Abstract
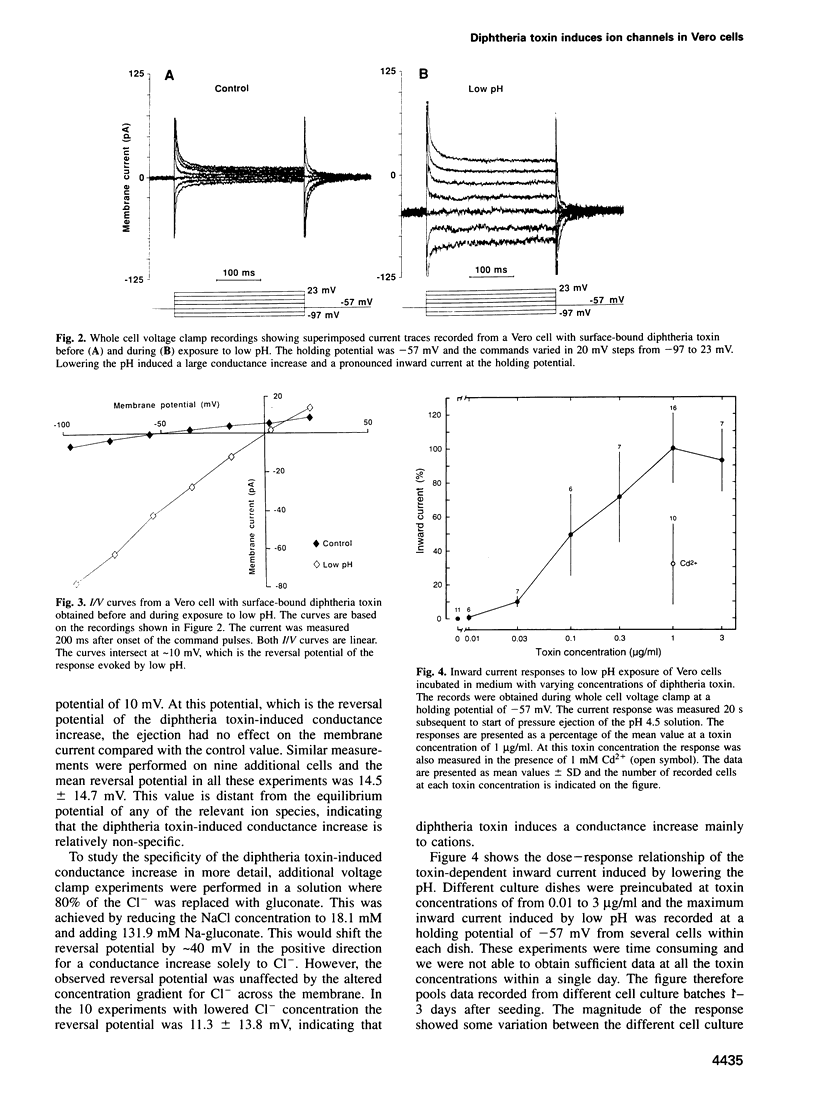
Receptor-dependent translocation of diphtheria toxin across the surface membrane of Vero cells was studied using patch clamp techniques. Translocation was induced by exposing cells with surface-bound toxin to low pH. Whole cell current and voltage clamp recordings showed that toxin translocation was associated with membrane depolarization and increased membrane conductance. The conductance increase was voltage independent, with a reversal potential of approximately 15 mV. This value was unaffected by changing the Cl- gradient across the membrane and microfluorometric measurements showed that the cytosolic Ca2+ concentration was only marginally elevated by the translocation. The conductance increase is thus mainly due to monovalent cations. Exposing outside-out and cell-attached patches with bound toxin to low pH induced a new type of ion channel in the membrane. The channel current was inward at negative membrane potentials and the single channel conductance was approximately 30 pS. This value is about three times larger than for receptor-independent channels induced by diphtheria toxin or toxin fragments in artificial lipid membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beise J., Hahnen J., Andersen-Beckh B., Dreyer F. Pore formation by tetanus toxin, its chain and fragments in neuronal membranes and evaluation of the underlying motifs in the structure of the toxin molecule. Naunyn Schmiedebergs Arch Pharmacol. 1994 Jan;349(1):66–73. doi: 10.1007/BF00178208. [DOI] [PubMed] [Google Scholar]
- Blaustein R. O., Koehler T. M., Collier R. J., Finkelstein A. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2209–2213. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov-Neori H., Yavin E., Montal M. Tetanus toxin forms channels in planar lipid bilayers containing gangliosides. Biophys J. 1984 Jan;45(1):83–85. doi: 10.1016/S0006-3495(84)84117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Donovan J. J., Middlebrook J. L. Ion-conducting channels produced by botulinum toxin in planar lipid membranes. Biochemistry. 1986 May 20;25(10):2872–2876. doi: 10.1021/bi00358a020. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Beasley E. M., Schatz G. Protein sorting in mitochondria. Trends Biochem Sci. 1992 Nov;17(11):453–459. doi: 10.1016/0968-0004(92)90487-t. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada E., Uchida T. Binding properties of diphtheria toxin to cells are altered by mutation in the fragment A domain. J Biol Chem. 1985 Oct 5;260(22):12148–12153. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978 Oct 25;253(20):7325–7330. [PubMed] [Google Scholar]
- Milne J. C., Collier R. J. pH-dependent permeabilization of the plasma membrane of mammalian cells by anthrax protective antigen. Mol Microbiol. 1993 Nov;10(3):647–653. doi: 10.1111/j.1365-2958.1993.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Misler S. Gating of ion channels made by a diphtheria toxin fragment in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4320–4324. doi: 10.1073/pnas.80.14.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Low pH-induced release of diphtheria toxin A-fragment in Vero cells. Biochemical evidence for transfer to the cytosol. J Biol Chem. 1988 Feb 15;263(5):2518–2525. [PubMed] [Google Scholar]
- Naglich J. G., Metherall J. E., Russell D. W., Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992 Jun 12;69(6):1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Kozlov J. V., van Deurs B., Sandvig K. Bacterial protein toxins acting on intracellular targets. Semin Cell Biol. 1991 Feb;2(1):7–14. [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. Interactions between diphtheria toxin entry and anion transport in Vero cells. II. Inhibition of anion antiport by diphtheria toxin. J Biol Chem. 1986 Feb 5;261(4):1553–1561. [PubMed] [Google Scholar]
- Papini E., Sandoná D., Rappuoli R., Montecucco C. On the membrane translocation of diphtheria toxin: at low pH the toxin induces ion channels on cells. EMBO J. 1988 Nov;7(11):3353–3359. doi: 10.1002/j.1460-2075.1988.tb03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- RUBINSTEIN D., RABINOWITCH E. ACTION SPECTRUM FOR THE APPEARANCE OF THE 520 MILLIMICRON DIFFERENCE BAND IN ILLUMINATED CHLORELLA CELLS. Biophys J. 1964 Mar;4:107–113. doi: 10.1016/s0006-3495(64)86772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin-induced channels in Vero cells selective for monovalent cations. J Biol Chem. 1988 Sep 5;263(25):12352–12359. [PubMed] [Google Scholar]
- Sandvig K., Tønnessen T. I., Sand O., Olsnes S. Requirement of a transmembrane pH gradient for the entry of diphtheria toxin into cells at low pH. J Biol Chem. 1986 Sep 5;261(25):11639–11644. [PubMed] [Google Scholar]
- Schiavo G., Poulain B., Rossetto O., Benfenati F., Tauc L., Montecucco C. Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. EMBO J. 1992 Oct;11(10):3577–3583. doi: 10.1002/j.1460-2075.1992.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver J. W., Donovan J. J. Interactions of diphtheria toxin with lipid vesicles: determinants of ion channel formation. Biochim Biophys Acta. 1987 Sep 18;903(1):48–55. doi: 10.1016/0005-2736(87)90154-4. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991 May 3;65(3):371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. Signal peptides open protein-conducting channels in E. coli. Cell. 1992 May 15;69(4):677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Olsnes S., Sandvig K. Requirement of specific receptors for efficient translocation of diphtheria toxin A fragment across the plasma membrane. J Biol Chem. 1988 Sep 15;263(26):13449–13455. [PubMed] [Google Scholar]
- Südhof T. C., De Camilli P., Niemann H., Jahn R. Membrane fusion machinery: insights from synaptic proteins. Cell. 1993 Oct 8;75(1):1–4. [PubMed] [Google Scholar]
- Zalman L. S., Wisnieski B. J. Mechanism of insertion of diphtheria toxin: peptide entry and pore size determinations. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3341–3345. doi: 10.1073/pnas.81.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]


