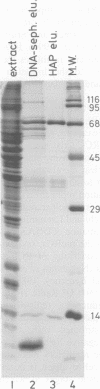
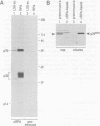
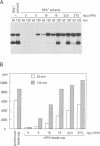
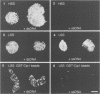
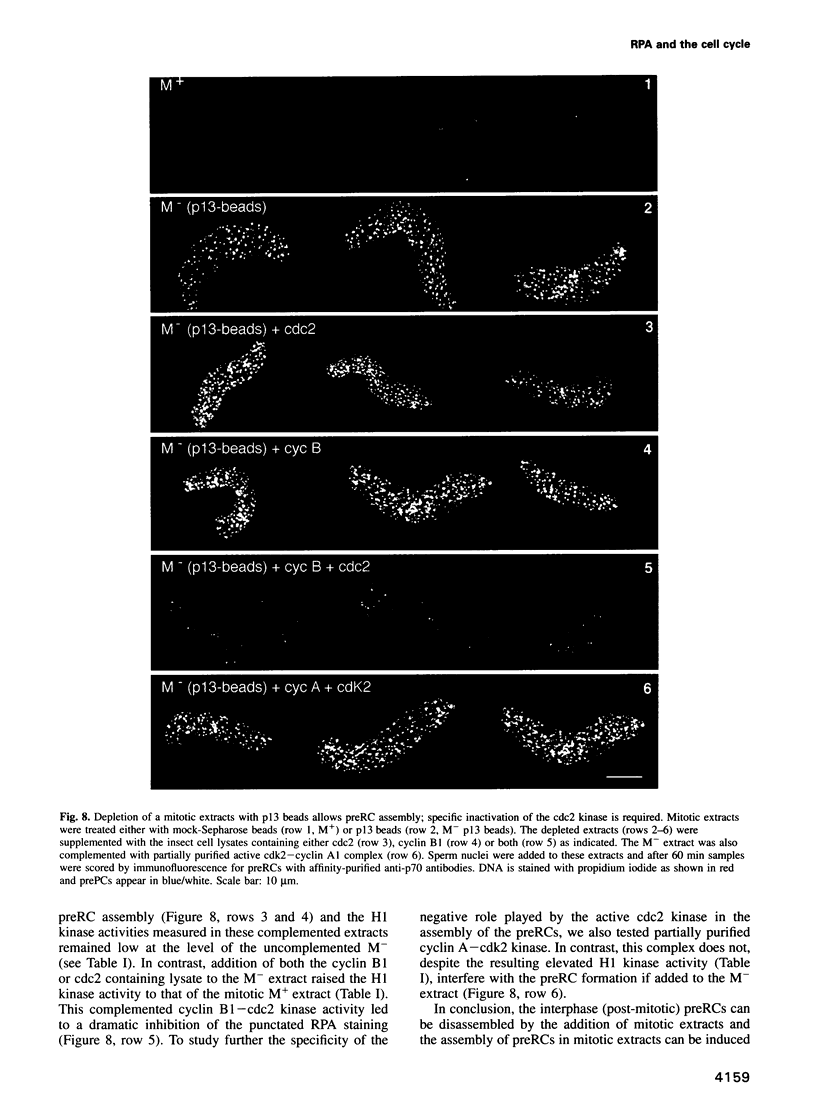
Abstract
RPA is a cellular, three-subunit, single-stranded (ss) DNA binding protein, which assists T-antigen in the assembly of the pre-priming complex in the SV40 replication system. By immunodepletion and complementation, we have identified RPA as an essential factor for cellular DNA replication in Xenopus extracts. RPA assembles post-mitotically on the decondensing chromosomes into numerous subnuclear pre-replication centres (preRCs) which serve, upon formation of the nuclear membrane, as RCs for the initiation of DNA synthesis. By a variety of experiments including the use of isolated components, we demonstrate that an inactive cdc2-cyclin B kinase complex is essential to allow post-mitotic assembly of the preRCs. In contrast, the active cdk2-cyclin A kinase does not impede or facilitate the assembly of preRCs. Digestion analysis using the single-strand-specific P1 nuclease as well as competition experiments with ssDNA, reveal that replication-associated unwinding of the DNA, assisted by RPA, requires the formation of the nuclear membrane. The p21 cdk-interacting protein Cip1 appears to inhibit DNA replication prior to the unwinding DNA step, but after assembly of preRC and nuclear reconstruction.
Full text
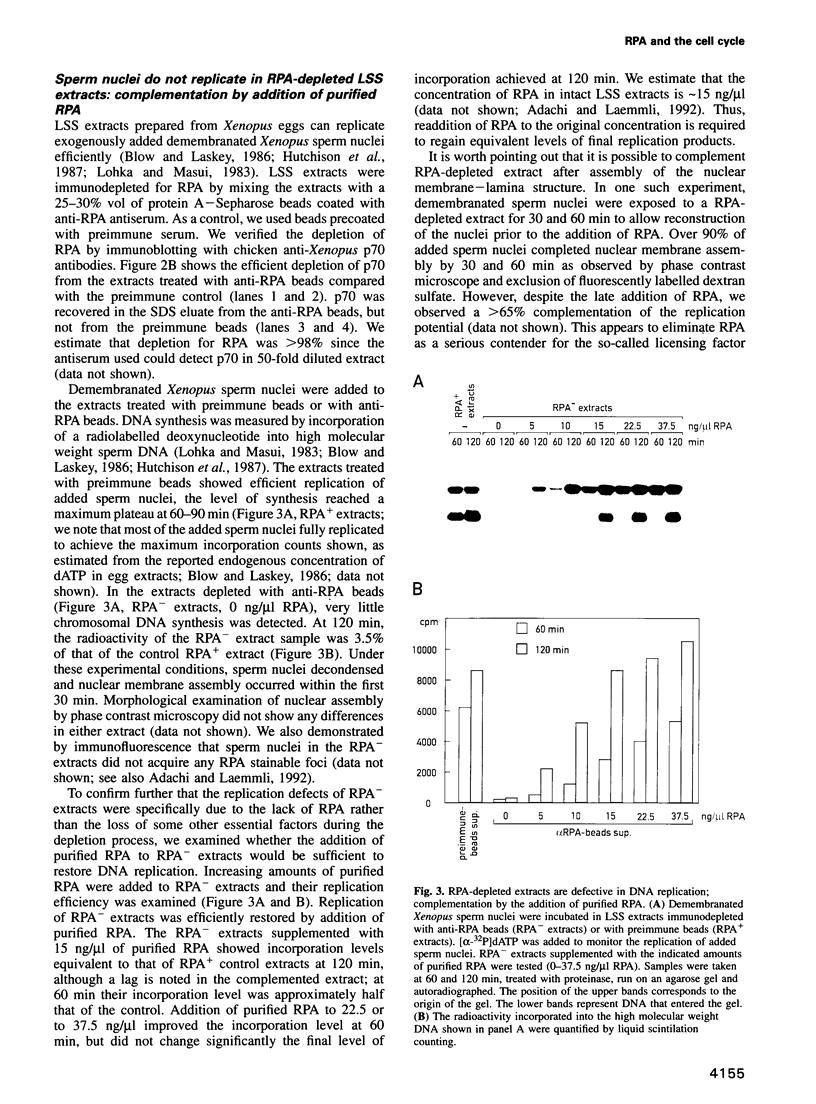
PDF
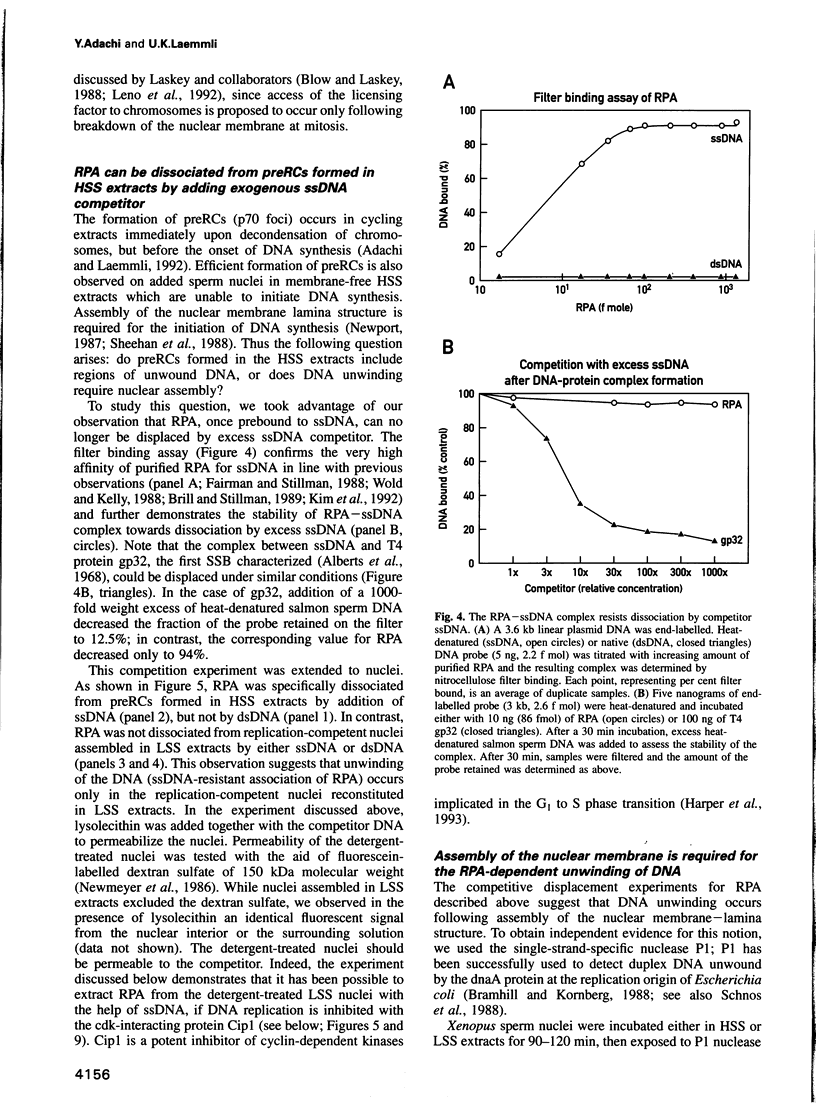





Images in this article
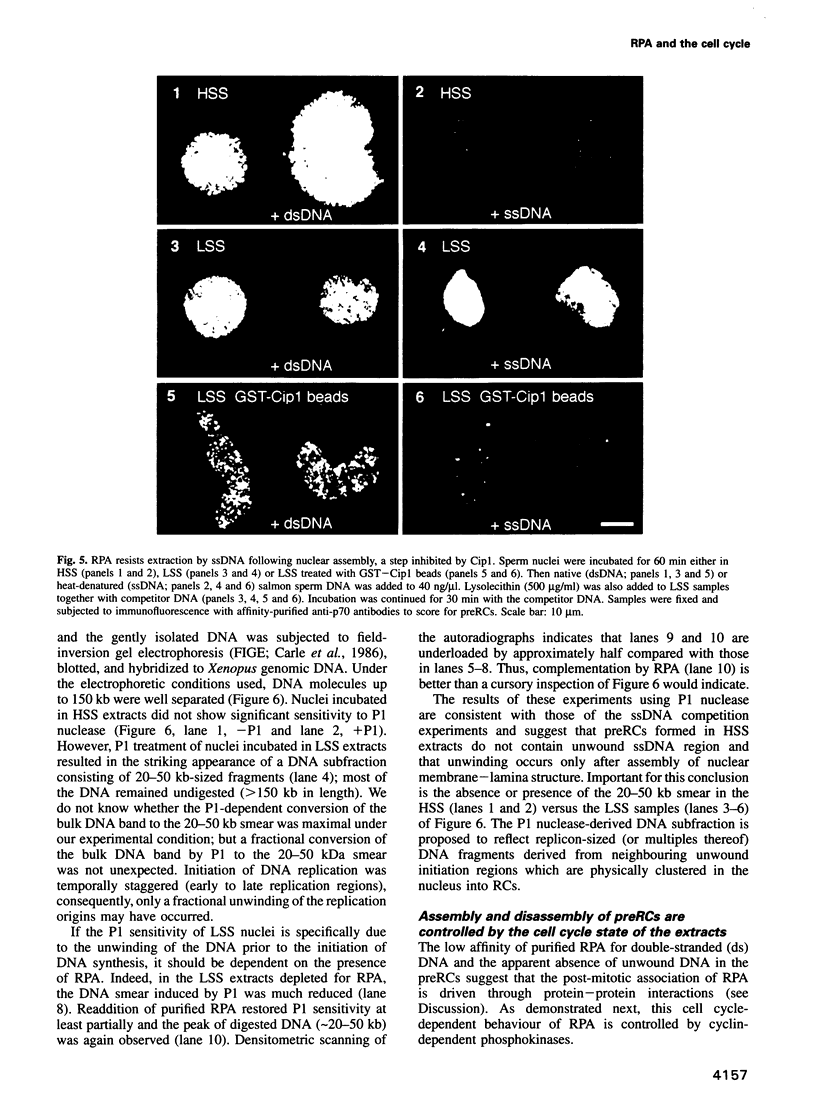
Selected References
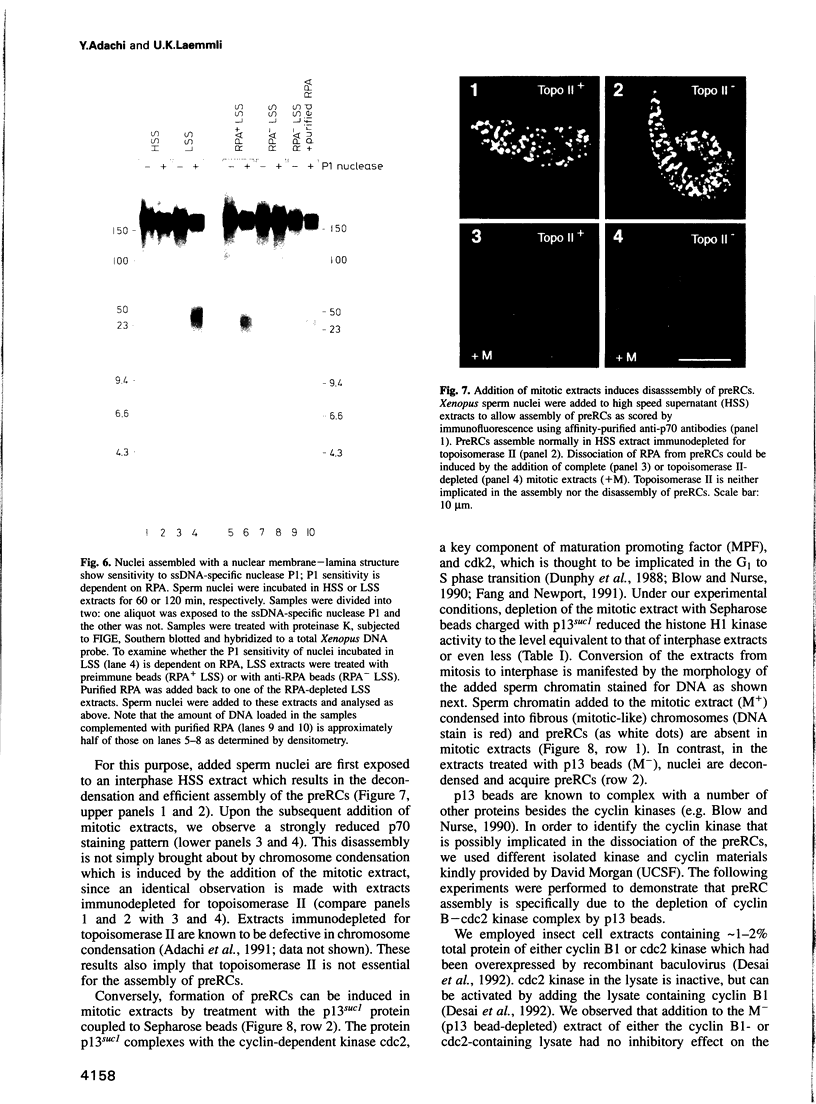
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Laemmli U. K. Identification of nuclear pre-replication centers poised for DNA synthesis in Xenopus egg extracts: immunolocalization study of replication protein A. J Cell Biol. 1992 Oct;119(1):1–15. doi: 10.1083/jcb.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y., Luke M., Laemmli U. K. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 1991 Jan 11;64(1):137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Wolffe A. P. Nuclear assembly, structure, and function: the use of Xenopus in vitro systems. Exp Cell Res. 1993 Mar;205(1):1–15. doi: 10.1006/excr.1993.1051. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988 Apr 7;332(6164):546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990 Sep 7;62(5):855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Stillman B. Replication factor-A from Saccharomyces cerevisiae is encoded by three essential genes coordinately expressed at S phase. Genes Dev. 1991 Sep;5(9):1589–1600. doi: 10.1101/gad.5.9.1589. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Stillman B. Yeast replication factor-A functions in the unwinding of the SV40 origin of DNA replication. Nature. 1989 Nov 2;342(6245):92–95. doi: 10.1038/342092a0. [DOI] [PubMed] [Google Scholar]
- Callan H. G. Replication of DNA in the chromosomes of eukaryotes. Proc R Soc Lond B Biol Sci. 1972 Apr 18;181(1062):19–41. doi: 10.1098/rspb.1972.0039. [DOI] [PubMed] [Google Scholar]
- Cardoso M. C., Leonhardt H., Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993 Sep 24;74(6):979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Coverley D., Kenny M. K., Munn M., Rupp W. D., Lane D. P., Wood R. D. Requirement for the replication protein SSB in human DNA excision repair. Nature. 1991 Feb 7;349(6309):538–541. doi: 10.1038/349538a0. [DOI] [PubMed] [Google Scholar]
- Cox L. S. DNA replication in cell-free extracts from Xenopus eggs is prevented by disrupting nuclear envelope function. J Cell Sci. 1992 Jan;101(Pt 1):43–53. doi: 10.1242/jcs.101.1.43. [DOI] [PubMed] [Google Scholar]
- Desai D., Gu Y., Morgan D. O. Activation of human cyclin-dependent kinases in vitro. Mol Biol Cell. 1992 May;3(5):571–582. doi: 10.1091/mbc.3.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din S., Brill S. J., Fairman M. P., Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990 Jun;4(6):968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- Dornreiter I., Erdile L. F., Gilbert I. U., von Winkler D., Kelly T. J., Fanning E. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992 Feb;11(2):769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Dutta A., Ruppert J. M., Aster J. C., Winchester E. Inhibition of DNA replication factor RPA by p53. Nature. 1993 Sep 2;365(6441):79–82. doi: 10.1038/365079a0. [DOI] [PubMed] [Google Scholar]
- Dutta A., Stillman B. cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 1992 Jun;11(6):2189–2199. doi: 10.1002/j.1460-2075.1992.tb05278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Distinct roles of cdk2 and cdc2 in RP-A phosphorylation during the cell cycle. J Cell Sci. 1993 Nov;106(Pt 3):983–994. doi: 10.1242/jcs.106.3.983. [DOI] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Felix M. A., Pines J., Hunt T., Karsenti E. A post-ribosomal supernatant from activated Xenopus eggs that displays post-translationally regulated oscillation of its cdc2+ mitotic kinase activity. EMBO J. 1989 Oct;8(10):3059–3069. doi: 10.1002/j.1460-2075.1989.tb08457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Forbes D. J. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990 Jan 12;60(1):17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- Fox M. H., Arndt-Jovin D. J., Jovin T. M., Baumann P. H., Robert-Nicoud M. Spatial and temporal distribution of DNA replication sites localized by immunofluorescence and confocal microscopy in mouse fibroblasts. J Cell Sci. 1991 Jun;99(Pt 2):247–253. doi: 10.1242/jcs.99.2.247. [DOI] [PubMed] [Google Scholar]
- Grieco F., Hay J. M., Hull R. An improved procedure for the purification of protein fused with glutathione S-transferase. Biotechniques. 1992 Dec;13(6):856–858. [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- He Z., Brinton B. T., Greenblatt J., Hassell J. A., Ingles C. J. The transactivator proteins VP16 and GAL4 bind replication factor A. Cell. 1993 Jun 18;73(6):1223–1232. doi: 10.1016/0092-8674(93)90650-f. [DOI] [PubMed] [Google Scholar]
- Heyer W. D., Rao M. R., Erdile L. F., Kelly T. J., Kolodner R. D. An essential Saccharomyces cerevisiae single-stranded DNA binding protein is homologous to the large subunit of human RP-A. EMBO J. 1990 Jul;9(7):2321–2329. doi: 10.1002/j.1460-2075.1990.tb07404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Galabru J., Rivière Y., Montagnier L. Efficiency of poly(A).poly(U) as an adjuvant. Immunol Today. 1988 Jun;9(6):161–162. doi: 10.1016/0167-5699(88)91288-1. [DOI] [PubMed] [Google Scholar]
- Hunter T. Braking the cycle. Cell. 1993 Dec 3;75(5):839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- Hutchison C. J., Cox R., Drepaul R. S., Gomperts M., Ford C. C. Periodic DNA synthesis in cell-free extracts of Xenopus eggs. EMBO J. 1987 Jul;6(7):2003–2010. doi: 10.1002/j.1460-2075.1987.tb02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O., Méchali M. Chromosomal replication initiates and terminates at random sequences but at regular intervals in the ribosomal DNA of Xenopus early embryos. EMBO J. 1993 Dec;12(12):4511–4520. doi: 10.1002/j.1460-2075.1993.tb06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins H., Hölman T., Lyon C., Lane B., Stick R., Hutchison C. Nuclei that lack a lamina accumulate karyophilic proteins and assemble a nuclear matrix. J Cell Sci. 1993 Sep;106(Pt 1):275–285. doi: 10.1242/jcs.106.1.275. [DOI] [PubMed] [Google Scholar]
- Kenny M. K., Lee S. H., Hurwitz J. Multiple functions of human single-stranded-DNA binding protein in simian virus 40 DNA replication: single-strand stabilization and stimulation of DNA polymerases alpha and delta. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9757–9761. doi: 10.1073/pnas.86.24.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. K., Schlegel U., Furneaux H., Hurwitz J. The role of human single-stranded DNA binding protein and its individual subunits in simian virus 40 DNA replication. J Biol Chem. 1990 May 5;265(13):7693–7700. [PubMed] [Google Scholar]
- Kim C., Snyder R. O., Wold M. S. Binding properties of replication protein A from human and yeast cells. Mol Cell Biol. 1992 Jul;12(7):3050–3059. doi: 10.1128/mcb.12.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käs E., Laemmli U. K. In vivo topoisomerase II cleavage of the Drosophila histone and satellite III repeats: DNA sequence and structural characteristics. EMBO J. 1992 Feb;11(2):705–716. doi: 10.1002/j.1460-2075.1992.tb05103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Cavadore J. C., Dorée M. M phase-specific cdc2 kinase: preparation from starfish oocytes and properties. Methods Enzymol. 1991;200:291–301. doi: 10.1016/0076-6879(91)00147-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leno G. H., Downes C. S., Laskey R. A. The nuclear membrane prevents replication of human G2 nuclei but not G1 nuclei in Xenopus egg extract. Cell. 1992 Apr 3;69(1):151–158. doi: 10.1016/0092-8674(92)90126-w. [DOI] [PubMed] [Google Scholar]
- Leno G. H., Laskey R. A. The nuclear membrane determines the timing of DNA replication in Xenopus egg extracts. J Cell Biol. 1991 Feb;112(4):557–566. doi: 10.1083/jcb.112.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Botchan M. R. The acidic transcriptional activation domains of VP16 and p53 bind the cellular replication protein A and stimulate in vitro BPV-1 DNA replication. Cell. 1993 Jun 18;73(6):1207–1221. doi: 10.1016/0092-8674(93)90649-b. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Roles of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol. 1984 Apr;98(4):1222–1230. doi: 10.1083/jcb.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbubani H. M., Paull T., Elder J. K., Blow J. J. DNA replication initiates at multiple sites on plasmid DNA in Xenopus egg extracts. Nucleic Acids Res. 1992 Apr 11;20(7):1457–1462. doi: 10.1093/nar/20.7.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J., Campbell K. H., Ford C. C., Stick R., Hutchison C. J. The role of lamin LIII in nuclear assembly and DNA replication, in cell-free extracts of Xenopus eggs. J Cell Sci. 1991 Mar;98(Pt 3):271–279. doi: 10.1242/jcs.98.3.271. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Newport J., Kirschner M. Maturation-promoting factor induces nuclear envelope breakdown in cycloheximide-arrested embryos of Xenopus laevis. J Cell Biol. 1983 Jul;97(1):81–91. doi: 10.1083/jcb.97.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A. D., Blow J. J., White J. G., Amos W. B., Wilcock D., Laskey R. A. Replication occurs at discrete foci spaced throughout nuclei replicating in vitro. J Cell Sci. 1989 Nov;94(Pt 3):471–477. doi: 10.1242/jcs.94.3.471. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Morita T., Sato C. Structural organizations of replicon domains during DNA synthetic phase in the mammalian nucleus. Exp Cell Res. 1986 Aug;165(2):291–297. doi: 10.1016/0014-4827(86)90583-5. [DOI] [PubMed] [Google Scholar]
- Nakayasu H., Berezney R. Mapping replicational sites in the eucaryotic cell nucleus. J Cell Biol. 1989 Jan;108(1):1–11. doi: 10.1083/jcb.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Finlay D. R., Forbes D. J. In vitro transport of a fluorescent nuclear protein and exclusion of non-nuclear proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2091–2102. doi: 10.1083/jcb.103.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. W., Wilson K. L., Dunphy W. G. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990 Dec;111(6 Pt 1):2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987 Jan 30;48(2):205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Newport J., Spann T. Disassembly of the nucleus in mitotic extracts: membrane vesicularization, lamin disassembly, and chromosome condensation are independent processes. Cell. 1987 Jan 30;48(2):219–230. doi: 10.1016/0092-8674(87)90425-9. [DOI] [PubMed] [Google Scholar]
- O'Keefe R. T., Henderson S. C., Spector D. L. Dynamic organization of DNA replication in mammalian cell nuclei: spatially and temporally defined replication of chromosome-specific alpha-satellite DNA sequences. J Cell Biol. 1992 Mar;116(5):1095–1110. doi: 10.1083/jcb.116.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnos M., Zahn K., Inman R. B., Blattner F. R. Initiation protein induced helix destabilization at the lambda origin: a prepriming step in DNA replication. Cell. 1988 Feb 12;52(3):385–395. doi: 10.1016/s0092-8674(88)80031-x. [DOI] [PubMed] [Google Scholar]
- Sheehan M. A., Mills A. D., Sleeman A. M., Laskey R. A., Blow J. J. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988 Jan;106(1):1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Wold M. S., Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J. Identification of cellular proteins required for simian virus 40 DNA replication. J Biol Chem. 1989 Feb 15;264(5):2801–2809. [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]