Abstract
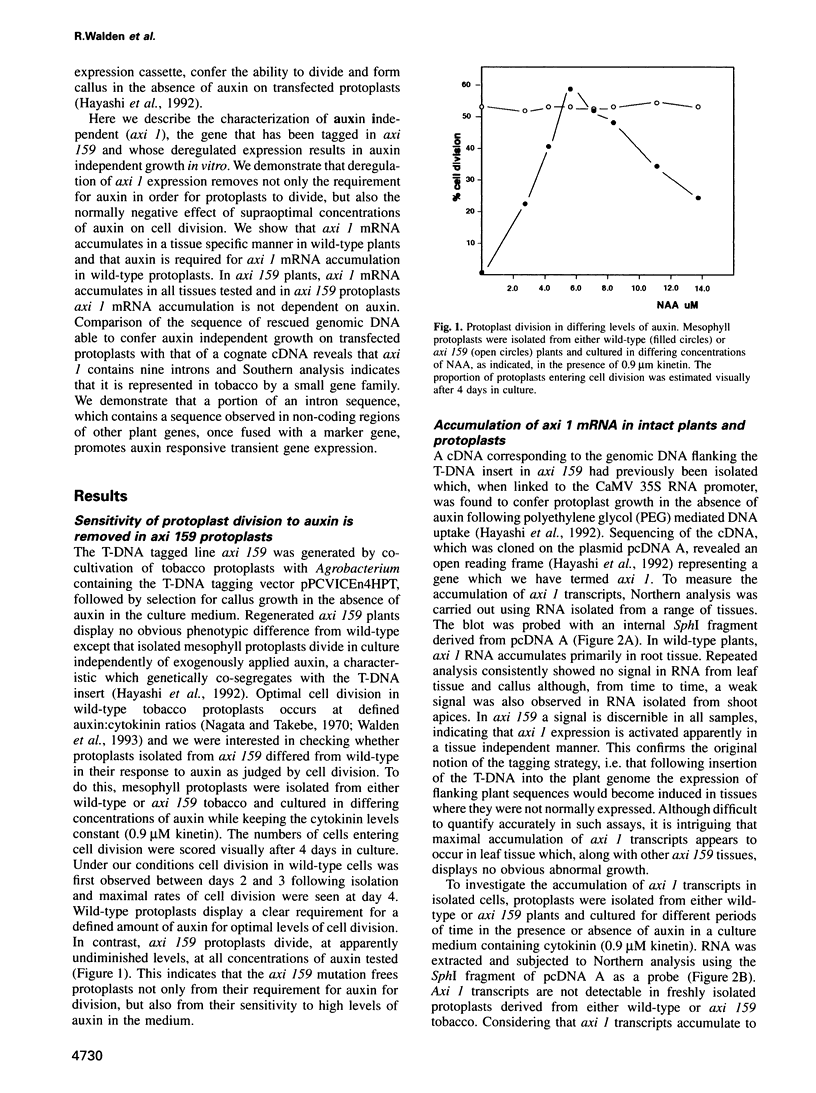
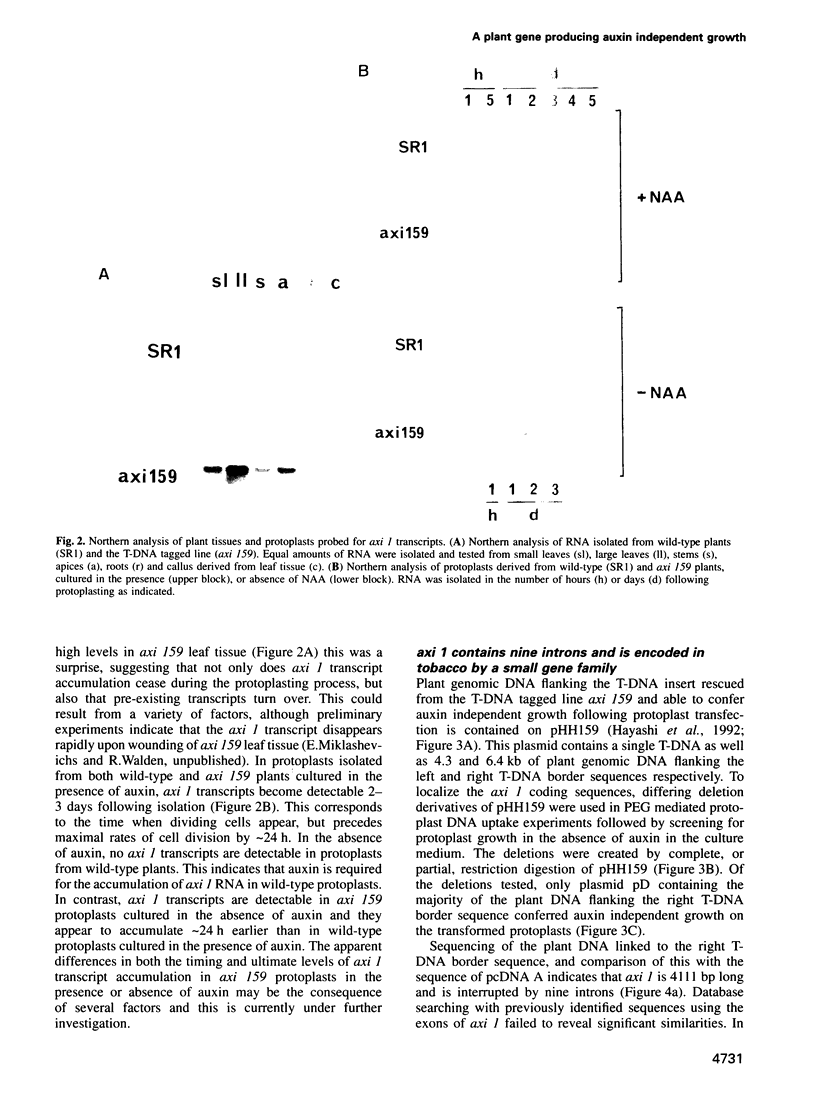
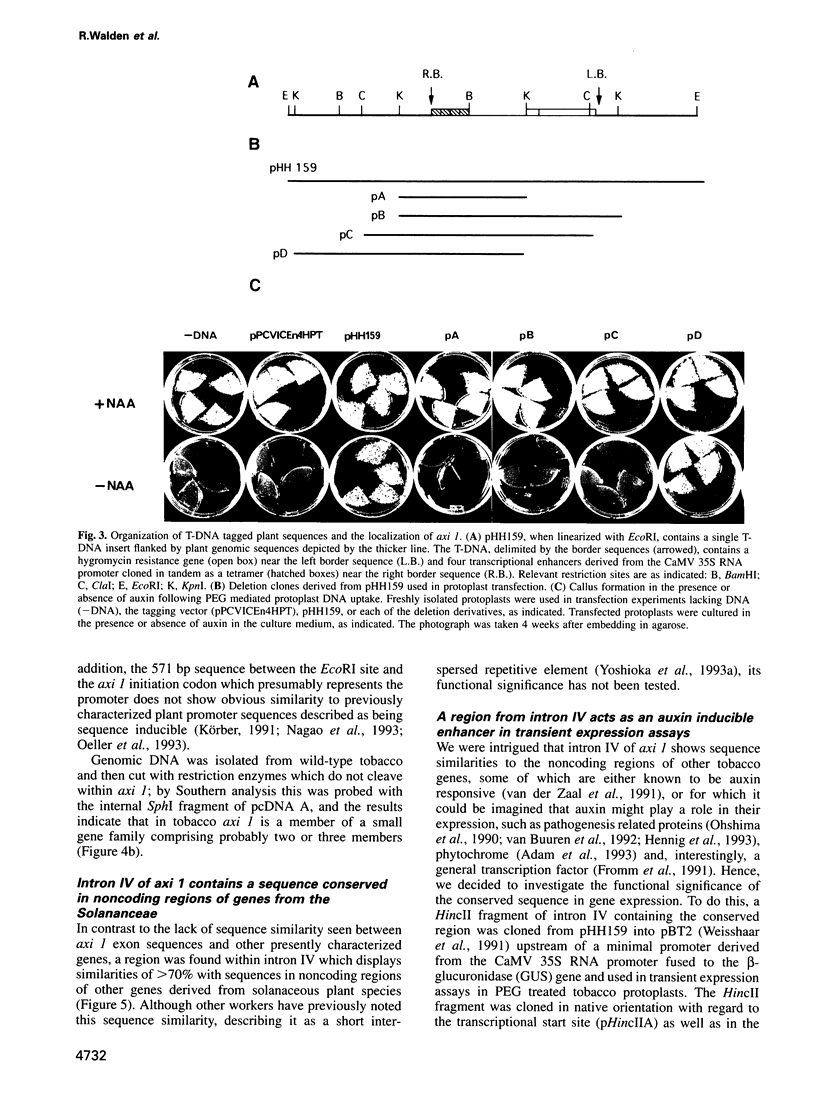
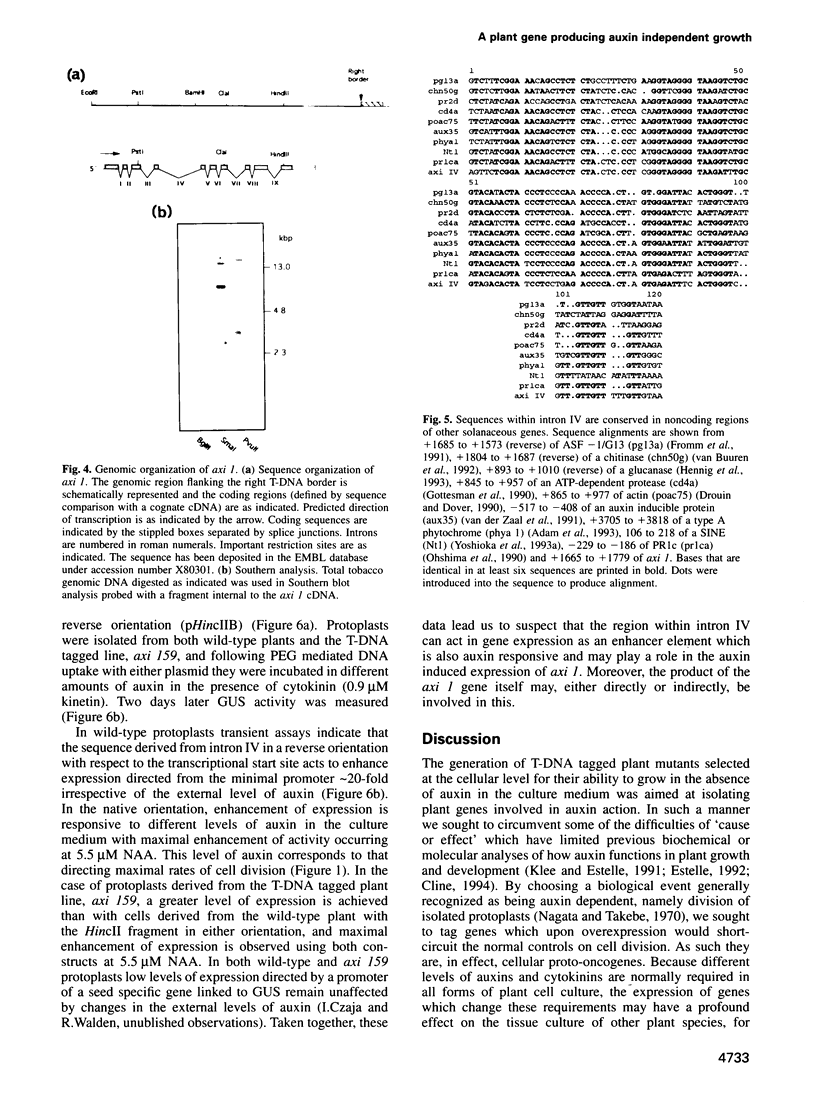
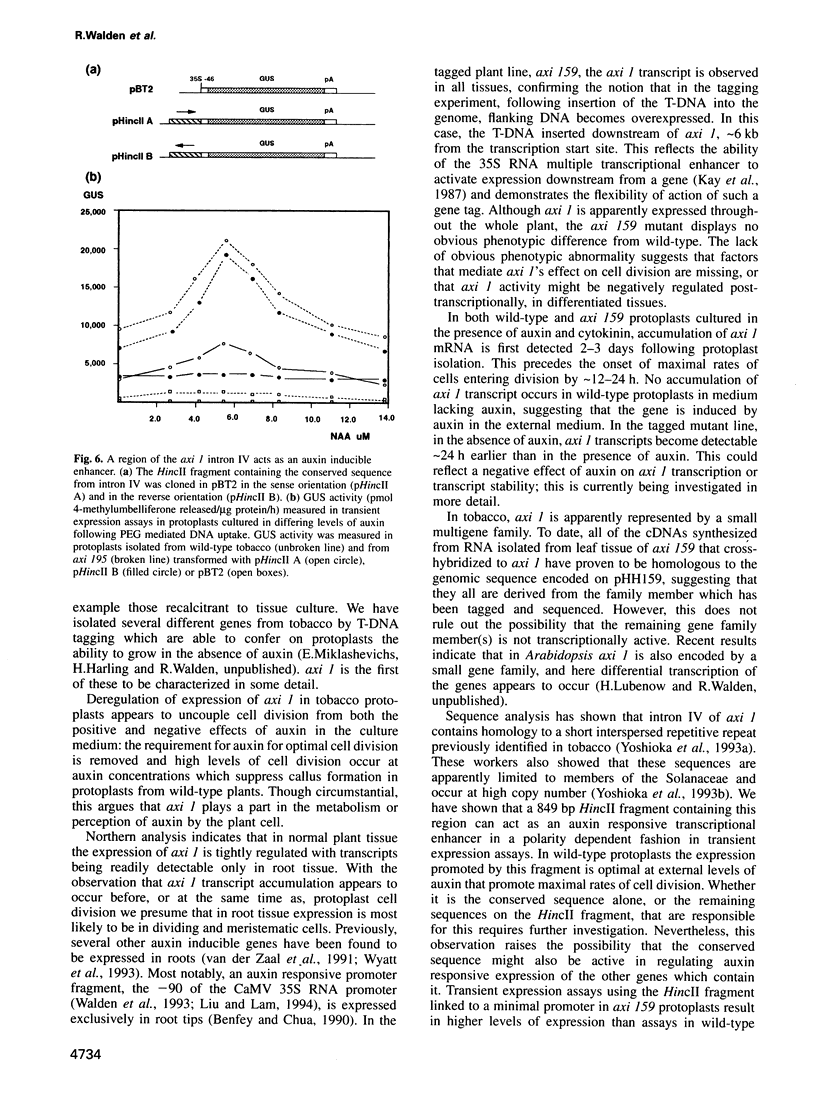
We describe the characterization of axi 1, a tobacco gene isolated by activation T-DNA tagging which apparently plays a role in auxin action. Upon deregulated expression, axi 1 confers on protoplasts the ability to grow in culture not only in the absence of auxin but also in high auxin concentrations where maximal frequencies of cell division are not observed in wild-type protoplasts. In wild-type plants axi 1 is transcribed principally in root tissue. In the tagged plant line, axi 159, axi 1 RNA can be detected in all tissues tested. Freshly isolated wild-type protoplasts require auxin for the accumulation of detectable levels of axi 1 transcript and this precedes maximal levels of cell division. In contrast, axi 1 RNA appears in protoplasts isolated from axi 159 plants in the absence of auxin. axi 1 was localized to 6.2 kb of plant genomic DNA flanking the right T-DNA border sequence. axi 1 is interrupted by nine introns and in tobacco it is a member of a small gene family. Database searching reveals no similarity within the coding region with other genes. Sequences within the fourth intron are similar to those located in the non-coding regions of other plant genes, some of which are known to be auxin inducible. A DNA fragment containing the conserved sequence acts as an auxin responsive element in transient expression assays in wild-type protoplasts and this response is higher in axi 159 protoplasts. This suggests that auxin induced axi 1 expression may be mediated by a region contained within an intron sequence and that the axi 1 product might play a role in this induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E., Deak M., Kay S., Chua N. H., Nagy F. Sequence of a tobacco (Nicotiana tabacum) gene coding for type A phytochrome. Plant Physiol. 1993 Apr;101(4):1407–1408. doi: 10.1104/pp.101.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P. N., Chua N. H. The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science. 1990 Nov 16;250(4983):959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin G., Dover G. A. Independent gene evolution in the potato actin gene family demonstrated by phylogenetic procedures for resolving gene conversions and the phylogeny of angiosperm actin genes. J Mol Evol. 1990 Aug;31(2):132–150. doi: 10.1007/BF02109482. [DOI] [PubMed] [Google Scholar]
- Estelle M. The plant hormone auxin: insight in sight. Bioessays. 1992 Jul;14(7):439–444. doi: 10.1002/bies.950140703. [DOI] [PubMed] [Google Scholar]
- Fromm H., Katagiri F., Chua N. H. The tobacco transcription activator TGA1a binds to a sequence in the 5' upstream region of a gene encoding a TGA1a-related protein. Mol Gen Genet. 1991 Oct;229(2):181–188. doi: 10.1007/BF00272154. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. H. Cellular signaling: new insights into the action of the plant growth hormone auxin. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11442–11445. doi: 10.1073/pnas.90.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Squires C., Pichersky E., Carrington M., Hobbs M., Mattick J. S., Dalrymple B., Kuramitsu H., Shiroza T., Foster T. Conservation of the regulatory subunit for the Clp ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci U S A. 1990 May;87(9):3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Czaja I., Lubenow H., Schell J., Walden R. Activation of a plant gene by T-DNA tagging: auxin-independent growth in vitro. Science. 1992 Nov 20;258(5086):1350–1353. doi: 10.1126/science.1455228. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hennig J., Dewey R. E., Cutt J. R., Klessig D. F. Pathogen, salicylic acid and developmental dependent expression of a beta-1,3-glucanase/GUS gene fusion in transgenic tobacco plants. Plant J. 1993 Sep;4(3):481–493. doi: 10.1046/j.1365-313x.1993.04030481.x. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R., Chan A., Daly M., McPherson J. Duplication of CaMV 35S Promoter Sequences Creates a Strong Enhancer for Plant Genes. Science. 1987 Jun 5;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Körber H., Strizhov N., Staiger D., Feldwisch J., Olsson O., Sandberg G., Palme K., Schell J., Koncz C. T-DNA gene 5 of Agrobacterium modulates auxin response by autoregulated synthesis of a growth hormone antagonist in plants. EMBO J. 1991 Dec;10(13):3983–3991. doi: 10.1002/j.1460-2075.1991.tb04973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser H. M., Lincoln C. A., Timpte C., Lammer D., Turner J., Estelle M. Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature. 1993 Jul 8;364(6433):161–164. doi: 10.1038/364161a0. [DOI] [PubMed] [Google Scholar]
- Liu X., Lam E. Two binding sites for the plant transcription factor ASF-1 can respond to auxin treatments in transgenic tobacco. J Biol Chem. 1994 Jan 7;269(1):668–675. [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Marton L Joo F. Non-Mendelian streptomycin-resistant tobacco mutant with altered chlorplasts and mitochondria. Nature. 1975 May 29;255(5507):401–402. doi: 10.1038/255401a0. [DOI] [PubMed] [Google Scholar]
- Nagao R. T., Goekjian V. H., Hong J. C., Key J. L. Identification of protein-binding DNA sequences in an auxin-regulated gene of soybean. Plant Mol Biol. 1993 Mar;21(6):1147–1162. doi: 10.1007/BF00023610. [DOI] [PubMed] [Google Scholar]
- Napier R. M., Venis M. A. From auxin-binding protein to plant hormone receptor? Trends Biochem Sci. 1991 Feb;16(2):72–75. doi: 10.1016/0968-0004(91)90028-t. [DOI] [PubMed] [Google Scholar]
- Oeller P. W., Keller J. A., Parks J. E., Silbert J. E., Theologis A. Structural characterization of the early indoleacetic acid-inducible genes, PS-IAA4/5 and PS-IAA6, of pea (Pisum sativum L.). J Mol Biol. 1993 Oct 20;233(4):789–798. doi: 10.1006/jmbi.1993.1555. [DOI] [PubMed] [Google Scholar]
- Ohshima M., Harada N., Matsuoka M., Ohashi Y. The nucleotide sequence of pathogenesis-related (PR) 1c protein gene of tobacco. Nucleic Acids Res. 1990 Jan 11;18(1):182–182. doi: 10.1093/nar/18.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K., Hesse T., Moore I., Campos N., Feldwisch J., Garbers C., Hesse F., Schell J. Hormonal modulation of plant growth: the role of auxin perception. Mech Dev. 1991 Feb;33(2):97–106. doi: 10.1016/0925-4773(91)90076-i. [DOI] [PubMed] [Google Scholar]
- Palme K. Molecular analysis of plant signaling elements: relevance of eukaryotic signal transduction models. Int Rev Cytol. 1992;132:223–283. doi: 10.1016/s0074-7696(08)62457-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Block A., Armstrong G. A., Herrmann A., Schulze-Lefert P., Hahlbrock K. Regulatory elements required for light-mediated expression of the Petroselinum crispum chalcone synthase gene. Symp Soc Exp Biol. 1991;45:191–210. [PubMed] [Google Scholar]
- Wyatt R. E., Ainley W. M., Nagao R. T., Conner T. W., Key J. L. Expression of the Arabidopsis AtAux2-11 auxin-responsive gene in transgenic plants. Plant Mol Biol. 1993 Aug;22(5):731–749. doi: 10.1007/BF00027361. [DOI] [PubMed] [Google Scholar]
- van Buuren M., Neuhaus J. M., Shinshi H., Ryals J., Meins F., Jr The structure and regulation of homeologous tobacco endochitinase genes of Nicotiana sylvestris and N. tomentosiformis origin. Mol Gen Genet. 1992 Apr;232(3):460–469. doi: 10.1007/BF00266251. [DOI] [PubMed] [Google Scholar]
- van der Zaal E. J., Droog F. N., Boot C. J., Hensgens L. A., Hoge J. H., Schilperoort R. A., Libbenga K. R. Promoters of auxin-induced genes from tobacco can lead to auxin-inducible and root tip-specific expression. Plant Mol Biol. 1991 Jun;16(6):983–998. doi: 10.1007/BF00016071. [DOI] [PubMed] [Google Scholar]





