Abstract
Gypsy displays striking similarities to vertebrate retroviruses, including the presence of a yet uncharacterized additional open reading frame (ORF3) and the recent evidence for infectivity. It is mobilized with high frequency in the germline of the progeny of females homozygous for the flamenco permissive mutation. We report the characterization of a gypsy subgenomic ORF3 RNA encoding typical retroviral envelope proteins. In females, env expression is strongly repressed by one copy of the non-permissive allele of flamenco. A less dramatic reduction in the accumulation of other transcripts and retrotranscripts is also observed. These effects correlate well with the inhibition of gypsy transposition in the progeny of these females, and are therefore likely to be responsible for this phenomenon. The effects of flamenco on gypsy expression are apparently restricted to the somatic follicle cells that surround the maternal germline. Moreover, permissive follicle cells display a typically polarized distribution of gypsy RNAs and envelope proteins, both being mainly accumulated at the apical pole, close to the oocyte. We propose a model suggesting that gypsy germinal transposition might occur only in individuals that have maternally inherited enveloped gypsy particles due to infection of the maternal germline by the soma.
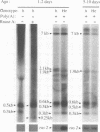
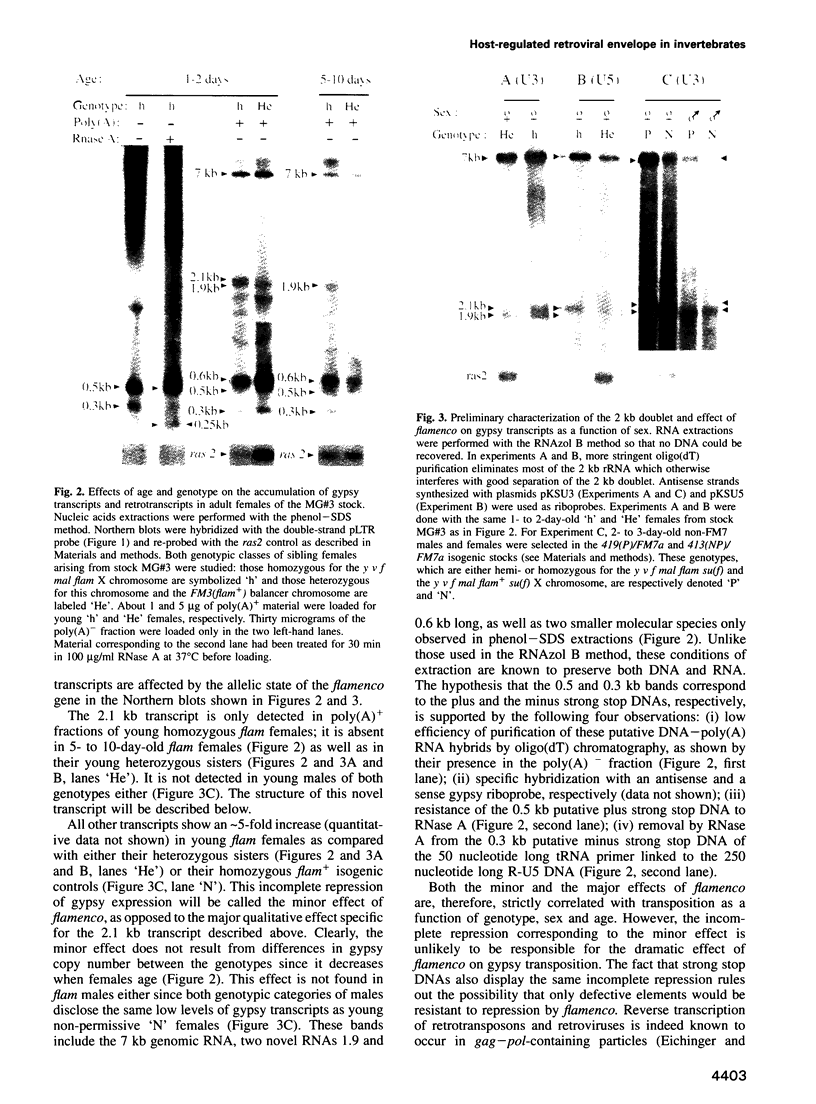
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhipova I. R., Mazo A. M., Cherkasova V. A., Gorelova T. V., Schuppe N. G., Llyin Y. V. The steps of reverse transcription of Drosophila mobile dispersed genetic elements and U3-R-U5 structure of their LTRs. Cell. 1986 Feb 28;44(4):555–563. doi: 10.1016/0092-8674(86)90265-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANG F. B. The development of Newcastle disease virus in cells of the chorio-allantoic membrane as studied by thin section. Bull Johns Hopkins Hosp. 1953 Apr;92(4):309–329. [PubMed] [Google Scholar]
- Bayev A. A., Jr, Lyubomirskaya N. V., Dzhumagaliev E. B., Ananiev E. V., Amiantova I. G., Ilyin Y. V. Structural organization of transposable element mdg4 from Drosophila melanogaster and a nucleotide sequence of its long terminal repeats. Nucleic Acids Res. 1984 Apr 25;12(8):3707–3723. doi: 10.1093/nar/12.8.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. G., 3rd, Corces V. G. Expression of an activated ras gene causes developmental abnormalities in transgenic Drosophila melanogaster. Genes Dev. 1988 May;2(5):567–577. doi: 10.1101/gad.2.5.567. [DOI] [PubMed] [Google Scholar]
- Brennan M. D., Weiner A. J., Goralski T. J., Mahowald A. P. The follicle cells are a major site of vitellogenin synthesis in Drosophila melanogaster. Dev Biol. 1982 Jan;89(1):225–236. doi: 10.1016/0012-1606(82)90309-8. [DOI] [PubMed] [Google Scholar]
- Bucheton A. I transposable elements and I-R hybrid dysgenesis in Drosophila. Trends Genet. 1990 Jan;6(1):16–21. doi: 10.1016/0168-9525(90)90044-7. [DOI] [PubMed] [Google Scholar]
- Busson D., Gans M., Komitopoulou K., Masson M. Genetic Analysis of Three Dominant Female-Sterile Mutations Located on the X Chromosome of DROSOPHILA MELANOGASTER. Genetics. 1983 Oct;105(2):309–325. doi: 10.1093/genetics/105.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Freund R., Meselson M. Long terminal repeat nucleotide sequence and specific insertion of the gypsy transposon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4462–4464. doi: 10.1073/pnas.81.14.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans M., Audit C., Masson M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 1975 Dec;81(4):683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Frank D., Bolce M. E., Brown B. D., Sive H. L., Harland R. M. Localization of specific mRNAs in Xenopus embryos by whole-mount in situ hybridization. Development. 1990 Oct;110(2):325–330. doi: 10.1242/dev.110.2.325. [DOI] [PubMed] [Google Scholar]
- Inouye S., Yuki S., Saigo K. Complete nucleotide sequence and genome organization of a Drosophila transposable genetic element, 297. Eur J Biochem. 1986 Jan 15;154(2):417–425. doi: 10.1111/j.1432-1033.1986.tb09414.x. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Bownes M. Ovarian and fat-body vitellogenin synthesis in Drosophila melanogaster. Eur J Biochem. 1982 Apr;123(3):527–534. doi: 10.1111/j.1432-1033.1982.tb06563.x. [DOI] [PubMed] [Google Scholar]
- Kim A. I., Belyaeva E. S., Aslanian M. M. Autonomous transposition of gypsy mobile elements and genetic instability in Drosophila melanogaster. Mol Gen Genet. 1990 Nov;224(2):303–308. doi: 10.1007/BF00271566. [DOI] [PubMed] [Google Scholar]
- Kim A., Terzian C., Santamaria P., Pélisson A., Purd'homme N., Bucheton A. Retroviruses in invertebrates: the gypsy retrotransposon is apparently an infectious retrovirus of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1285–1289. doi: 10.1073/pnas.91.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty T. R., Lim J. K. Site-specific instability in Drosophila melanogaster: evidence for transposition of destabilizing element. Genetics. 1982 Jul-Aug;101(3-4):461–476. doi: 10.1093/genetics/101.3-4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch R. A., Friesen P. D. The baculovirus-integrated retrotransposon TED encodes gag and pol proteins that assemble into viruslike particles with reverse transcriptase. J Virol. 1992 Mar;66(3):1590–1601. doi: 10.1128/jvi.66.3.1590-1601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Newnam G. A ubiquitin-conjugating enzyme, RAD6, affects the distribution of Ty1 retrotransposon integration positions. Genetics. 1993 Mar;133(3):499–508. doi: 10.1093/genetics/133.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirskaya N. V., Arkhipova I. R., Ilyin Y. V., Kim A. I. Molecular analysis of the gypsy (mdg4) retrotransposon in two Drosophila melanogaster strains differing by genetic instability. Mol Gen Genet. 1990 Sep;223(2):305–309. doi: 10.1007/BF00265067. [DOI] [PubMed] [Google Scholar]
- MURPHY J. S., BANG F. B. Observations with the electron microscope on cells of the chick chorio-allantoic membrane infected with influenza virus. J Exp Med. 1952 Mar;95(3):259–268. doi: 10.1084/jem.95.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlor R. L., Parkhurst S. M., Corces V. G. The Drosophila melanogaster gypsy transposable element encodes putative gene products homologous to retroviral proteins. Mol Cell Biol. 1986 Apr;6(4):1129–1134. doi: 10.1128/mcb.6.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrokhi L. J., Mazo A. M. Cloning and analysis of the mobile element gypsy from D. virilis. Nucleic Acids Res. 1991 Feb 25;19(4):913–916. doi: 10.1093/nar/19.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Burks C., Hertz G., Stormo G. D., White O., Fields C. Splicing signals in Drosophila: intron size, information content, and consensus sequences. Nucleic Acids Res. 1992 Aug 25;20(16):4255–4262. doi: 10.1093/nar/20.16.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mével-Ninio M., Mariol M. C., Gans M. Mobilization of the gypsy and copia retrotransposons in Drosophila melanogaster induces reversion of the ovo dominant female-sterile mutations: molecular analysis of revertant alleles. EMBO J. 1989 May;8(5):1549–1558. doi: 10.1002/j.1460-2075.1989.tb03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. J., Dubay J. W., Hunter E., Compans R. W. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3987–3991. doi: 10.1073/pnas.88.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L. G., Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein that block processing to gp85 and gp37. J Virol. 1987 May;61(5):1609–1614. doi: 10.1128/jvi.61.5.1609-1614.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S., Lemaitre B., Coen D. Maternal inheritance of P cytotype in Drosophila melanogaster: a "pre-P cytotype" is strictly extra-chromosomally transmitted. Mol Gen Genet. 1993 Oct;241(1-2):115–123. doi: 10.1007/BF00280208. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Saigo K., Kugimiya W., Matsuo Y., Inouye S., Yoshioka K., Yuki S. Identification of the coding sequence for a reverse transcriptase-like enzyme in a transposable genetic element in Drosophila melanogaster. Nature. 1984 Dec 13;312(5995):659–661. doi: 10.1038/312659a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. U., Gerasimova T., Kurkulos M., Boeke J. D., Corces V. G. An env-like protein encoded by a Drosophila retroelement: evidence that gypsy is an infectious retrovirus. Genes Dev. 1994 Sep 1;8(17):2046–2057. doi: 10.1101/gad.8.17.2046. [DOI] [PubMed] [Google Scholar]
- Spence S. E., Gilbert D. J., Swing D. A., Copeland N. G., Jenkins N. A. Spontaneous germ line virus infection and retroviral insertional mutagenesis in eighteen transgenic Srev lines of mice. Mol Cell Biol. 1989 Jan;9(1):177–184. doi: 10.1128/mcb.9.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell. 1979 Mar;16(3):589–598. doi: 10.1016/0092-8674(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Tanda S., Mullor J. L., Corces V. G. The Drosophila tom retrotransposon encodes an envelope protein. Mol Cell Biol. 1994 Aug;14(8):5392–5401. doi: 10.1128/mcb.14.8.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Origin of retroviruses from cellular moveable genetic elements. Cell. 1980 Oct;21(3):599–600. doi: 10.1016/0092-8674(80)90420-1. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Boeke J. D. Host genes that influence transposition in yeast: the abundance of a rare tRNA regulates Ty1 transposition frequency. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8360–8364. doi: 10.1073/pnas.87.21.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]