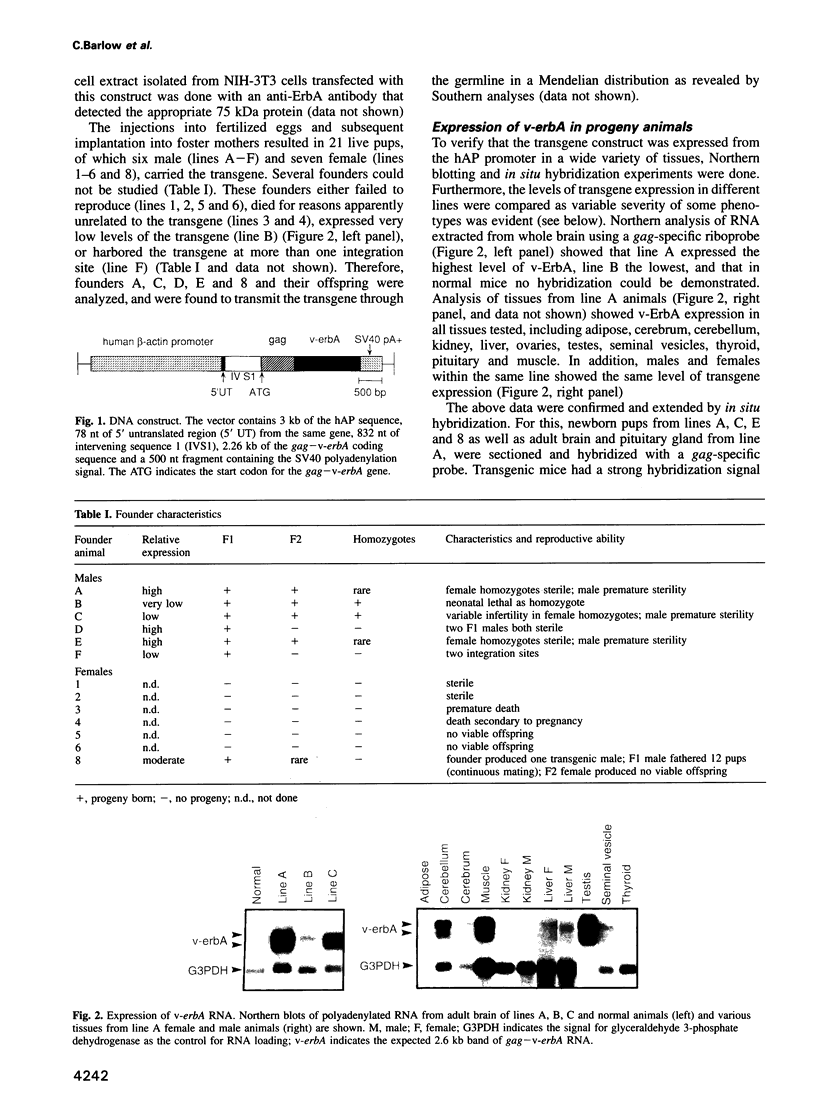
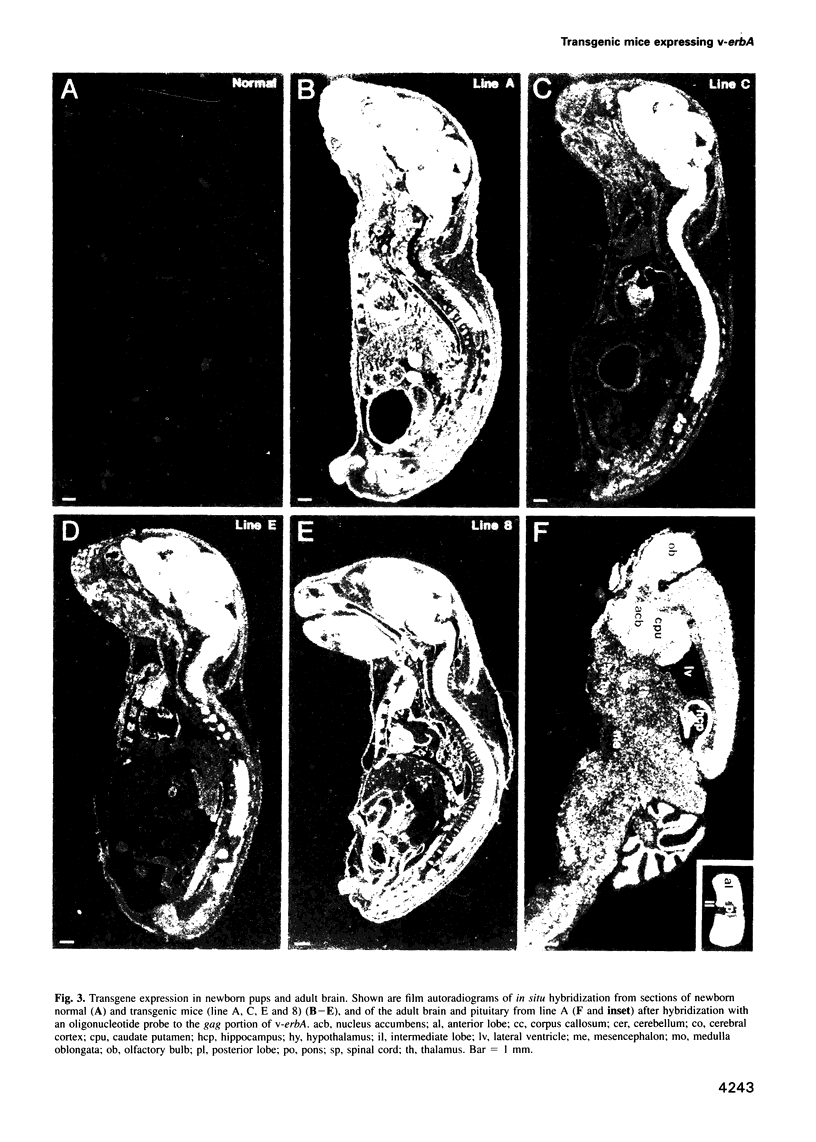
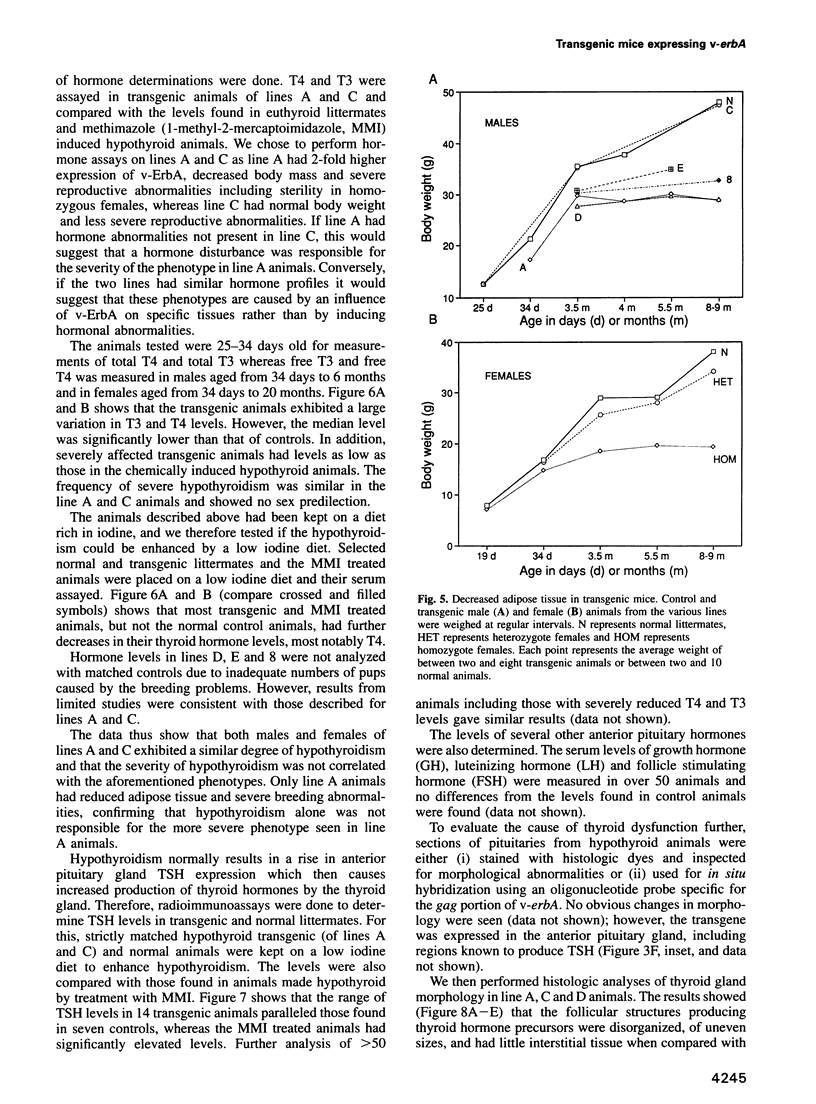
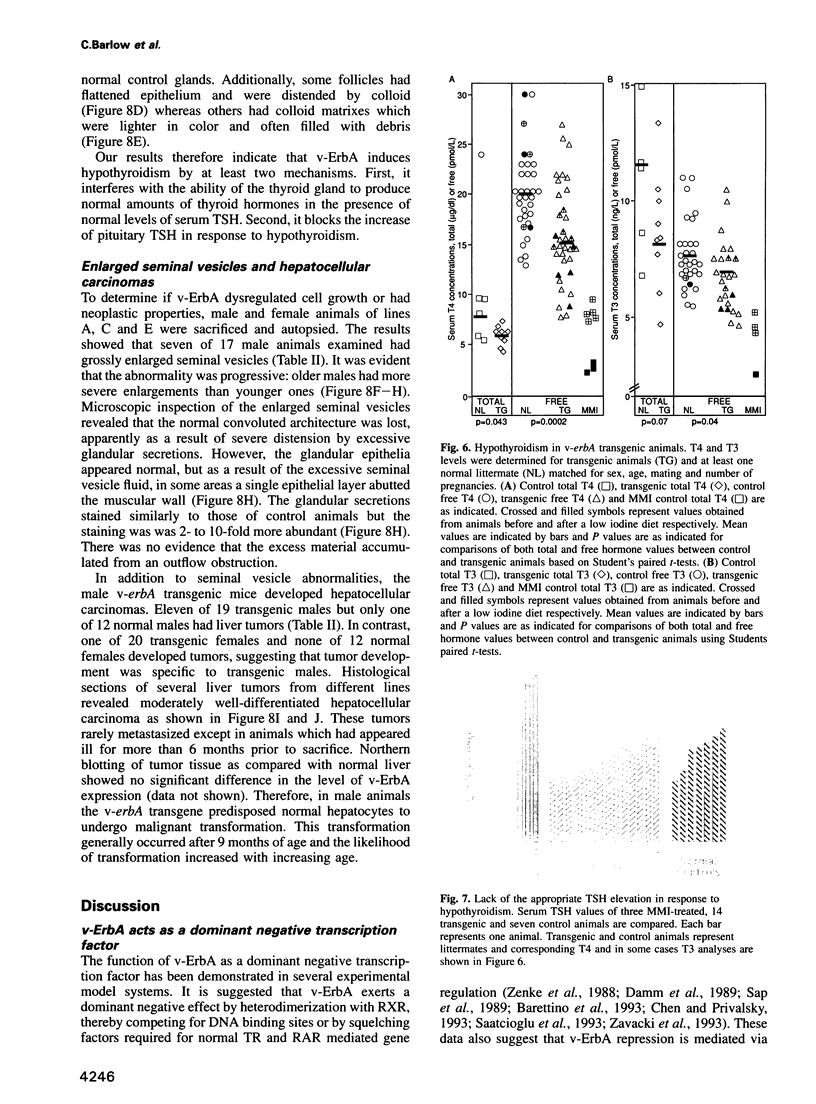
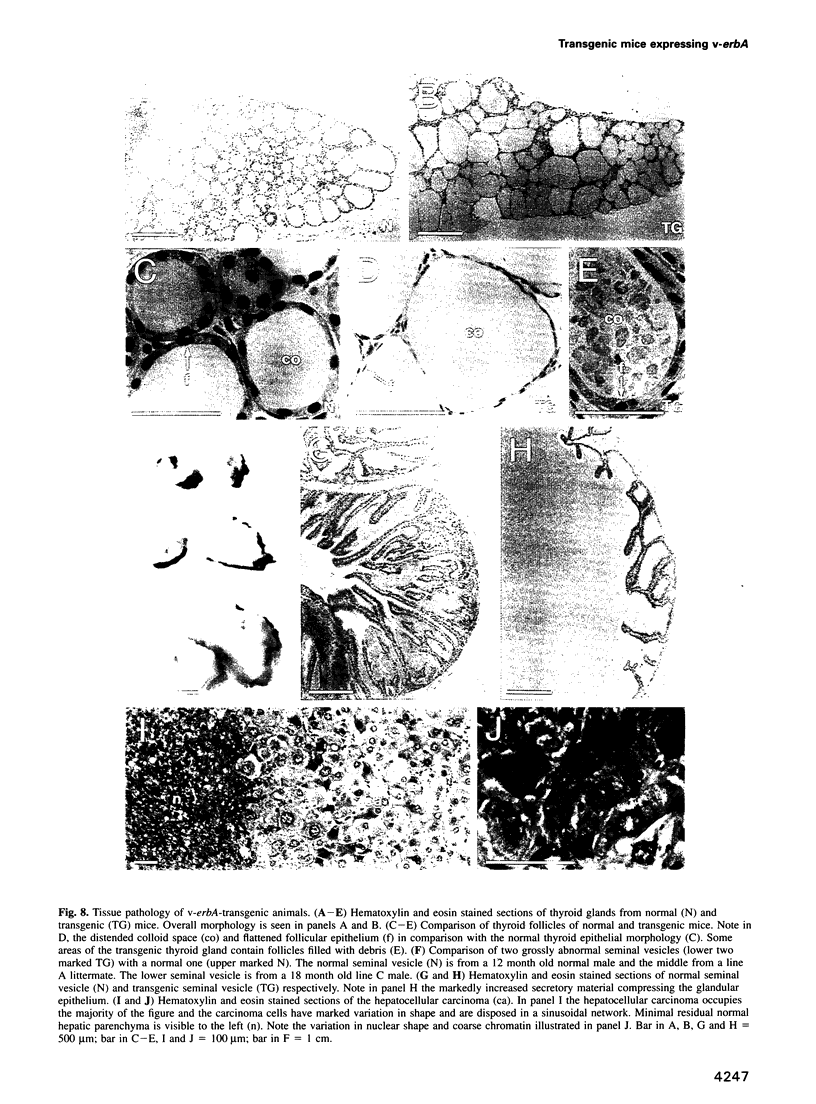
Abstract
The v-erbA oncogene consists of an avian retroviral gag gene fused to a mutated thyroid hormone receptor. To define better its role as an oncogene in mammals and its ability to function as a dominant negative transcription factor, transgenic mice expressing v-erbA ubiquitously were generated. The effects of v-erbA are pleiotropic, tissue-specific and dose dependent. Mice have breeding disorders, abnormal behavior, reduced adipose tissue, hypothyroidism with inappropriate TSH response, and enlarged seminal vesicles. This provides an animal model consistent with the proposal that v-ErbA functions as a dominant negative receptor by transcriptional interference or squelching of normal receptors or associated proteins. Finally, male animals develop hepatocellular carcinoma, demonstrating that v-erbA can promote neoplasia in mammals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiguchi I., Strauss K., Borges M., Silva J. E., Moses A. C. Thyroid hormone receptors and 3,5,3'-triiodothyronine biological effects in FRTL5 thyroid follicular cells. Endocrinology. 1992 Sep;131(3):1279–1287. doi: 10.1210/endo.131.3.1324156. [DOI] [PubMed] [Google Scholar]
- Ambrosino C., Cicatiello L., Cobellis G., Addeo R., Sica V., Bresciani F., Weisz A. Functional antagonism between the estrogen receptor and Fos in the regulation of c-fos protooncogene transcription. Mol Endocrinol. 1993 Nov;7(11):1472–1483. doi: 10.1210/mend.7.11.8114761. [DOI] [PubMed] [Google Scholar]
- Barettino D., Bugge T. H., Bartunek P., Vivanco Ruiz M. D., Sonntag-Buck V., Beug H., Zenke M., Stunnenberg H. G. Unliganded T3R, but not its oncogenic variant, v-erbA, suppresses RAR-dependent transactivation by titrating out RXR. EMBO J. 1993 Apr;12(4):1343–1354. doi: 10.1002/j.1460-2075.1993.tb05779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde B. G., Sharif M., Privalsky M. L. Ontogeny of the v-erbA oncoprotein from the thyroid hormone receptor: an alteration in the DNA binding domain plays a role crucial for v-erbA function. J Virol. 1991 Apr;65(4):2037–2046. doi: 10.1128/jvi.65.4.2037-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Privalsky M. L. The erbA oncogene represses the actions of both retinoid X and retinoid A receptors but does so by distinct mechanisms. Mol Cell Biol. 1993 Oct;13(10):5970–5980. doi: 10.1128/mcb.13.10.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dagerlind A., Friberg K., Bean A. J., Hökfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992 Aug;98(1):39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Desbois C., Aubert D., Legrand C., Pain B., Samarut J. A novel mechanism of action for v-ErbA: abrogation of the inactivation of transcription factor AP-1 by retinoic acid and thyroid hormone receptors. Cell. 1991 Nov 15;67(4):731–740. doi: 10.1016/0092-8674(91)90068-a. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D., Sjöberg M., Vennström B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. EMBO J. 1990 May;9(5):1519–1528. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Fuerstenberg S., Leitner I., Schroeder C., Schwarz H., Vennström B., Beug H. Transcriptional repression of band 3 and CAII in v-erbA transformed erythroblasts accounts for an important part of the leukaemic phenotype. EMBO J. 1992 Sep;11(9):3355–3365. doi: 10.1002/j.1460-2075.1992.tb05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Benchaibi M., Xiao J. H., Ghysdael J., Samarut J. Expression of the v-erbA oncogene in chicken embryo fibroblasts stimulates their proliferation in vitro and enhances tumor growth in vivo. Cell. 1987 Jun 5;49(5):687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M. Regulation of gene expression by the thyroid hormone receptor. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):157–176. doi: 10.1016/0304-419x(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P., Zametkin A. J., Martinez P., Vitiello B., Matochik J. A., Mixson A. J., Weintraub B. D. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993 Apr 8;328(14):997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- Hermann T., Hoffmann B., Piedrafita F. J., Zhang X. K., Pfahl M. V-erbA requires auxiliary proteins for dominant negative activity. Oncogene. 1993 Jan;8(1):55–65. [PubMed] [Google Scholar]
- Iñiguez M. A., Rodriguez-Peña A., Ibarrola N., Morreale de Escobar G., Bernal J. Adult rat brain is sensitive to thyroid hormone. Regulation of RC3/neurogranin mRNA. J Clin Invest. 1992 Aug;90(2):554–558. doi: 10.1172/JCI115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson M., Beug H., Gray C., Graf T., Vennström B. Defective v-erbB genes can be complemented by v-erbA in erythroblast and fibroblast transformation. Oncogene. 1987 May;1(2):167–173. [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Köhne A. C., Baniahmad A., Renkawitz R. NeP1. A ubiquitous transcription factor synergizes with v-ERBA in transcriptional silencing. J Mol Biol. 1993 Aug 5;232(3):747–755. doi: 10.1006/jmbi.1993.1428. [DOI] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D., Kastner P., Dierich A., Mark M., LeMeur M., Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993 May 21;73(4):643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Zenke M., Gehring U., Sap J., Beug H., Vennström B. Characterization of the hormone-binding domain of the chicken c-erbA/thyroid hormone receptor protein. EMBO J. 1988 Jan;7(1):155–159. doi: 10.1002/j.1460-2075.1988.tb02795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M. Nuclear receptor/AP-1 interaction. Endocr Rev. 1993 Oct;14(5):651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- Ray P., Higgins K. M., Tan J. C., Chu T. Y., Yee N. S., Nguyen H., Lacy E., Besmer P. Ectopic expression of a c-kitW42 minigene in transgenic mice: recapitulation of W phenotypes and evidence for c-kit function in melanoblast progenitors. Genes Dev. 1991 Dec;5(12A):2265–2273. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- Refetoff S., DeWind L. T., DeGroot L. J. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967 Feb;27(2):279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- Saatcioglu F., Bartunek P., Deng T., Zenke M., Karin M. A conserved C-terminal sequence that is deleted in v-ErbA is essential for the biological activities of c-ErbA (the thyroid hormone receptor). Mol Cell Biol. 1993 Jun;13(6):3675–3685. doi: 10.1128/mcb.13.6.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Palmiter R. D., Brinster R. L., Lee D. C. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Sideras P., Nilsson L., Islam K. B., Quintana I. Z., Freihof L., Rosén A., Juliusson G., Hammarström L., Smith C. I. Transcription of unrearranged Ig H chain genes in human B cell malignancies. Biased expression of genes encoded within the first duplication unit of the Ig H chain locus. J Immunol. 1992 Jul 1;149(1):244–252. [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yen P. M., Ikeda M., Brubaker J. H., Forgione M., Sugawara A., Chin W. W. Roles of v-erbA homodimers and heterodimers in mediating dominant negative activity by v-erbA. J Biol Chem. 1994 Jan 14;269(2):903–909. [PubMed] [Google Scholar]
- Yu M. W., Chen C. J. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res. 1993 Feb 15;53(4):790–794. [PubMed] [Google Scholar]
- Zavacki A. M., Harney J. W., Brent G. A., Larsen P. R. Dominant negative inhibition by mutant thyroid hormone receptors is thyroid hormone response element and receptor isoform specific. Mol Endocrinol. 1993 Oct;7(10):1319–1330. doi: 10.1210/mend.7.10.8264663. [DOI] [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Wills K. N., Husmann M., Hermann T., Pfahl M. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol. 1991 Dec;11(12):6016–6025. doi: 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Metzger D. The lack of transcriptional activation of the v-erbA oncogene is in part due to a mutation present in the DNA binding domain of the protein. Nucleic Acids Res. 1990 Aug 11;18(15):4489–4497. doi: 10.1093/nar/18.15.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]