Abstract
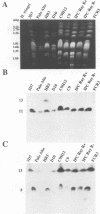
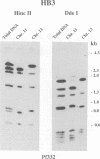
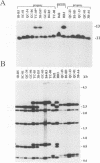
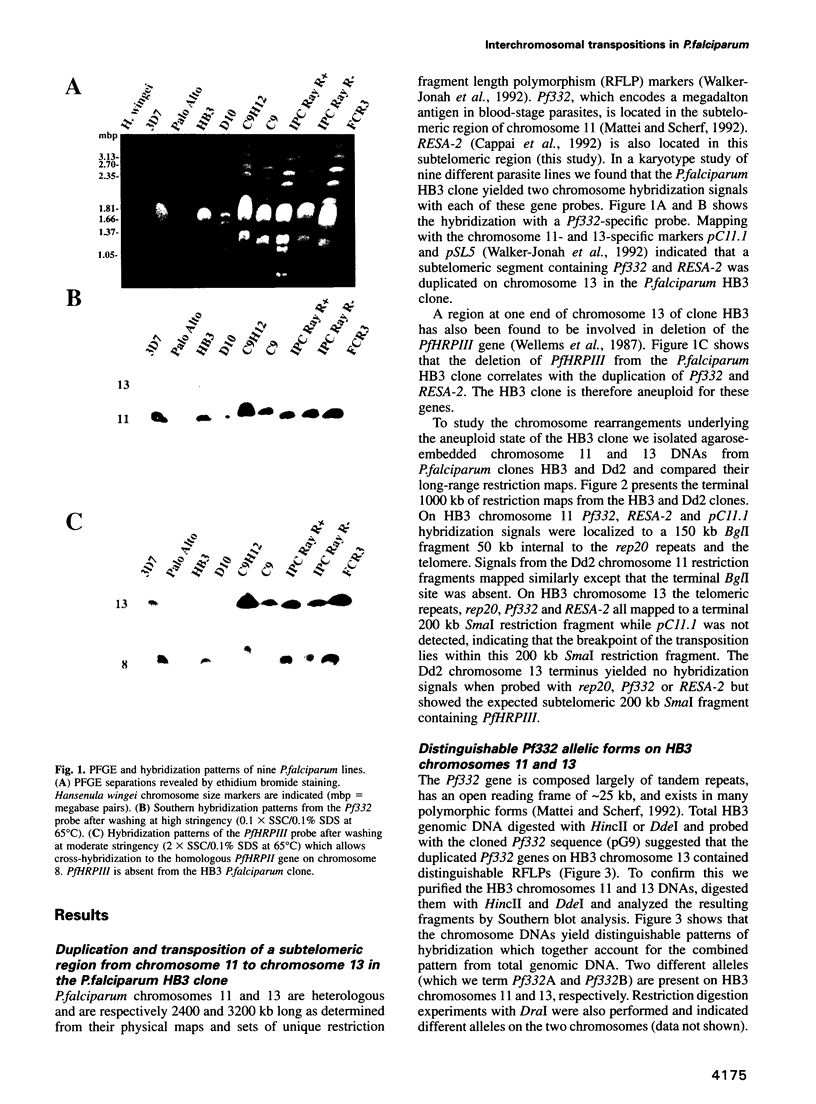
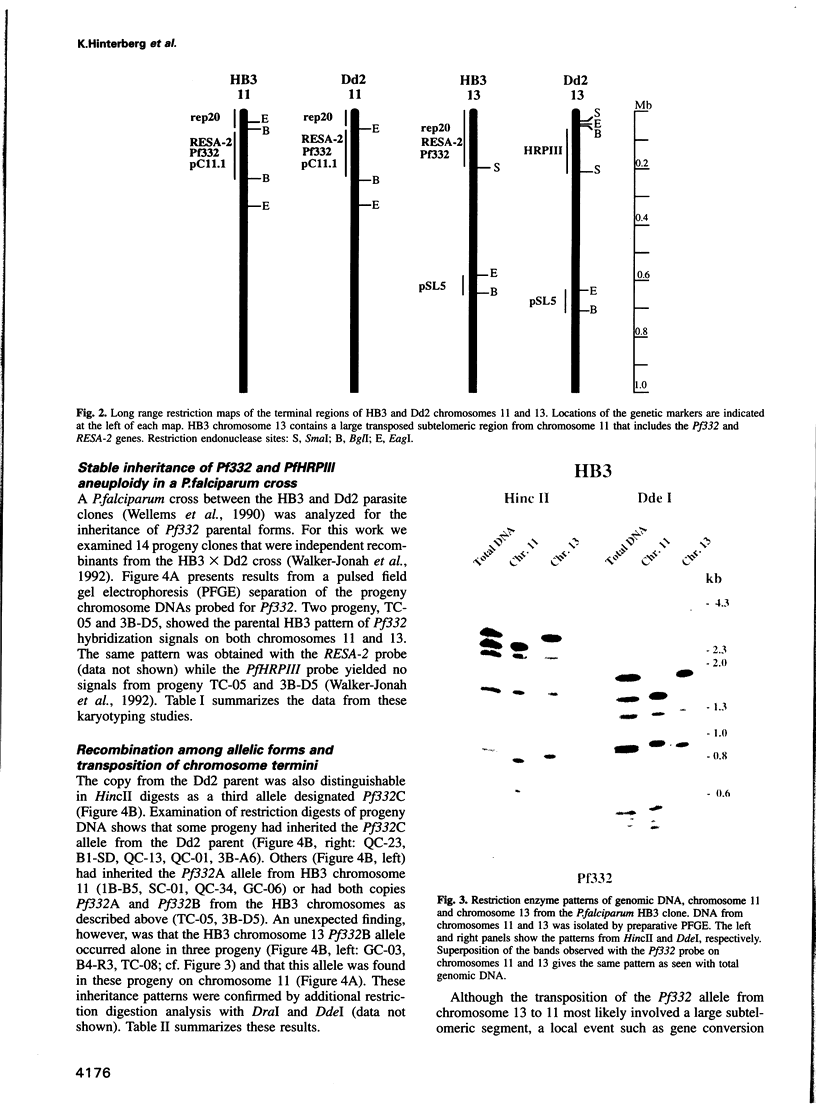
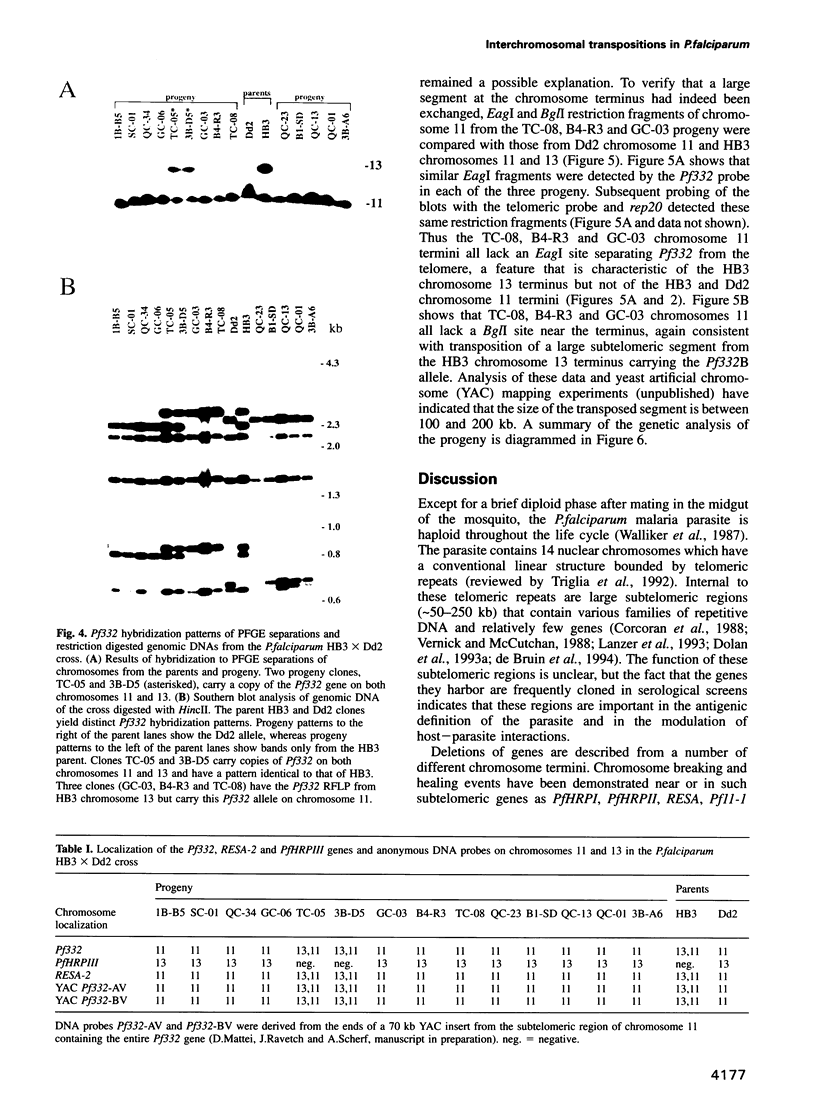
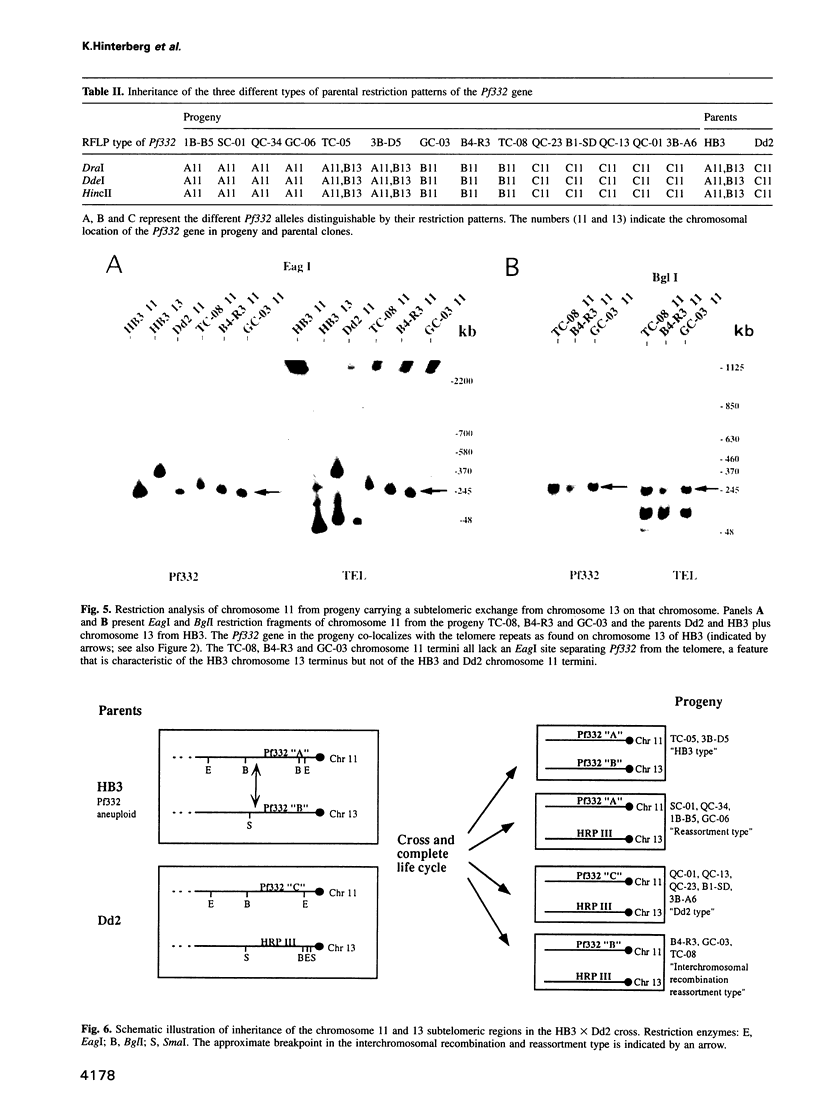
Duplications and interchromosomal transpositions of chromosome segments are implicated in the genetic variability of Plasmodium falciparum malaria parasites. One parasite clone, HB3, was shown to lack a subtelomeric region of chromosome 13 that normally carries a PfHRPIII gene. We show here that the chromosome 13 segment carrying PfHRPIII was replaced in HB3 by a duplicated terminal segment from chromosome 11. Mapping results indicate that the segment includes at least 100-200 kb of subtelomeric DNA and contains duplicated copies of the Pf332 and RESA-2 genes. We followed inheritance of this duplication in a genetic cross between the HB3 and another P.falciparum clone, Dd2, that is euploid for the Pf332, RESA-2 and PfHRPIII genes. Three types of progeny from the cross showed expected inheritance forms: a Dd2 euploid parent type, an HB3 aneuploid parent type, and a recombinant euploid type that carried PfHRPIII from Dd2 chromosome 13 and Pf332 from HB3 chromosome 11. However, a fourth euploid progeny type was also observed, in which the chromosome 13 segment from HB3 was transposed back to replace the terminus of chromosome 11. Three of 14 individual progeny were of this type. These findings suggest a mechanism of recombination from subtelomeric pairing and exchange between non-homologous chromosomes in meiosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Nash T. E., Wellems T. E. Telomeric location of Giardia rDNA genes. Mol Cell Biol. 1991 Jun;11(6):3326–3330. doi: 10.1128/mcb.11.6.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Cappai R., Kaslow D. C., Peterson M. G., Cowman A. F., Anders R. F., Kemp D. J. Cloning and analysis of the RESA-2 gene: a DNA homologue of the ring-infected erythrocyte surface antigen gene of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Sep;54(2):213–221. doi: 10.1016/0166-6851(92)90113-x. [DOI] [PubMed] [Google Scholar]
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T. Gene conversion, recombination nodules, and the initiation of meiotic synapsis. Bioessays. 1987 May;6(5):232–236. doi: 10.1002/bies.950060510. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Dancis B. M., Holmquist G. P. Telomere replication and fusion in eukaryotes. J Theor Biol. 1979 May 21;78(2):211–224. doi: 10.1016/0022-5193(79)90265-0. [DOI] [PubMed] [Google Scholar]
- Dolan S. A., Herrfeldt J. A., Wellems T. E. Restriction polymorphisms and fingerprint patterns from an interspersed repetitive element of Plasmodium falciparum DNA. Mol Biochem Parasitol. 1993 Sep;61(1):137–142. doi: 10.1016/0166-6851(93)90166-u. [DOI] [PubMed] [Google Scholar]
- Gysin J., Dubois P., Pereira da Silva L. Protective antibodies against erythrocytic stages of Plasmodium falciparum in experimental infection of the squirrel monkey, Saimiri sciureus. Parasite Immunol. 1982 Nov;4(6):421–430. doi: 10.1111/j.1365-3024.1982.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Janse C. J., Ramesar J., Mons B. Chromosome translocation in Plasmodium berghei. Nucleic Acids Res. 1992 Feb 11;20(3):581–586. doi: 10.1093/nar/20.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- Lanzer M., de Bruin D., Ravetch J. V. Transcriptional differences in polymorphic and conserved domains of a complete cloned P. falciparum chromosome. Nature. 1993 Feb 18;361(6413):654–657. doi: 10.1038/361654a0. [DOI] [PubMed] [Google Scholar]
- Loidl J., Scherthan H., Kaback D. B. Physical association between nonhomologous chromosomes precedes distributive disjunction in yeast. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):331–334. doi: 10.1073/pnas.91.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D., Scherf A. The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene. 1992 Jan 2;110(1):71–79. doi: 10.1016/0378-1119(92)90446-v. [DOI] [PubMed] [Google Scholar]
- Nolte D., Hundt E., Langsley G., Knapp B. A Plasmodium falciparum blood stage antigen highly homologous to the glycophorin binding protein GBP. Mol Biochem Parasitol. 1991 Dec;49(2):253–264. doi: 10.1016/0166-6851(91)90069-i. [DOI] [PubMed] [Google Scholar]
- Patarapotikul J., Langsley G. Chromosome size polymorphism in Plasmodium falciparum can involve deletions of the subtelomeric pPFrep20 sequence. Nucleic Acids Res. 1988 May 25;16(10):4331–4340. doi: 10.1093/nar/16.10.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Janse C. J., Dore E., Scotti R., Pace T., Reterink T. J., van der Berg F. M., Mons B. Generation of chromosome size polymorphism during in vivo mitotic multiplication of Plasmodium berghei involves both loss and addition of subtelomeric repeat sequences. Mol Biochem Parasitol. 1990 Jun;41(1):73–82. doi: 10.1016/0166-6851(90)90098-7. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Pace T., Dore E., Frontali C. Identification of a telomeric DNA sequence in Plasmodium berghei. EMBO J. 1985 Nov;4(11):2991–2995. doi: 10.1002/j.1460-2075.1985.tb04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. J., Biggs B. A., Brown G., Newbold C. I. Protection, pathogenesis and phenotypic plasticity in Plasmodium falciparum malaria. Parasitol Today. 1993 Aug;9(8):281–286. doi: 10.1016/0169-4758(93)90121-u. [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Craig A. G., Berendt A. R., Pinches R., Nash G., Marsh K., Newbold C. I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992 Jun 25;357(6380):689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A., Carter R., Petersen C., Alano P., Nelson R., Aikawa M., Mattei D., Pereira da Silva L., Leech J. Gene inactivation of Pf11-1 of Plasmodium falciparum by chromosome breakage and healing: identification of a gametocyte-specific protein with a potential role in gametogenesis. EMBO J. 1992 Jun;11(6):2293–2301. doi: 10.1002/j.1460-2075.1992.tb05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A., Hilbich C., Sieg K., Mattei D., Mercereau-Puijalon O., Müller-Hill B. The 11-1 gene of Plasmodium falciparum codes for distinct fast evolving repeats. EMBO J. 1988 Apr;7(4):1129–1137. doi: 10.1002/j.1460-2075.1988.tb02922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A., Mattei D. Cloning and characterization of chromosome breakpoints of Plasmodium falciparum: breakage and new telomere formation occurs frequently and randomly in subtelomeric genes. Nucleic Acids Res. 1992 Apr 11;20(7):1491–1496. doi: 10.1093/nar/20.7.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P., Wellems T. E. Long-range restriction maps of Plasmodium falciparum chromosomes: crossingover and size variation among geographically distant isolates. Genomics. 1988 Nov;3(4):287–295. doi: 10.1016/0888-7543(88)90117-6. [DOI] [PubMed] [Google Scholar]
- Speed R. M. The possible role of meiotic pairing anomalies in the atresia of human fetal oocytes. Hum Genet. 1988 Mar;78(3):260–266. doi: 10.1007/BF00291673. [DOI] [PubMed] [Google Scholar]
- Tourneur N., Scherf A., Wahlgren M., Gysin J. The squirrel monkey as an experimental model for Plasmodium falciparum erythrocyte rosette formation. Am J Trop Med Hyg. 1992 Nov;47(5):633–642. doi: 10.4269/ajtmh.1992.47.633. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Triglia T., Foote S. J., Kemp D. J., Cowman A. F. Amplification of the multidrug resistance gene pfmdr1 in Plasmodium falciparum has arisen as multiple independent events. Mol Cell Biol. 1991 Oct;11(10):5244–5250. doi: 10.1128/mcb.11.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Wellems T. E., Kemp D. J. Towards a high-resolution map of the Plasmodium falciparum genome. Parasitol Today. 1992 Jul;8(7):225–229. doi: 10.1016/0169-4758(92)90118-l. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Morrisey J., Plowe C. V., Kaslow D. C., Wellems T. E. Unidirectional dominance of cytoplasmic inheritance in two genetic crosses of Plasmodium falciparum. Mol Cell Biol. 1993 Dec;13(12):7349–7357. doi: 10.1128/mcb.13.12.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Gottesdiener K., Lee M. G. Antigenic variation in African trypanosomes. Trends Genet. 1992 Dec;8(12):452–457. doi: 10.1016/0168-9525(92)90330-7. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Walker-Jonah A., Dolan S. A., Gwadz R. W., Panton L. J., Wellems T. E. An RFLP map of the Plasmodium falciparum genome, recombination rates and favored linkage groups in a genetic cross. Mol Biochem Parasitol. 1992 Apr;51(2):313–320. doi: 10.1016/0166-6851(92)90081-t. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990 May 17;345(6272):253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Walliker D., Smith C. L., do Rosario V. E., Maloy W. L., Howard R. J., Carter R., McCutchan T. F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987 Jun 5;49(5):633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- Wilkie A. O., Higgs D. R., Rack K. A., Buckle V. J., Spurr N. K., Fischel-Ghodsian N., Ceccherini I., Brown W. R., Harris P. C. Stable length polymorphism of up to 260 kb at the tip of the short arm of human chromosome 16. Cell. 1991 Feb 8;64(3):595–606. doi: 10.1016/0092-8674(91)90243-r. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Blanton H. M. Distribution of telomere-associated sequences on natural chromosomes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 May;8(5):2257–2260. doi: 10.1128/mcb.8.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin D., Lanzer M., Ravetch J. V. Characterization of yeast artificial chromosomes from Plasmodium falciparum: construction of a stable, representative library and cloning of telomeric DNA fragments. Genomics. 1992 Oct;14(2):332–339. doi: 10.1016/s0888-7543(05)80223-x. [DOI] [PubMed] [Google Scholar]
- de Bruin D., Lanzer M., Ravetch J. V. The polymorphic subtelomeric regions of Plasmodium falciparum chromosomes contain arrays of repetitive sequence elements. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):619–623. doi: 10.1073/pnas.91.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]