Abstract
Background
Recurrent medulloblastoma is a daunting therapeutic challenge as it is almost universally fatal. Recent studies confirmed that medulloblastoma comprises four distinct subgroups. We sought to delineate subgroup specific differences in medulloblastoma recurrence patterns.
Methods
We retrospectively identified a discovery cohort of all recurrent medulloblastomas at the Hospital for Sick Children between 1994-2012, and performed molecular subgrouping on FFPE tissues using a nanoString-based assay. The anatomical site of recurrence (local tumour bed or leptomeningeal metastasis), time to recurrence and survival post-recurrence were determined in a subgroup specific fashion. Subgroup specific recurrence patterns were confirmed in two independent, non-overlapping FFPE validation cohorts. Where possible molecular subgrouping was performed on tissue obtained from both the initial surgery and at recurrence.
Results
A screening cohort of 30 recurrent medulloblastomas was assembled; nine with local recurrences, and 21 metastatic. When re-analysed in a subgroup specific manner, local recurrences were more frequent in SHH tumours (8/9, 88%) and metastatic recurrences were more common in Group 3 and 4 (17/20 [85%] with one WNT, p=0.0014, local vs metastatic recurrence, SHH vs Group 3 vs Group 4). The subgroup specific location of recurrence was confirmed in a multicenter validation cohort (p=0·0013 for local vs metastatic recurrence SHH vs Group 3 vs Group 4, n=77), and a second independent validation cohort comprising 96 recurrences (p<0·0001 for local vs metastatic recurrence SHH vs Group 3 vs Group 4, n=96). Treatment with craniospinal irradiation at diagnosis was not significantly associated with the anatomical pattern of recurrence. Survival post recurrence was significantly longer in Group 4 patients (p=0·013) as confirmed in a multicenter validation cohort (p=0·0075). Strikingly, subgroup affiliation remained stable at recurrence in all 34 cases with available matched primary and recurrent pairs.
Conclusions
Medulloblastoma does not switch subgroup at the time of recurrence further highlighting the stability of the four principle medulloblastoma subgroups. Significant differences in the location and timing of recurrence across medulloblastoma subgroups were observed which have potential treatment ramifications. Specifically, intensified local (posterior fossa) therapy should be tested in the initial treatment of SHH patients. Refinement of therapy for Groups 3 and 4 should focus on the metastatic compartment, as it is the near universal cause of patient deaths.
Introduction
Medulloblastoma is the most common malignant brain tumour of childhood.1, 2 Using multimodal therapy consisting of surgery, craniospinal irradiation and adjuvant chemotherapy, five-year overall survivals approach 85% for average risk disease and 70% for high risk disease.3-5 However, recurrent medulloblastoma represents a tremendous challenge, as it is almost uniformly fatal in previously irradiated patients despite a multitude of therapies including re-resection, re-irradiation, high dose chemotherapy with autologous stem-cell support and enrolment in clinical trials.6 Recent integrative genomic studies have shown that medulloblastoma comprises at least four distinct subgroups (WNT, SHH, Group 3 and Group 4) that are clinically, transcriptionally, and genetically distinct.2, 7-18 Of these four subgroups, patients with WNT subgroup tumours have an excellent prognosis while patients with Group 3 tumours have the worst prognosis and more commonly present with disseminated disease at diagnosis.1, 2, 12 Although these integrative genomic studies have shown that there are significant differences in survival between the four subgroups, little is known with respect to subgroup specific anatomical and temporal characteristics of recurrence.
In glioblastoma, molecular subgroup affiliation may change at recurrence in part due to intratumoural heterogeneity based on geographical location.19, 20 Although medulloblastoma subgroups have been shown to arise from distinct cells of origin, the stability of subgroup affiliation at recurrence remains unknown.21-23 Moreover, the clinical behaviour of the individual subgroups at recurrence has yet to be determined. As such, an understanding of the temporal and spatial details in a subgroup specific manner can help develop our treatment of recurrent medulloblastoma, as the next generation of subgroup specific clinical trials will likely be initially based in the context of recurrent medulloblastoma.
In order to characterize the subgroup specific clinical patterns of recurrence in medulloblastoma, we assembled a discovery cohort of 30 recurrent medulloblastomas at the Hospital for Sick Children, followed by the assembly of two independent, non-overlapping validation cohorts consisting of 173 recurrent medulloblastomas, representing the largest cohort of recurrent medulloblastomas ever assembled (n=203, Supplemental Table 1). This includes 51 samples where tissue was available from both diagnosis and at recurrence. Our analysis of three cohorts of recurrent medulloblastoma demonstrates clear subgroup specific difference in both the temporal and spatial patterns of recurrence.
Materials and Methods
Patient Cohorts
All samples were obtained in accordance with the Research Ethics Board at the Hospital for Sick Children (Toronto, Canada) and collaborating centres. Samples were obtained after approval from research ethics boards at each participating institution. Three independent, non-overlapping cohorts were assembled. An institutional discovery cohort was collected, comprising all medulloblastoma patients with either frozen or FFPE material along with clinical variables and survival data treated between 1994-2012 at the Hospital for Sick Children (131 total medulloblastoma cases subgrouped of which 30 were recurrent, herein referred to as the Toronto Discovery Cohort). These tumours were subgrouped by nanoString.17 A validation cohort of only recurrent medulloblastomas from 12 centres (Munich University Hospital, Hospital de Santa Maria in Lisbon, Duke University Medical Center, Lucille Packard Children's Hospital, Children's National Medical Center, BC Children's Hospital, A. I. duPont Hospital for Children, Brain Tumour Bank of Canada, Hospital Sant Joan de Deu de Barcelona, McGill University Health Centre, Children's Hospital Boston, New York University Langone Medical Center, and Cincinnati Children's Hospital) was obtained and subgrouped using nanoString (n=77, herein referred to as Validation Cohort 1, samples collected from 1991-2012). Matched samples from diagnosis and recurrence were obtained whenever possible. A second validation cohort of recurrent medulloblastoma obtained from the NN Burdenko Neurosurgical Institute in Moscow (samples collected from 1994-2011) was subgrouped according to gene expression, or IHC-based subclassification (n=96, herein referred to as Validation Cohort 2). Neuroimaging to determine the pattern of recurrence were reviewed by a multidisciplinary tumour board which included a neuroradiologist blinded of molecular subgroup at both the Hospital for Sick Children and the Burdenko Neurosurgical Institute. The pattern of recurrence in Validation Cohort 1 was provided by individual contributing institutions based on review of neuroimaging. Where available, treatment at diagnosis for all three cohorts are summarized in Table 1 and in Supplemental Table 6. Specifically, adjuvant chemotherapy regiments were all cisplatin based across the three cohorts. Treatment in Validation Cohort 2 was uniform as per the German HIT protocols in Validation Cohort 2 as previously described.24, 25
Table 1. Overall comparison of the demographics of the three independent cohorts of recurrent medulloblastoma.
| Discovery Cohort | Validation Cohort 1 | Validation Cohort 2 | p-value | |
|---|---|---|---|---|
| N | 30 | 77 | 96 | |
|
| ||||
| Male Gender | 20 (66.6%) | 39 (70%) | 61 (64%) | 0.76 |
|
| ||||
| Age (Median) | 5.4 (3·6-8·8) | 7 (3·9-11·6) | 7 (4-11) | 0·31 |
|
| ||||
| 0-3 | 6 (20%) | 10 (13%) | 12 (12.5%) | |
|
| ||||
| 3-16 | 24 (80%) | 53 (69%) | 81 (84%) | |
|
| ||||
| >16 | 0 | 14 (18%) | 3 (3%) | |
|
| ||||
| Histology | 0·19 | |||
|
| ||||
| LCA | 6 (20%) | 18 (30%) | 26 (27%) | |
|
| ||||
| Classic | 21 (70%) | 31 (52%) | 63 (66%) | |
|
| ||||
| Desmoplastic/MBEN | 3 (10%) | 11 (18%) | 7 (7%) | |
|
| ||||
| Metastases at Diagnosis | 8 (27%) | 19 (32%) | 44 (46%) | 0·11 |
|
| ||||
| Extent of Resection | n/a | 0·26 | ||
| Gross Total | 21 (75% | 60 (63%) | ||
| Subtotal | 7 (25%) | 36 (37%) | ||
|
| ||||
| Treatment | 0·49 | |||
| CSI+/-Chemo | 22 (80%) | 50 (71%) | 75 (78%) | |
| Chemo Only | 6 (20%) | 21a (29%) | 21 (22%) | |
|
| ||||
| Pattern of Recurrence | 0·20 | |||
|
| ||||
| Tumour Bed | 9 (30%) | 26 (34%) | 24 (25%) | |
|
| ||||
| Metastatic | 18 (60%) | 42 (54%) | 49 (51%) | |
|
| ||||
| Mixed | 3 (10%) | 9 (12%) | 23 (24%) | |
|
| ||||
| Subgroup | 0·15 | |||
|
| ||||
| WNT | 1 (3%) | 0 | 2 (2%) | |
|
| ||||
| SHH | 11 (37%) | 30 (39%) | 21 (22%) | |
|
| ||||
| Group 3 | 9 (30%) | 22 (29%) | 37 (38%) | |
|
| ||||
| Group 4 | 9 (30%) | 25 (32%) | 36 (37%) | |
|
| ||||
| Median Time to Recurrence (Months) | 18·3 (11·4-44·8) | 19·9 (10·9-32·6) | 12 (8-22) | 0·0038* |
|
| ||||
| Overall Followup Time (Months) | 32·9 (13·7-72·3) | 33·2 (170-57·8) | 30 (18-64·8) | 0·91 |
|
| ||||
| Survival | 0·38 | |||
|
| ||||
| Alive | 6 (20%) | 24 (34%) | 32 (33%) | |
|
| ||||
| Dead | 24 (80%) | 49 (66%) | 64 (67%) | |
p-values are Fisher Exact Test for categorical variables, and Kruskal Wallis for continuous variables. p-values in bold and starred denote a significant p-value of <0·05. Continuous variables reported as median (IQR).
LCA = Large Cell/Anaplastic Histology. MBEN = Medulloblastoma with extensive nodularity.
CSI=Craniospinal Irradiation. Gender unavailable on 21 cases, M+ status at diagnosis unavailable in 18 cases, and Histology unavailable in 17 cases in Validation Cohort 1.
- Two patients in Validation Cohort 1 received radiation therapy to the posterior fossa only followed by chemotherapy
Isolation of Nucleic Acids
RNA was extracted from fresh-frozen tissue using the Trizol method (Invitrogen) according to the manufacturer's instructions. RNA from FFPE samples (five to seven paraffin sections per sample or the equivalent in scrolls) was extracted using the RNeasy FFPE kit (Qiagen), according to the manufacturer's instructions.
Subgroup Analysis
Subgroup affiliation was determined using nanoString targeted gene expression profiling as previously described in all cases from the Hospital for Sick Children and from Validation Cohort 1.14 Subgroup determination for Validation Cohort 2 was performed in all samples by immunohistochemistry using the four antibody method as described previously (WNT=nuclear β-catenin, SHH=SFRP1, Group 3=NPR3, Group 4=KCNA1).9, 10, 17 Cases were ascribed a subgroup if they were immunoreactive for only a single marker. Of the 96 cases in Validation Cohort 2 subgrouped by immunohistochemistry none were immunoreactive for more than one marker, and 39 were also subgrouped using whole genome expression profiling, or nanoString without any re-classifications of subgroup.
Statistical Analysis
Survival time post-recurrence between subgroups was performed using the Kaplan-Meier estimate and a logrank test was used to test for differences between subgroups. Time to recurrence was plotted using the Kaplan-Meier method and the generalized Wilcoxon test was used to compare for differences between subgroups due to the absence of censoring and absence of a right bias in these data. Comparisons of binary and categorical patient characteristics between subgroups and cohorts were performed using the Fisher's exact test. Continuous variables were analyzed using the Kruskal Wallis test or Mann Whitney U test. In all cases a p-value of under 0·05 was required to be considered statistically significant. All statistical analyses except nanoString class prediction were performed using StataSE 12 (Stata Corp. College Station, TX). Principle component analysis (PCA) was performed in the Partek Genomic Suite and negative matrix factorization (NMF) was performed using Gene Pattern 2·0.26 NanoString-based class prediction and normalization of nanoString data were performed using the R statistical environment (v 2·15), as previously described.14
Role of Funding source
The funding sources of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study Population and Demographics
A discovery cohort was assembled consisting of 30 patients with recurrent medulloblastoma treated at the Hospital for Sick Children between 1994-2012. To account for unobserved variables and a potential bias due to variable subgrouping methodology, two non-overlapping validation cohorts were also assembled consisting of 77 and 96 patients with recurrent medulloblastoma. This represents the largest cohort of recurrent medulloblastoma assembled to date (Supplemental Table 1) and the largest cohort of recurrent medulloblastoma with molecular correlation. The demographics of all three cohorts are provided in Table 1. The adult age group (age >16 years) is significantly overrepresented in Validation Cohort 1 (Multicentre). Metastatic dissemination at diagnosis is higher in Validation Cohort 2 (Burdenko). Median time to recurrence is significantly shorter in Validation Cohort 2. Histological classification was not significantly different across the three cohorts.
Subgroup affiliation at diagnosis and recurrence
In order to determine if subgroup affiliation remains stable at recurrence, 34 paired samples with FFPE tissue available from the initial surgery and recurrence were analysed by nanoString. Five pairs were obtained from the Hospital for Sick Children, and 29 pairs were obtained from various centres as part of Validation Cohort 1. In all 34 paired samples, subgroup affiliation remained stable between diagnosis and the corresponding local or metastatic recurrence (Figure 1A, Supplemental Figure 1). One local recurrence was subgrouped as a Group 4 at diagnosis and SHH at recurrence; however, upon review of the histology by a senior neuropathogist (AK), the recurrence was determined to be a glioblastoma (Supplemental Figure 2). To further confirm this finding, an orthogonal technique of subgroup determination using immunohistochemistry was performed on 17 paired samples from the initial surgery and recurrence as part of Validation Cohort 2 using a 4 antibody method as previously described (Figure 1B).17, 27 In all 17 paired samples, the initial pattern of immunoreactivity remained stable at recurrence. Therefore, we conclude that medulloblastoma does not change subgroup at the time of recurrence.
Figure 1.
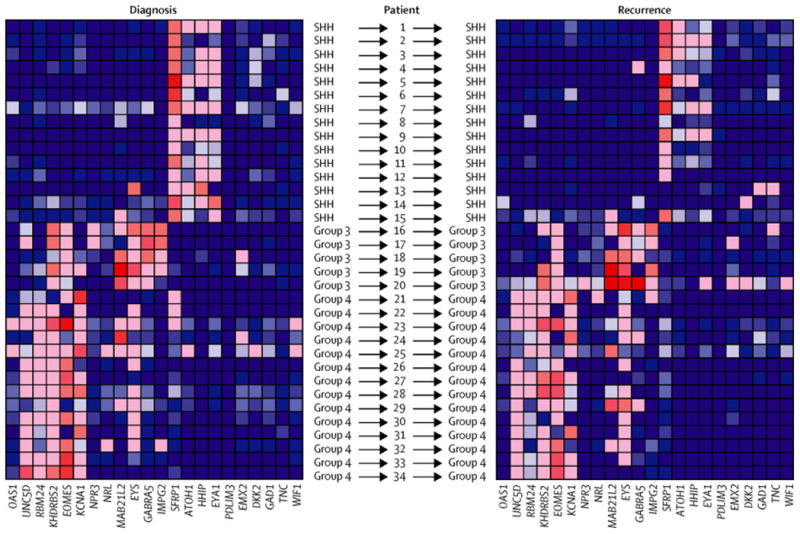
Subgroup affiliation does not change at recurrence. A) Heatmap of relative gene expression of 22 nanoString probes normalised to three housekeeping genes (ACTB, GAPDH, LDHA) across 34 medulloblastoma samples collected at diagnosis, and 34 matched recurrent medulloblastoma samples horizontally aligned. At recurrence, tissue was obtained from the primary site in 12 of 15 SHH; and 4 of 14 Group 4; the remaining samples are biopsies from leptomeningeal metastases. Subgroup affiliation of both primary and recurrence is shown to the left and right respectively for each sample. Relative gene expression is plotted on a blue-red gradient where red indicates high expression and blue low expression. B) Representative immunohistochemistry for 4 markers of medulloblastoma subgroup affiliation (WNT - ß-catenin, nuclear expression. SHH - SFRP1, membranous and perimembranous cytoplasmic expression. Group 3 -NPR3, membranous and perimembranous cytoplasmic expression. Group 4 - KCNA1, cytoplasmic and nuclear expression). Primary and recurrent pairs where available were stained with the four antibodies to determine subgroup affiliation.
Subgroup Specific Survival Analysis of Recurrences
Median time to recurrence for the Discovery cohort was 1·49 years (95% CI 0·99-2·39 years, Supplemental Figure 3). When time to recurrence is analysed in a subgroup specific manner, Group 4 tumours recurred significantly later than both Group 3 and SHH tumours (Supplementary Table 2, Supplemental Figure 4A, p=0·0080, Generalised Wilcoxon). Of the 30 recurrences, six were alive with a median post-recurrence survival of 0·70 years (95% CI 0·18-2·05 years, Supplemental Figure 4) for the Discovery cohort calculated as the time to death or last follow-up after the first recurrence. There are two long-term survivors (>5 years) post recurrence of 8·47 and 13·75 years, with subgroup affiliations of WNT and Group 4, respectively. Both were initially treated with reduced dose craniospinal irradiation and chemotherapy. The WNT long-term survivor relapsed 1.49 years post-diagnosis and was salvaged with re-irradiation and high dose chemotherapy with autologous stem cell support followed by temozolomide/etoposide at a second recurrence one year later. The Group 4 long-term survivor was salvaged with re-resection followed by high dose chemotherapy with autologous stem cell support. Interestingly, when survival post recurrence was analysed in a subgroup specific manner, Group 4 tumours had a significantly longer survival post-recurrence as compared to Group 3 and SHH tumours (Figure 2A, p=0·013, Log-Rank). The two significant findings that Group 4 tumours recur later than Group 3 and SHH tumours, and have a significantly longer survival post-recurrence were validated in Validation Cohort 1 but not in Validation Cohort 2 (Figure 2B and 2C). Time to recurrence was significantly shorter for Validation Cohort 2 suggesting possible differences in therapy account for this discrepancy although treatment regiments were similar across cohorts (Supplemental Table 2, Supplemental Figure 3B and 3C). We therefore conclude that time to recurrence and survival post-recurrence may differ significantly between subgroups.
Figure 2.
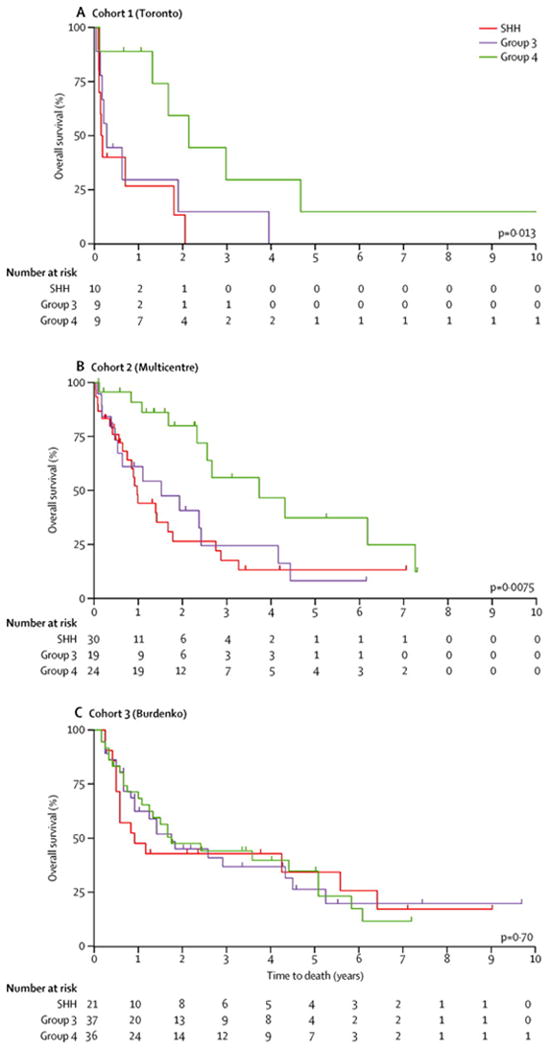
Subgroup specific survival post-recurrence across three non-overlapping cohorts of recurrent medulloblastoma. Kaplan Meier Survival estimates of overall survival post-recurrence for the A) Discovery Cohort, B) Validation Cohort 1, and C) Validation Cohort 2. p-values were determined using the log-rank test across the three subgroups.
Survival data were available in 73 of 77 cases in Validation Cohort 1. Four patients were alive at five years post-recurrence, with one patient each from the SHH and Group 3 and 2 patients with a Group 4 tumour. Two patients in Validation Cohort 1 both with Group 4 tumours died of disease at 6·2 and 7·4 years after the initial recurrence. Survival data were available in all 96 cases in Validation Cohort 2. Long-term survivors post recurrence were more common in this cohort with 17 cases surviving more than five years after recurrence of which 15 received radiation at initial therapy. The subgroup distribution of these 17 cases was one WNT, four SHH, six Group 3 and six Group 4. Two recurrent WNT cases were also identified in Validation Cohort 2 one long-term survivor of 5·4 years post recurrence (time to recurrence 3·2 years), while the other died 0·67 years post recurrence (time to recurrence 1·25 years). Where treatment information was available, we do not observe any consistent differences in treatment regimens in these long-term survivors compared to the remainder of the cohort.
Location of Recurrence
In order to determine if the location of recurrence differs between subgroups, we reviewed the location of first recurrence in the Toronto discovery cohort. The pattern of recurrence was available in all 30 recurrent cases. All patients were followed by serial magnetic resonance imaging of the craniospinal axis after completion of therapy as per treatment protocol. Recurrences were divided into local recurrences (tumour bed only without involvement of the cerebellar leptomeninges and if CSF examinations were available, no malignant cells in the CSF), metastatic recurrences (relapse at distant sites outside the tumour bed) and metastatic plus local recurrences (relapse at both distant sites and the tumour bed). We observed nine local recurrences and 21 metastatic recurrences. Strikingly, when we re-analysed this pattern in a subgroup specific manner, eight of nine local recurrences were SHH tumours, and 17 of 20 metastatic recurrences were either Group 3 or Group 4 with one WNT metastatic recurrences (p=0·0014 Fisher's Exact Test; local vs metastatic recurrence SHH vs Group 3 vs Group 4, Table 2 and Supplementary Figure 5A). In the two independent, non-overlapping validation cohorts, this pattern was also observed and was statistically significant in both cohorts (p=0·0013 [Table 2, Validation Cohort 1] and p<0.0001 [Table 2, Validation Cohort 2] Fisher's Exact Test; Supplementary Figure 5B and 5C). There is no statistically significant difference in the pattern of relapse between Group 3 and Group 4 (Supplemental Table 3). In an exploratory analysis combining all three cohorts, we observe a trend towards a higher incidence of local recurrences in Group 4 in non-irradiated cases suggesting that non-irradiated Group 4 infants may recur locally (Table 3, p=0.031; Supplemental Table 4 and 5, Supplemental Figure 6). When comparing the location of recurrence of patients treated with chemotherapy only versus craniospinal irradiation (with or without adjuvant chemotherapy), we observe no difference in SHH and Group 3 in any of the three cohorts, and we observe no difference in these two treatment regiments when combining all three cohorts (SHH p=0·83, Group 3 p=0·34, Figure 4, Supplemental Figure 6 and Supplemental Table 4/5/6) CSF examinations in 5 of 9 local recurrences in the Discovery Cohort and in all local recurrences in Validation Cohort 2 revealed no metastatic dissemination. In Validation Cohort 1, CSF examinations were not available on all patients, however in one local Group 4 recurrence a CSF examination revealed M1 disease. Two extraneural metastases occurred in Validation Cohort 1. The first case was a SHH tumour in an adult patient who received radiation and chemotherapy. The second extraneural metastasis occurred in a child treated with radiation only. Three WNT tumours recurred across all three cohorts, where two recurred with distant metastases only and one recurred in the tumour bed only (Supplemental Table 4/5). No significant differences were observed in either time to recurrence, or overall survival when comparing by location of recurrence in any of the three subgroups. SHH tumours failed with metastases in 25% of cases across all three cohorts. In order to determine if age was a factor in disseminated relapse in SHH tumours, we determine the presence of metastatic recurrences by age across all three cohorts and found no statistically significant difference (Supplementary Figure 7). Across all three cohorts, the presence of metastatic dissemination was not significantly associated with the pattern of relapse in SHH tumours (p=0.25). In Group 3 and 4 patients, 40% of Group 3 and 58% of Group 4 patients with metastatic dissemination at relapse had M0 disease at diagnosis (Supplemental Table 5). We therefore conclude that SHH tumours more frequently have isolated tumour bed recurrences, and that Group 3 and 4 tumours usually fail in the metastatic compartment.
Table 2. Anatomical patterns of recurrence across medulloblastoma subgroups.
| Discovery Cohort (p=0.0014) | Local | Mixed | Metastatic |
|---|---|---|---|
| SHH (n = 11) | 8 (72·7%) | 1 (9·1%) | 2 (18·2%) |
| Group 3 (n = 9) | 1 (11·1%) | 1 (11·1%) | 7 (77·8%) |
| Group 4 (n = 9) | 0 | 1 (11·1%) | 8 (88·9%) |
| Validation Cohort 1 (p=0.0013) | |||
| SHH (n = 30) | 18 (60%) | 2 (6·7%) | 10 (33·3%) |
| Group 3 (n = 22) | 4 (18·2%) | 5 (22·7%) | 13 (59·1%) |
| Group 4 (n = 25) | 4 (16%) | 2 (8%) | 19 (76%) |
| Validation Cohort 2 (p<0.0001) | |||
| SHH (n = 21) | 18 (85·7%) | 2 (9·5%) | 1 (4·8%) |
| Group 3 (n = 37) | 1 (2·7%) | 10 (27%) | 26 (70·3%) |
| Group 4 (n = 36) | 3 (8·3%) | 11(30·6%) | 22 (61·1%) |
*p-values determined by the Fisher's exact test; comparison of local vs mixed vs metastatic pattern of recurrence. 1 WNT recurrence in the discovery cohort and 2 WNT recurrences in Validation Cohort 2 are described in the results section
Table 3. Anatomical pattern of recurrence stratified by treatment with craniospinal irradiation across medulloblastoma subgroups combining all three cohorts.
| SHH (p=0.83) | Local | Mixed | Metastatic |
|---|---|---|---|
| Chemotherapy (n = 20) | 14 (70%) | 1 (5%) | 5 (25%) |
| CSI+/-Chemotherapy (n = 38) | 26 (68·4%) | 4 (10·5%) | 8 (21·1%) |
| Group 3 (p=0.32) | |||
| Chemotherapy (n = 19) | 2 (10%) | 7 (35%) | 11 (55%) |
| CSI+/-Chemotherapy (n = 48) | 4 (8·3%) | 9 (18.8%) | 35 (72·9%) |
| Group 4 (p=0.031) | |||
| Chemotherapy (n = 8) | 3 (37·5%) | 2 (25%) | 3 (38%) |
| CSI+/-Chemotherapy (n = 59) | 4 (6·8%) | 12 (20%) | 43 (73%) |
p-values determined by the Fisher's exact test; comparison of local vs mixed vs metastatic pattern of recurrence.
Note: Two patients in Validation Cohort 1 received focal RT with chemotherapy, one SHH with a metastatic recurrence and one Group 4 with a metastatic recurrence. Both cases are included in the chemotherapy only category. CSI=Craniospinal Irradiation
Discussion
Our study represents to our knowledge the first comprehensive analysis of medulloblastoma recurrence in a subgroup specific manner. Importantly we demonstrate that medulloblastoma does not change subgroup at recurrence. This was not dependent on location of recurrence, as subgroup affiliation remained stable at in local tumour bed and metastatic samples at recurrence. Retaining subgroup provides further evidence supporting the notion that medulloblastoma arises from distinct cells of origin, the characteristics of which are carried forward from ontogeny into oncology. We also find significant differences exist across subgroups with respect to the anatomical and temporal patterns of recurrence, specifically SHH tumours recur mostly in the local tumour bed and Group 3 and 4 tumours recur almost exclusively with metastases. Group 4 tumours also have an increased time to death post-recurrence. These findings have significant implications in the care of children with recurrent medulloblastoma and provide insight into the planning of future clinical trials.
Our finding that medulloblastoma does not change subgroup at recurrence highlights the stability of medulloblastoma subgroups, and further strengthens the notion that medulloblastoma subgroups arise from distinct cells of origin within the posterior fossa. Indeed, this is in agreement with murine models whereby WNT, SHH and Group 3 have unique cells of origin, specifically the lower rhombic lip, the external granule layer, and postnatal cerebellar progenitor cells respectively. Our findings suggest that Group 4 tumours also arise from a yet to be identified cell of origin distinct from the other three subgroups.21-23 Unlike glioblastoma where there are some initial suggestions that subgroup affiliation can change at recurrence, we observed all 34 cases where we had matching tissue from both diagnosis and recurrence that subgroup affiliation remains stable.19, 20 Similar to breast cancer and renal cell carcinoma, metastases from medulloblastoma have been shown to be highly genetically divergent from their matched primary tumor.16, 28, 29 Genetic divergence of medulloblastoma metastases from their primary in the face of subgroup stability across both compartments suggests that subgroup identity may be established in the cell of origin; which has been suggested to be distinct for each subgroup. Further investigation of paired samples from diagnosis and matched metastatic recurrences using next generation methodologies such as RNA-seq and whole genome sequencing will be required to determine the full spectrum of heterogeneity between the primary and metastatic compartments.
Previously, it has been suggested that local recurrences are more common in younger children, and metastatic recurrences are more common in older children.30, 31 Infant protocols consisting of chemotherapy only have been associated with a higher propensity for local recurrence. However, our data suggest a simple, proximate explanation that this is likely driven by subgroup, as SHH tumours are more common in infants and Group 4 are more common in older children. Overall, we find no association between the treatment regiment and the site of recurrence with the exception of a possible association between chemotherapy only approaches and local recurrences in Group 4 tumours. This suggests that in younger children who are not irradiated, local Group 4 recurrences should not be unexpected albeit this is limited by a small sample size of infant Group 4 patients and warrants further investigation in a prospective trial. Treatment was uniform across cohorts and subgroups where young infants under three years of age are treated with chemotherapy only approaches and children over three years of age are treated with radiation followed by cisplatin based adjuvant chemotherapy. Taken together, our data suggests that subgroup affiliation, rather than treatment effects seems to be the primary driver of location of recurrence particularly in Group 3 and SHH patients. A limitation of our study is the lack of knowledge with respect to M1 assessments at relapse and lack of detailed treatment information at relapse. Future prospective studies will need to rigorously determine that the CSF is free of metastatic disease in local recurrences, specifically in the rare situations where local recurrences occur in Group 3 and 4 tumours to exclude metastatic dissemination. Prospective multicentre longitudinal studies of recurrent medulloblastoma in a subgroup specific manner are required to determine if current or future salvage therapies confer any benefit. Our finding that the pattern of recurrence is highly subgroup specific requires prospective validation in a multicentre cooperative study of homogenously treated patients.
Previous cross-species genomic studies have shown that metastatic medulloblastoma is a bicompartmental disease in which the metastases are highly divergent from their matched primary in both human and mouse.16, 32 This suggests that response and susceptibility to therapy are likely to be different in the two compartments. Our data demonstrate the excellent tumour control for Group 3 and Group 4 medulloblastoma in the posterior fossa using current therapies. However, the alarming fact that most Group 3 and Group 4 recurrences are metastatic, in combination with the fact that metastases are genetically divergent from the primary tumour, and that medulloblastoma metastases are understudied suggests that future basic science and clinical trials of Group 3 and 4 should be more highly focused on the metastatic compartment. The majority of average risk Group 3 and 4 patients in our cohort were treated with high dose craniospinal irradiation further reinforcing the importance of generating novel approaches to therapy in the metastatic compartment. Moreover, our observation that most Group 3 and 4 metastatic relapses do not relapse in the primary site suggests that microscopic leptomeningeal metastases not visible by neuroimaging or CSF examination are resistant to current therapy. As such, additional local therapies targeting the primary site in the posterior fossa are unlikely to increase the cure rate for patients with Group 3 or Group 4 medulloblastoma. Possible strategies aimed at the metastatic compartment include intrathecal consolidation regiments in addition to current therapies, which achieve excellent local tumour bed control. Current clinical trials for previously irradiated relapsed metastatic medulloblastoma are very heterogeneous and commonly involve enrolment in a Phase 2 clinical trial. As any future subgroup specific clinical trial will likely begin with relapsed patients, trials for relapsed Group 3 or 4 medulloblastoma are poised to fail if they are based on the biology of the primary tumour. As SHH tumours predominantly recur in the posterior fossa, consideration of a clinical trial intensifying treatment to the posterior fossa (to treat local recurrence) while simultaneously reducing craniospinal doses (as leptomeningeal failure is rare) should be considered. Ongoing trials will help to elucidate the effects of Smoothened inhibition on local tumour control for SHH subgroup patients.
Panel: Research in Context
Systematic review
We reviewed available English studies of medulloblastoma in Pubmed and Google Scholar specifically with a focus on recurrent medulloblastoma without any date restrictions. We identify several studies over the past 30 years reporting the anatomical pattern of recurrence in medulloblastoma, specifically that recurrences can occur either in the tumour bed, along the leptomeninges or both, however all these previous studies are limited by both small numbers and absence of biological correlation.39 Several recent integrated genomic studies have identifies four subgroups of medulloblastoma with distinct demographics, genetics, transcriptomes and outcomes, however little is known with regards to the clinical implications of these subgroups at the time of disease recurrence.
Interpretation
Our study represents the largest single study of recurrent medulloblastoma and the first to evaluate recurrent medulloblastoma in a subgroup specific manner. Many in the pediatric neuro-oncology have suggested recently that medulloblastomas might recur as more aggressive subgroups (i.e., a SHH or Group 4 tumour becoming a Group 3 tumour). In this study we demonstrate that at the time of recurrence, medulloblastomas maintain their subgroup affiliation. Excitingly, we show that the anatomic pattern of recurrence is highly subgroup specific. SHH medulloblastomas almost always recur locally, suggesting that additional therapies aimed at the posterior fossa might be most beneficial. Most importantly, we show that almost all recurrences of Group 3 and Group 4 medulloblastoma are metastatic, with failure in the posterior fossa in radiated patients being vanishingly rare. Moreover we show this subgroup specific pattern of relapse to be highly consistent across three independent cohorts, and this is not dependent on therapy at diagnosis. As such subgroup affiliation, rather than treatment effects seems to be the primary driver of location of recurrence. The current clinical data on metastatic recurrence, in combination with previous publications showing that metastases are clinically and genetically distinct from the primary tumour16, 32 suggest that the paediatric neuro-oncology community needs to radically shift our focus away from the primary tumour to focus on the metastases as Group 3 and Group 4 patients are dying almost exclusively from metastatic disease.
Supplementary Material
Acknowledgments
MDT is supported by a CIHR Clinician Scientist Phase II award, funds from the Garron Family Chair in Childhood Cancer Research at The Hospital for Sick Children and The University of Toronto, and operating funds from the Canadian Institutes of Health Research, the National Institutes of Health (R01CA159859 and R01CA148699) and the Pediatric Brain Tumor Foundation. VR is supported by a CIHR fellowship and an Alberta Innovates-Health Solutions Clinical Fellowship. SG, RM, DB are supported by the Pediatric Brain Tumor Foundation. MK, SG, DZ are supported in part by grant UL1TR000038 from the National Center for Research Resources, National Institutes of Health and grant 5P30CA016087-32 from the National Cancer Institute. MR is supported by a fellowship from the Mildred Scheel Cancer Foundation. SMP is supported by a grant from the Deutsche Kinderkrebsstiftung. We thank Susan Archer for technical writing.
Funding: Canadian Institutes of Health Research, National Institutes of Health, Pediatric Brain Tumor Foundation, Garron Family Chair in Childhood Cancer Research at The Hospital for Sick Children and The University of Toronto.
Footnotes
Author Contributions: VR, MR, EB, SMP, AK and MDT designed the study. MDT procured financial support. VR, MR, BL, CCF, SP, Y-JC, US, SG, RM, DB, MF, KLL, SLP, SD, JT, NJ, AF, DTWJ, MK, MAK, SLG, DZ, SN, JP, JM, EL, AWW, MR, OZ, EK, JA, SEC, JTR, CH, UT, KTC, RJP, AK collected data and provided study materials. VR, MR, EB, DS, AMD, PAN, SMP, AK, MDT analysed and interpreted the data. VR, MR, EB, SMP and MDT wrote the report. All authors approved the final manuscript.
Conflict of Interests: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramaswamy V, Northcott PA, Taylor MD. FISH and chips: the recipe for improved prognostication and outcomes for children with medulloblastoma. Cancer genetics. 2011;204(11):577–88. doi: 10.1016/j.cancergen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–34. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–20. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 5.Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-Risk Medulloblastoma: A Pediatric Oncology Group Randomized Trial of Chemotherapy Before or After Radiation Therapy (POG 9031) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(23):2936–41. doi: 10.1200/JCO.2012.43.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizer B, Donachie PH, Robinson K, et al. Treatment of recurrent central nervous system primitive neuroectodermal tumours in children and adolescents: results of a Children's Cancer and Leukaemia Group study. Eur J Cancer. 2011;47(9):1389–97. doi: 10.1016/j.ejca.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubuc AM, Morrissy AS, Kloosterhof NK, et al. Subgroup-specific alternative splicing in medulloblastoma. Acta Neuropathol. 2012;123(4):485–99. doi: 10.1007/s00401-012-0959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remke M, Hielscher T, Korshunov A, et al. FSTL5 Is a Marker of Poor Prognosis in Non-WNT/Non-SHH Medulloblastoma. J Clin Oncol. 2011;29(29):3852–61. doi: 10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 10.Remke M, Hielscher T, Northcott PA, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–23. doi: 10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 11.Jones DT, Jager N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–5. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–84. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–26. doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–10. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Northcott PA, Dubuc A, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482(7386):529–33. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma Comprises Four Distinct Molecular Variants. J Clin Oncol. 2011;29(11):1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–8. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–14. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Pei Y, Moore CE, Wang J, et al. An Animal Model of MYC-Driven Medulloblastoma. Cancer Cell. 2012;21(2):155–67. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawauchi D, Robinson G, Uziel T, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21(2):168–80. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson P, Tong Y, Robinson G, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–9. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfister S, Remke M, Benner A, et al. Outcome Prediction in Pediatric Medulloblastoma Based on DNA Copy-Number Aberrations of Chromosomes 6q and 17q and the MYC and MYCN Loci. J Clin Oncol. 2009;27(10):1627–36. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 25.Korshunov A, Remke M, Werft W, et al. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28(18):3054–60. doi: 10.1200/JCO.2009.25.7121. [DOI] [PubMed] [Google Scholar]
- 26.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nature genetics. 2006;38(5):500–1. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 27.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29(11):1400–7. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparicio S, Caldas C. The implications of clonal genome evolution for cancer medicine. N Engl J Med. 2013;368(9):842–51. doi: 10.1056/NEJMra1204892. [DOI] [PubMed] [Google Scholar]
- 30.Warmuth-Metz M, Blashofer S, Bueren AO, et al. Recurrence in childhood medulloblastoma. J Neurooncol. 2010;103(3):705–11. doi: 10.1007/s11060-010-0452-x. [DOI] [PubMed] [Google Scholar]
- 31.Tabori U, Sung L, Hukin J, et al. Distinctive clinical course and pattern of relapse in adolescents with medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;64(2):402–7. doi: 10.1016/j.ijrobp.2005.07.962. [DOI] [PubMed] [Google Scholar]
- 32.Korshunov A, Benner A, Remke M, Lichter P, von Deimling A, Pfister S. Accumulation of genomic aberrations during clinical progression of medulloblastoma. Acta Neuropathol. 2008;116(4):383–90. doi: 10.1007/s00401-008-0422-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


