Identification and characterization of Smyd2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer. 2006;3:26. doi: 10.1186/1476-4598-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T, Berger SL. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;3:629–632. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- Saddic LA, West LE, Aslanian A, Yates JR 3rd, Rubin SM, Gozani O, Sage J. Methylation of the retinoblastoma tumor suppressor by SMYD2. J Biol Chem. 2010;3:37733–37740. doi: 10.1074/jbc.M110.137612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, Brown MA, Van Amerongen MJ, Novoyatleva T, Wietelmann A, Harriss J, Ferrazzi F, Bottger T, Harvey RP, Tucker PW, Engel FB. Cardiac deletion of Smyd2 is dispensable for mouse heart development. PLoS One. 2010;3:e9748. doi: 10.1371/journal.pone.0009748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aller GS, Reynoird N, Barbash O, Huddleston M, Liu S, Zmoos AF, McDevitt P, Sinnamon R, Le B, Mas G. et al. Smyd3 regulates cancer cell phenotypes and catalyzes histone H4 lysine 5 methylation. Epigenetics. 2012;3:340–343. doi: 10.4161/epi.19506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Tsunesumi S, Yamaguchi K, Watanabe S, Furukawa Y. Smyd3 is required for the development of cardiac and skeletal muscle in zebrafish. PLoS One. 2011;3:e23491. doi: 10.1371/journal.pone.0023491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R, Udit S, Batzer MA, Feschotte C. Birth of a chimeric primate gene by capture of the transposase gene from a mobile element. Proc Natl Acad Sci USA. 2006;3:8101–8106. doi: 10.1073/pnas.0601161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Buard J, Grey C, Fledel-Alon A, Ober C, Przeworski M, Coop G, De Massy B. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science. 2010;3:836–840. doi: 10.1126/science.1183439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S, Bowden R, Tumian A, Bontrop RE, Freeman C, MacFie TS, McVean G, Donnelly P. Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science. 2010;3:876–879. doi: 10.1126/science.1182363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvanov ED, Petkov PM, Paigen K. Prdm9 controls activation of mammalian recombination hotspots. Science. 2010;3:835. doi: 10.1126/science.1181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Liang J, Xiong Y, Shi F, Zhang Y, Lu W, He Q, Yang D, Chen R, Liu D. et al. The trithorax group protein Ash2l is essential for pluripotency and maintaining open chromatin in embryonic stem cells. J Biol Chem. 2013;3:5039–5048. doi: 10.1074/jbc.M112.424515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;3:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol. 2012;3:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- Sinha KM, Yasuda H, Coombes MM, Dent SY, De Crombrugghe B. Regulation of the osteoblast-specific transcription factor Osterix by NO66, a Jumonji family histone demethylase. EMBO J. 2010;3:68–79. doi: 10.1038/emboj.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Wolf A, Feng T, Ho CH, Sekirnik R, Zayer A, Granatino N, Cockman ME, Loenarz C, Loik ND. et al. Oxygenase-catalyzed ribosome hydroxylation occurs in prokaryotes and humans. Nat Chem Biol. 2012;3:960–962. doi: 10.1038/nchembio.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Gocke CB, Luo X, Borek D, Tomchick DR, Machius M, Otwinowski Z, Yu H. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol Cell. 2006;3:377–387. doi: 10.1016/j.molcel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I. et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;3:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- Wang J, Hevi S, Kurash JK, Lei H, Gay F, Bajko J, Su H, Sun W, Chang H, Xu G. et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;3:125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- Foster CT, Dovey OM, Lezina L, Luo JL, Gant TW, Barlev N, Bradley A, Cowley SM. Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol Cell Biol. 2010;3:4851–4863. doi: 10.1128/MCB.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Alzayady K, Stewart R, Ye P, Yang S, Li W, Shi Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol Cell Biol. 2010;3:1997–2005. doi: 10.1128/MCB.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musri MM, Carmona MC, Hanzu FA, Kaliman P, Gomis R, Parrizas M. Histone demethylase LSD1 regulates adipogenesis. J Biol Chem. 2010;3:30034–30041. doi: 10.1074/jbc.M110.151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jang H, Kim H, Kim ST, Cho EJ, Youn HD. Histone demethylase LSD1 is required to induce skeletal muscle differentiation by regulating myogenic factors. Biochem Biophys Res Commun. 2010;3:327–332. doi: 10.1016/j.bbrc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Macfarlan TS, Gifford WD, Agarwal S, Driscoll S, Lettieri K, Wang J, Andrews SE, Franco L, Rosenfeld MG, Ren B, Pfaff SL. Endogenous retroviruses and neighboring genes are coordinately repressed by LSD1/KDM1A. Genes Dev. 2011;3:594–607. doi: 10.1101/gad.2008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karytinos A, Forneris F, Profumo A, Ciossani G, Battaglioli E, Binda C, Mattevi A. A novel mammalian flavin-dependent histone demethylase. J Biol Chem. 2009;3:17775–17782. doi: 10.1074/jbc.M109.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone DN, Su H, Hevi S, Gay F, Lei H, Bajko J, Xu G, Li E, Chen T. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature. 2009;3:415–418. doi: 10.1038/nature08315. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jiang J, Stewart DM, Qi S, Yamane K, Li J, Zhang Y, Wong J. AOF1 is a histone H3K4 demethylase possessing demethylase activity-independent repression function. Cell Res. 2010;3:276–287. doi: 10.1038/cr.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Qi S, Xu M, Yu L, Tao Y, Deng Z, Wu W, Li J, Chen Z, Wong J. Structure-function analysis reveals a novel mechanism for regulation of histone demethylase LSD2/AOF1/KDM1b. Cell Res. 2013;3:225–241. doi: 10.1038/cr.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeo-Jones D, Huang PS, Jones RE, Haskell KM, Vuocolo GA, Hanobik MG, Huber HE, Oliff A. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991;3:251–254. doi: 10.1038/352251a0. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yan Q, Tothova Z, Yamane K, Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y, Kaelin WG Jr. The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;3:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Pasini D, Hansen KH, Christensen J, Agger K, Cloos PA, Helin K. Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and Polycomb-Repressive Complex 2. Genes Dev. 2008;3:1345–1355. doi: 10.1101/gad.470008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, Wysocka J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;3:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshiri ML, Holmes KB, Richter WF, Hess S, Islam AB, Yan Q, Plante L, Litovchick L, Gevry N, Lopez-Bigas N. et al. Coordinated repression of cell cycle genes by KDM5A and E2F4 during differentiation. Proc Natl Acad Sci USA. 2012;3:18499–18504. doi: 10.1073/pnas.1216724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Shi L, Zhou Y, Liu Y, Ma GE, Jiang Y, Xu Y, Zhang X, Feng H. Inhibition of osteogenic differentiation of human adipose-derived stromal cells by retinoblastoma binding protein 2 repression of RUNX2-activated transcription. Stem Cells. 2011;3:1112–1125. doi: 10.1002/stem.663. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Sundquist K, Baeckstrom D, Poulsom R, Hanby A, Meier-Ewert S, Jones T, Mitchell M, Pitha-Rowe P, Freemont P, Taylor-Papadimitriou J. A novel gene (PLU-1) containing highly conserved putative DNA/chromatin binding motifs is specifically up-regulated in breast cancer. J Biol Chem. 1999;3:15633–15645. doi: 10.1074/jbc.274.22.15633. [DOI] [PubMed] [Google Scholar]
- Xie L, Pelz C, Wang W, Bashar A, Varlamova O, Shadle S, Impey S. KDM5B regulates embryonic stem cell self-renewal and represses cryptic intragenic transcription. EMBO J. 2011;3:1473–1484. doi: 10.1038/emboj.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey BK, Stalker L, Schnerch A, Bhatia M, Taylor-Papidimitriou J, Wynder C. The histone demethylase KDM5b/JARID1b plays a role in cell fate decisions by blocking terminal differentiation. Mol Cell Biol. 2008;3:5312–5327. doi: 10.1128/MCB.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz SU, Albert M, Malatesta M, Morey L, Johansen JV, Bak M, Tommerup N, Abarrategui I, Helin K. Jarid1b targets genes regulating development and is involved in neural differentiation. EMBO J. 2011;3:4586–4600. doi: 10.1038/emboj.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M, Schmitz SU, Kooistra SM, Malatesta M, Morales Torres C, Rekling JC, Johansen JV, Abarrategui I, Helin K. The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3. PLoS Genet. 2013;3:e1003461. doi: 10.1371/journal.pgen.1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ellison J, Salido E, Yen P, Mohandas T, Shapiro LJ. Isolation and characterization of XE169, a novel human gene that escapes X-inactivation. Hum Mol Genet. 1994;3:153–160. doi: 10.1093/hmg/3.1.153. [DOI] [PubMed] [Google Scholar]
- Jensen LR, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, Janecke AR, Tariverdian G, Chelly J, Fryns JP. et al. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet. 2005;3:227–236. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C. et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;3:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outchkourov NS, Muino JM, Kaufmann K, Van Ijcken WF, Koerkamp MJ, Van Leenen D, De Graaf P, Holstege FC, Grosveld FG, Timmers HT. Balancing of Histone H3K4 Methylation States by the Kdm5c/SMCX Histone Demethylase Modulates Promoter and Enhancer Function. Cell Rep. 2013;3:1071–1079. doi: 10.1016/j.celrep.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Akasaka T, Takahashi N, Suzuki M, Koseki H, Bodmer R, Koga H. MBLR, a new RING finger protein resembling mammalian Polycomb gene products, is regulated by cell cycle-dependent phosphorylation. Genes Cells. 2002;3:835–850. doi: 10.1046/j.1365-2443.2002.00565.x. [DOI] [PubMed] [Google Scholar]
- Brien GL, Gambero G, O'Connell DJ, Jerman E, Turner SA, Egan CM, Dunne EJ, Jurgens MC, Wynne K, Piao L. et al. Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat Struct Mol Biol. 2012;3:1273–1281. doi: 10.1038/nsmb.2449. [DOI] [PubMed] [Google Scholar]
- Lynch JT, Harris WJ, Somervaille TC. LSD1 inhibition: a therapeutic strategy in cancer? Expert Opin Ther Targets. 2012;3:1239–1249. doi: 10.1517/14728222.2012.722206. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;3:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]


