Abstract
Objectives:
The rates of sleep related breathing disorders (SRBD) and treatment outcomes of depression were compared among insomnia patients who had stratified levels of hypnotic use during a 10-year follow-up (2001-2010).
Design:
A nationwide population-based cohort study.
Setting:
A nationally representative cohort of 1,000,000 enrollees.
Participants:
Data were collected from patients with major depressive disorder (MDD) and comorbid insomnia during January 2001 to December 2003 (study cohort N = 3,235). The mean dosage of hypnotics at baseline in the study cohort was calculated, and this information was used to categorize the cohort into three equal-sized groups based on levels of hypnotic dosage.
Main outcome measures:
Patient response to antidepressants during a period that extended from 1 year before to 1 year after the study (short-term outcome) and patient psychiatric and non-psychiatric visits and hospitalizations during follow-up (long-term outcome) were analyzed.
Results:
High-dosage patients presented the highest rates of subsequent SRBD diagnosis (3.9%), compared to medium-dosage patients (2.2%) and low-dosage patients (2.0%) (P = 0.011). Significantly more patients in the high-dosage group were difficult to treat with antidepressants compared to the other 2 groups (8.7% vs. 4.1% vs. 3.0%, P < 0.001), and their long-term depression outcome was worse for most parameters. Logistic regression showed that high-dosage hypnotics predicted the development of SRBD later (OR 1.678 [CI, 1.051 to 2.680], P = 0.030).
Conclusions:
There is a reliable association between a history of high dosages of hypnotics, subsequent diagnosis of sleep related breathing disorder, and worse depression outcomes.
Citation:
Li CT; Bai YM; Lee YC; Mao WC; Chen MH; Tu PC; Chen YS; Chen TJ; Chang WH; Su TP. High dosage of hypnotics predicts subsequent sleep-related breathing disorders and is associated with worse outcomes for depression. SLEEP 2014;37(4):803-809.
Keywords: Insomnia, hypnotics, depression, sleep-related, sleep apneas, antidepressants
INTRODUCTION
Sleep related breathing disorders (SRBD), such as obstructive sleep apnea, are commonly found in patients who have chronic insomnia with or without psychiatric history. A retrospective chart review examined a consecutive series of chronic insomnia patients who regularly used hypnotics (N = 218) and found that groups with and without psychiatric comorbidity exhibited high rates of obstructive sleep apnea (79% vs. 74%, respectively).1 However, the subjective perception of insomnia severity was worse in the group that had psychiatric comorbidity. In a study that had a special focus on patients with depression, the frequency of sleep apnea was up to 39% in patients with comorbid depression and insomnia (N = 51) and could be higher than was previously found among people with either depression or insomnia alone.2 Sedatives/hypnotics are reportedly used with increased frequency in obese patients with insomnia, and this association may reflect the presence of underlying SRBD.3 However, whether the increased hypnotic use in depression is related to SRBD remains unclear. Given the substantial overlap in symptoms between depression and SRBD, early identification and adequate treatment of SRBD in the context of chronic insomnia in depression can be even more challenging for clinical practice.4
A higher severity of SRBD could be associated with a higher severity of depression and increased hypnotic use. One study examined the depression severity prior to the initiation of continuous positive airway pressure (CPAP) treatment in patients with obstructive sleep apnea (N = 93).5 They reported that baseline apnea/apnea severity was significantly related to depressive rating scores. Because insomnia is highly prevalent in depression and polysomnography (PSG) is rarely assessed on a regular basis in the presence of insomnia,4 under-diagnosis of SRBD could be common in patients with comorbid insomnia and depression. The unrecognized SRBD in depression could result in poor responses to an adequate dosage of hypnotics and could be associated with treatment-refractory depression. Evidence suggests that SRBD plays a pivotal role in treatment-refractory depression. CPAP therapy in obstructive sleep apnea has resulted in a sustained improvement of depression symptoms.6 A few studies have reported that patients who had comorbid depression and sleep apnea showed a significant decrease in depression after the initiation of CPAP.4 SRBD-related hypopnea/apnea is also associated with a poor response to antidepressant medication in patients who have coronary heart disease.7 Recently a case report demonstrated that a patient with refractory depression improved substantially after receiving rapid palatal expansion for upper airway resistance without prominent snoring.8 This young adult with chronic severe depression showed no response to repeated antidepressant trials and even failed to respond to electroconvulsive therapy before palatal expansion. This case further supports the possibility that unrecognized SRBD in depression may play a role in treatment resistance to antidepressant treatments. However, to our knowledge, there is no large-scale prospective study investigating the associations between insomnia, SRBD, and depression outcomes.
Therefore, the present study aimed to follow a cohort of patients comorbid with insomnia and depression by utilizing our nationwide, population-based insurance database and to investigate the incidence of SRBD among patients with different historical levels of prescribed hypnotics. Our primary hypothesis was that a higher incidence of SRBD could be related to increased hypnotic amounts in depressed patients with insomnia. Second, a higher resistance to hypnotics in depressed patients, as demonstrated by increased hypnotic needs, could be associated with antidepressant refractoriness and worse long-term outcomes.
MATERIALS AND METHODS
Data Sources
We used the 1996-2010 National Health Insurance (NHI) database, which was published by the National Health Research Institute (NHRI) of Taiwan. Taiwan began its NHI program in 1995 to finance health care for all of its residents. The coverage rate was already 99% at the end of 2004. In the present study, we adopted the sample of 1 million representatives randomly selected from the 23 million beneficiaries from the National Health Insurance Research Database (NHIRD) in Taiwan. The database contains comprehensive information about clinical visits for each insured subject, including demographic data, dates of visits, diagnostic codes according to the clinical modification of the International Classifications of Disease-9 (ICD-9-CM), and prescription details.9 Because the dataset was released for research purposes and included only scrambled information on patient and physician identification, a signature of informed consent was not necessary and the study was exempt from full-committee review by the local ethics review committee.
Study Subjects
Identification of the Study Cohort
Our cohort (MDD-study cohort) included all patients in Taiwan who were diagnosed with MDD (ICD-9-CM code: 296.2 and 296.3) from January 2001 to December 2003 (Figure 1) using criteria identical to that in our previously published paper.10 In short, to ensure the validity of the diagnosis of MDD, we excluded patients whose diagnosis of MDD was not made by psychiatrists and included only those having ≥ 2 diagnoses of MDD made by psychiatrists. Only adult patients (age ≥ 18 years old) were included. To prevent the misdiagnosis of major depression, we excluded patients who were diagnosed as having bipolar disorder (ICD-9-CM code: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, and 296.8) and affective psychosis (ICD-9-CM code: 296.9) before enrollment.
Figure 1.
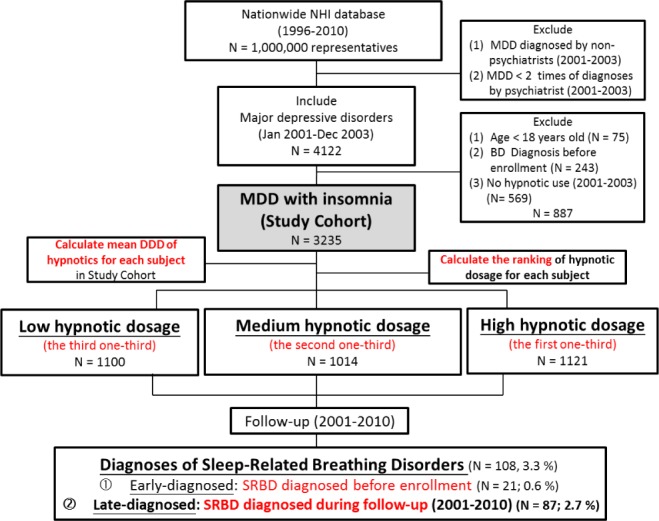
Study flow chart.
Because the primary hypothesis of the present study was to investigate the incidence of SRBD in depressed patients with insomnia, depressed patients who needed no hypnotic use during the enrollment period (January 2001 to December 2003) were deemed as having no or minimal insomnia problems and were therefore excluded.
Stratification According to Patterns of Mean Daily Dosage of Hypnotics
For each subject, mean defined daily dose (DDD) of hypnotics prescribed 1 year after enrollment was calculated. The DDD is defined by the World Health Organization (WHO) and is a unit for measuring a prescribed amount of a drug, and it is a method of standardizing drug dose across multiple drug types so that they can be compared. Then, all patients were categorized into 3 separate groups (i.e., high, medium, and low hypnotic dosage) according to their ranking of the prescribed hypnotic dosage in the whole study cohort. The high-dosage group included patients who ranked in the first one-third of the whole study population, the medium-dosage group was those in the second one-third, and the low-dosage group was those who ranked in the last one-third (Figure 1).
Identification of SRBD
The identification criteria for SRBD had been validated and were based on the ICD-9-CM codes 780.51, 780.53, and 780.57.11,12 SRBD patients were further divided into 2 groups, early-diagnosed SRBD and late-diagnosed SRBD (Figure 1). The definition of early-diagnosed SRBD was new diagnosis of SRBD in the period before the enrollment, while the definition of late-diagnosed SRBD was new diagnosis of SRBD during follow-up.
Since the details about PSG results such as apnea-hypopnea index (AHI) cannot be retrieved in this nationwide insurance database, we used the rate of CPAP treatment, surgical interventions, and clinical observation or life style changes (i.e., no specific treatment) as an indirect measure of SRBD severity. Because most Taiwanese people tend to be conservative about treatment and are reluctant to receive interventions until illness severity is high, CPAP treatment is mostly prescribed to patients with moderate-to-severe obstructive sleep apnea syndrome (AHI ≥ 15). In addition, if patients cannot tolerate CPAP, or clinical symptoms are severe or intolerable (e.g., waking up breath holding, gasping, choking), surgical interventions (e.g., uvulopalatopharyngoplasty, pharyngoplasty, and tonsillectomy and/or adenoidectomy) would be considered.
Depression Outcome Measurements
The short-term and long-term outcomes of depression were investigated. Regarding the short-term outcome, antidepressant responses were inspected. We used the stratification method used in the previous study.10,13 To summarize here, we defined patients as difficult to treat (DTT), intermediately difficult to treat (ITT), and easy to treat (ETT), according to the characteristics of their antidepressant use during a period that extended from 1 year before to 1 year after the study year. The adopted criteria for classifying patients into different groups of treatment outcome are widely accepted and have been validated.10,13 That is, although varying degrees of treatment refractoriness exist,14 MDD is usually considered as treatment-resistant depression (TRD) when ≥ 2 adequate trials with different antidepressants fail to achieve a significant clinical improvement.15 Therefore, in the present study, patients were defined as DTT if ≥ 2 antidepressant trials at an adequate dosage (for example, fluoxetine ≥ 20 mg/day) and duration (≥ 60 consecutive days) were prescribed during the 2-year period. In contrast, ETT patients were those who did not take any antidepressants or remained on a single anti-depressant. Patients who changed their antidepressants only once were defined as ITT. By utilizing such a stratification method, DTT patients at baseline enrollment were found to be associated with a higher risk of having more psychiatric hospitalizations and more suicide attempts during follow-up, which was a maximum of 9 years.13 Our results were consistent with previous findings that TRD leads to worsened outcomes including more hospitalizations and even enhanced mortality rates.14,16,17
In addition to the levels of antidepressant refractoriness, we measured severity of depression by investigating a combination use of antipsychotics (i.e., augmentation therapy by antipsychotics) and the rate of psychiatric admission for depression at baseline. Since patients with higher severity of depression would need medication augmentation (e.g., atypical antipsychotics) in combination with their antidepressants, we calculated the mean DDD of antipsychotics for each subject. Low-dose quetiapine (25-150 mg/day) may be prescribed for the treatment of insomnia, instead of depression. Therefore, patients prescribed with a quetiapine equivalent dose of 200 mg/day (i.e., 1/2 DDD of quetiapine) were identified in each hypnotic group.
With regard to the long-term outcomes of depression, we adopted the criteria that we used previously,13 and in the present study, we focused on emergency room (ER) visits, psychiatric hospitalizations, non-psychiatric hospitalizations, and suicide attempts during follow-up (from the enrollment to December 2010).
Statistical Analysis
SAS statistical package (SAS System for Windows, version 9.2; SAS Institute, Cary, NC, USA) and SPSS statistics (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL, USA) were used to perform the statistical analysis of the data in the present study. One-way ANOVA (or Student t test) and χ2 tests were applied to compare the continuous and categorical variables among groups, respectively. The significance was set at a P-value less than 0.05. Post hoc analysis was performed by LSD. Kaplan-Meier survival analysis was used to estimate SRBD-free survival curves among 3 different hypnotic groups because the Kaplan-Meier analysis allows estimation of survival over time even when subjects were followed for different lengths of time were dropped during follow-up. A log-rank test was used to compute a test of equality of the survival functions among groups, based on a χ2 distribution. The χ2 value was reported and the lower the corresponding P-value, the more significant the differences among the groups. Multivariate logistic regression was carried out using variables that differed significantly between groups with and without SRBD (e.g., age, hyperlipidemia, diabetes mellitus, psychiatric comorbidities, and groups with different levels of hypnotic use) as independent factors and the occurrence of SRBD as the dependent factor. Psychiatric comorbidities included dysthymia, anxiety disorders, and substance abuse and dependence based on the corresponding ICD-9-CM codes.13 The adjusted odds ratio (OR) of the SRBD risks and their 95% confidence intervals (CI) were reported. P < 0.05 (two-sided tests) was deemed to be statistically significant.
RESULTS
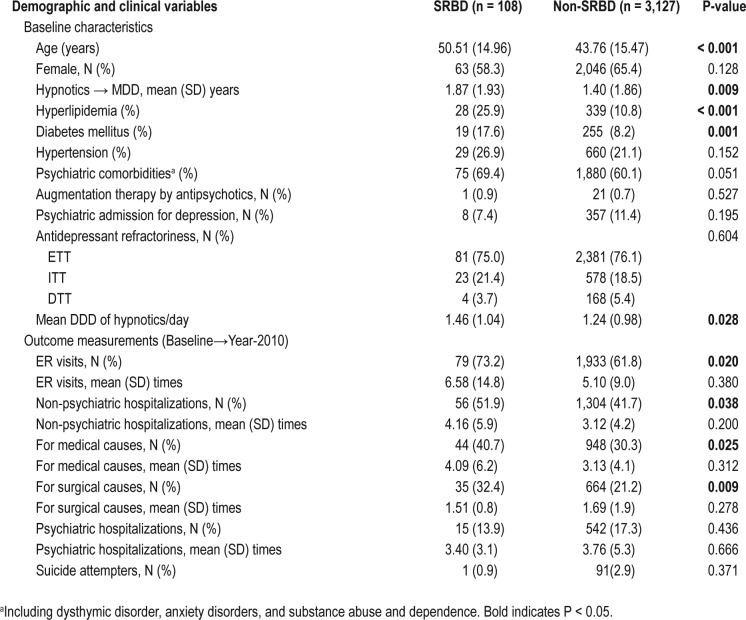
The study cohort included 3,235 depression patients with concurrent insomnia; these patients were identified from the 1,000,000 sampling cohort dataset at baseline (between January 2001 and December 2003). Three separate groups (low, medium, and high hypnotic dosage), according to their average hypnotic dosage, were compared. The demographic data and clinical variables are listed in Table 1. There was no difference in the age, age at onset of depression, age at first-time hypnotic use, urbanization of the living areas, and the medical comorbidities of hyperlipidemia, diabetes mellitus, and sleep apnea at baseline (Table 1). However, a high dosage of hypnotic use was associated with male gender (P = 0.021), lower personal income (P < 0.001), and the medical comorbidity of hypertension (P = 0.020), as well as higher severity of depression as demonstrated by higher rates of antipsychotic augmentation therapy, higher rates of psychiatric admission for depression, and higher levels of antidepressant refractoriness (P < 0.001) at baseline (Table 1).
Table 1.
Baseline demographic and clinical variables among different levels of hypnotic prescriptions in MDD patients with insomnia
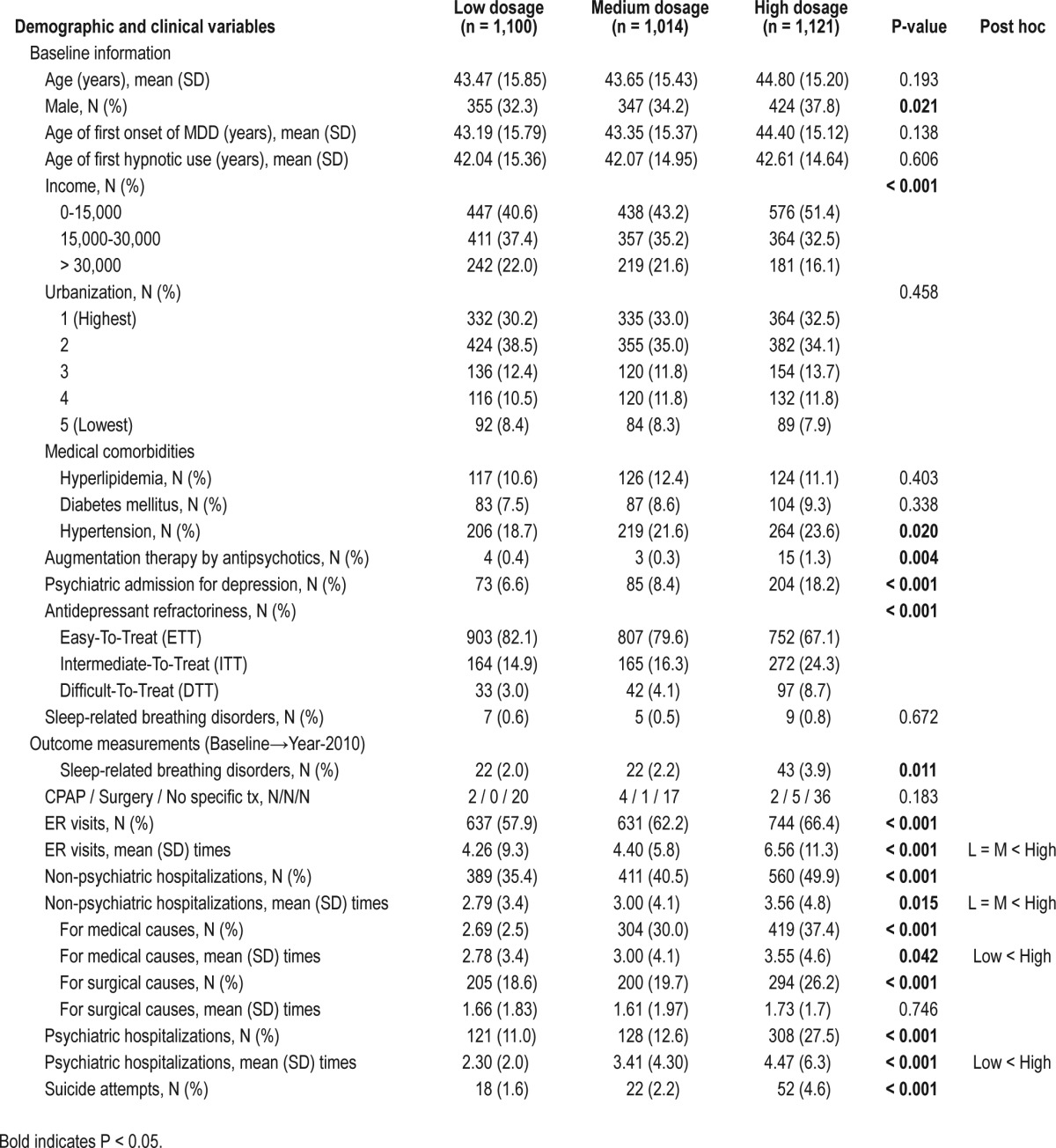
A total of 108 patients (3.3%) with newly diagnosed SRBD were identified, of whom 87 patients (2.7%) developed a diagnosis of SRBD during follow-up. As for long-term outcome measurements, a high dosage of hypnotic use was significantly associated with a higher risk for developing SRBD during the follow-up period (Table 1). Kaplan-Meier survival analysis also demonstrated that patients with a high dosage of hypnotic at baseline had a significantly higher chance for a diagnosis of SRBD during the follow-up than the other 2 groups (χ2 = 8.918, P = 0.012, post hoc analysis: high > medium and high > low) (Figure 2, Table S1, supplemental material).
Figure 2.

High dosage of hypnotic use predicts the diagnosis of SRBD during the follow-up.
As for SRBD severity, we found that no significant difference existed in the rate of CPAP use, surgical interventions, and no specific treatment among the 3 groups (Table 1). Most of the patients with SRBD received no specific treatment, ranging from 77.3% (medium-dosage group), 83.7% (high-dosage group), to 90.9% (low-dosage group); no significant between-group difference existed. The findings suggested that the SRBD patients were mostly mild in severity. The use of high hypnotic dosages was also associated with more ER visits, more psychiatric and non-psychiatric hospitalizations, and more suicide attempts (Table 1), supporting the idea that a higher dosage of hypnotics is a predictor for depression treatment outcome.
Patients with SRBD had a significantly higher mean DDD of hypnotics than patients without SRBD (1.46 ± 1.04 vs. 1.24 ± 0.98, P = 0.028), suggesting that the comorbidity of SRBD and depression complicates the sleep problems associated with depression. The finding of a higher hypnotic dosage in MDD patients with SRBD than those without SRBD remained unchanged, if SRBD patients were further categorized into an early-diagnosed SRBD group (i.e., a diagnosis of SRBD at baseline) and a late-diagnosed SRBD group (i.e., a development of a SRBD diagnosis during follow-up). It was because both early-diagnosed and late-diagnosed SRBD groups with MDD (1.49 ± 1.10 vs.1.45 ± 1.02, P = 0.889) had higher mean DDD of hypnotics than the non-SRBD group with MDD (P = 0.023, post hoc analysis: early-diagnosed SRBD > non-SRBD, late-diagnosed SRBD > non-SRBD) (Figure S1, supplemental material).
The early-diagnosed SRBD group was associated with fewer psychiatric hospitalizations than the non-SRBD group during follow-up (Figure 3). The late-diagnosed SRBD group was significantly associated with worse medical outcomes during follow-up, including more ER visits and more non-psychiatric hospitalizations (Figure 3). Although fewer patients in the early-diagnosed SRBD group presented with antidepressant refractoriness, there was no significant difference between groups.
Figure 3.
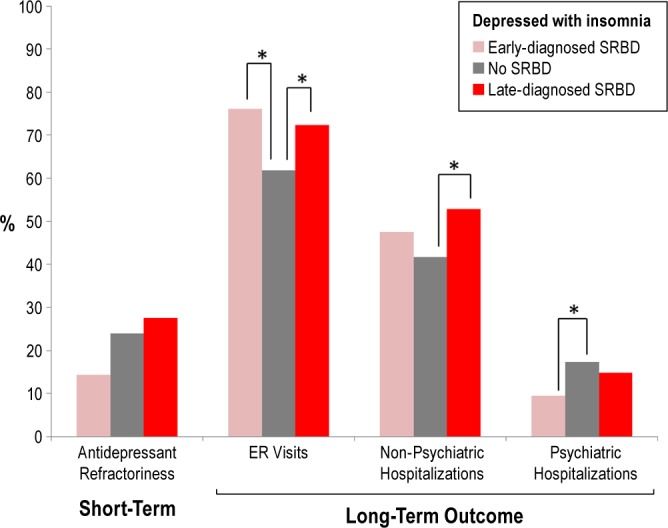
Early identification of SRBD (i.e., early-diagnosed SRBD) in unipolar depression was associated with better long-term outcomes of depression (*P < 0.05) than late-diagnosed SRBD patients.
The between-group comparison of SRBD and non-SRBD groups revealed that patients with SRBD were significantly older (P < 0.001), had a higher percentage of hyperlipidemia (P < 0.001) and diabetes mellitus (P = 0.001), and had a higher mean DDD of hypnotics (P = 0.028) at baseline (Table S2, supplemental material). The baseline severity of depression was not associated with the occurrence of SRBD later, since only one subject who needed antipsychotic augmentation therapy at baseline developed a diagnosis of SRBD during the follow-up; and patients who subsequently developed SRBD had no higher rates of psychiatric admission for depression at baseline (Table S2, supplemental material).
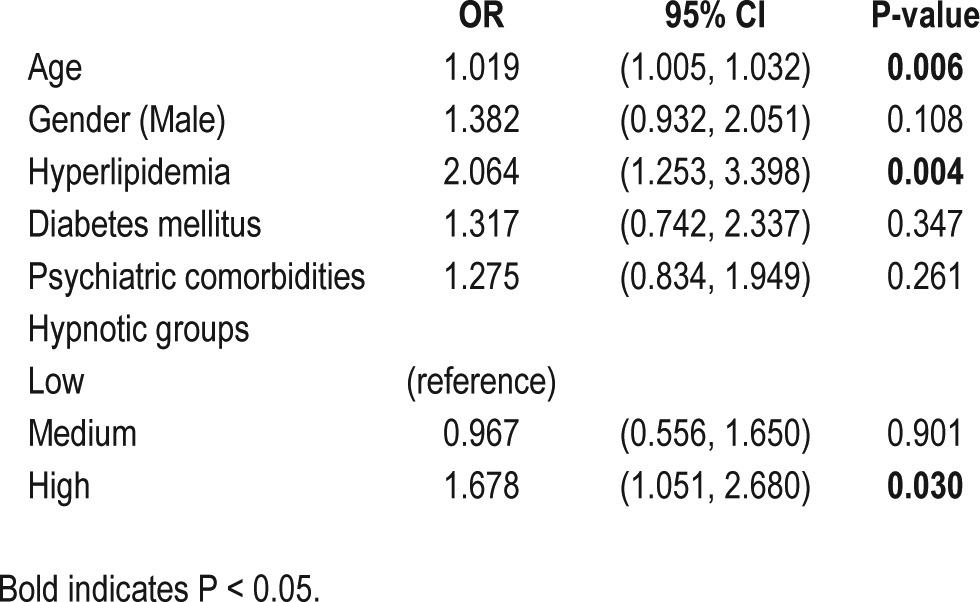
Patients with SRBD were associated with worsened medical outcomes during the follow-up such as higher rates of ER visits (P = 0.020), and higher rates of non-psychiatric hospitalizations (P = 0.038) for both medical and surgical causes. Patients with SRBD were also associated with more psychiatric comorbidities at baseline in a trend significance (P = 0.051). Logistic regression showed that age (O.R = 1.019), hyperlipidemia (O.R = 2.064), and high hypnotic dosage (O.R = 1.678) were the most important predictors of SRBD (Table 2). These findings support the idea that hypnotic use in unipolar depression is a useful predictor for the later diagnosis of SRBD.
Table 2.
Logistic regression of SRBD
DISCUSSION
To our knowledge, this is the first large-scale study to investigate the association between hypnotic use history in depressed patients and subsequent SRBD using a nationwide database. As hypothesized, a higher incidence of SRBD was related to a higher dosage of hypnotics at baseline in depressed patients with insomnia (Figure 2). The adjusted OR for the high hypnotic group to develop an SRBD diagnosis was 1.678 (95% CI = 1.051 to 2.680, P = 0.030; Table 2). Second, increased hypnotic needs were associated with higher antidepressant refractoriness and worse long-term outcomes, such as more ER visits, more psychiatric and non-psychiatric hospitalizations, and more suicide attempts (Table 1). Because more than 30% of depression is refractory to sequential anti-depressant treatments18 and insomnia is extremely common in patients with depression,19 our results imply that early identification of the use of high dosages of hypnotics in depressed patients is important because high use of hypnotics is a useful predictor for worse depression outcomes.
To summarize here, the present study showed that the increased hypnotic use in the MDD patients can carry two clinical implications: (1) a comorbidity of SRBD that might be unrecognized and (2) a reflection of severe insomnia in association with antidepressant-resistant depression. We discuss them in the following paragraphs.
High Hypnotic Dosage and SRBD
Our first major finding, that there is an association between high hypnotic dosage and the later diagnosis of SRBD, could have two potential causes. First, such an association could reflect the presence of an underlying but unrecognized SRBD in insomnia patients with more severe sleep problems. Patients with an unrecognized SRBD for a long period of time may result in persistent insomnia that responds poorly to hypnotics and, in turn, end up with a large amount of hypnotics. In fact, un-recognition and under-diagnosis of SRBD in patients with insomnia are not uncommon in clinical settings. Second, a higher dosage of hypnotics could result in the occurrence or exacerbation of an occult SRBD. In some cases, treatment with a benzodiazepine and hypnotic for comorbid insomnia and depression could adversely affect breathing during sleep, potentially by decreasing muscle tone in upper airway muscles, decreasing the ventilator response to hypoxia, and increasing the arousal threshold for the apneas.20,21
However, we favor the first possibility primarily because of the following observations. The severity of SRBD in the MDD patients was primarily mild, as most of the patients did not require specific treatment such as CPAP and surgical intervention (Table 1). A depressed patient with an underlying SRBD that is unrecognized may complain of insomnia as their major problem, leave emotional symptoms less concerned, and result in a delay in the diagnosis and treatment of MDD. This notion could be corroborated by our findings that the duration, in years, from initial use of a hypnotic to the first depression diagnosis was significantly longer in the high hypnotic dosage group (high vs. medium vs. low = 1.79 ± 1.98 vs. 1.28 ± 1.83 vs. 1.15 ± 1.72, P < 0.001). These findings suggest that SRBD in depression is easy to be under-diagnosed and the duration of influence of such a “hidden SRBD” on depression could be long. However, future research is still warranted to directly investigate the rate of “unrecognized” SRBD in depression.
Our data further revealed that in depressed patients with chronic insomnia, a reevaluation of the comorbidity of SRBD is important because it complicated the insomnia problem in depression; thus, an increased dosage of hypnotics was needed. This notion is supported by a retrospective chart review conducted by Krakow et al.,22 in which the investigators reported a high comorbidity rate of obstructive sleep apnea (71%) in chronic insomnia patients who experienced unsuccessful pharmacotherapy.22 Despite our finding that there was an increased amount of hypnotic use in both groups of patients with SRBD (Figure S1, supplemental material), the early detection of SRBD in unipolar depression is still important because late detection of SRBD worsened the medical outcomes. The evidence supporting this importance was that the delayed diagnosis of SRBD in depression increased non-psychiatric hospitalizations compared to those without SRBD, while the early identification of SRBD was associated with fewer psychiatric hospitalizations later (Figure 3). A large body of evidence has shown that subjects with SRBD have increased cardiovascular risk, such as hypertension, arrhythmias, and even heart failure, potentially through mechanisms such as sympathetic hyperactivation, oxidative stress, and systemic inflammation.23–27
High Hypnotic Dosage and Antidepressant-Resistant Depression
The second major finding of the present study was the higher severity of insomnia problems, as demonstrated by increased hypnotic use, was associated with antidepressant refractoriness and worse depression outcomes in patients with depression. Bi-directional relationships exist between sleep problems and depression.28 Chronic insomnia could lead to depression, and insomnia could occur in the context of depression. Despite inconsistency, some believe that sleep disturbance is a core pathophysiology of depression. The supporting evidence for this idea includes the alteration of sleep architecture in depression, such as decreased deep sleep, decreased REM sleep latency, increase in the proportion of REM sleep in the early part of the night,19 and the rapidly effective response to anti-depressants after sleep deprivation in even depressed patients who do not have prominent insomnia.29 Recent evidence from the STAR*D trial provided further support for the importance of insomnia symptoms in major depression, as the investigators found that insomnia symptoms were associated with several indicators of severe depression.30 Severe and non-remitting depression had more biological deficits than remitting depression.31 The present study provided further support that the use of high dosages of hypnotics is an independent predictor for worse depression outcomes.
In addition, we found no direct association between a diagnosis of SRBD and poor response to antidepressants (DTT patients; SRBD vs. non-SRBD = 3.7% vs. 5.4%), while an association was found between high hypnotic dosage and SRBD and between high hypnotic dosage and antidepressant refractoriness.
The strengths of the current study are the use of a large sample size from a nationwide database and a long (to a maximum of 10 years) naturalistic follow-up period. Therefore, because we used an anonymous database, the issue of unrecognized or hidden SRBD and related medical and physical outcomes could be disclosed without any bias from the experimenters. There is one limitation to the use of a registry-based database for this study. That is, we cannot ensure the adherence to CPAP in the treatment of SRBD. However, most of the patients in our study were not prescribed CPAP. Furthermore, even if adherence to CPAP was as poor as expected,32 our main results would not change, as CPAP was used as an index for the estimation of SRBD severity. There is a general agreement that prescription of CPAP is indicated for patients with moderate-to-severe objective obstructive sleep apnea (e.g., apnea hypopnea index ≥ 15 per hour).
CONCLUSION
Strong associations between high hypnotic dosage and the subsequent diagnosis of SRBD and between high hypnotic dosage and worsened short-term and long-term outcomes of depression were found in this study using a nationwide database in Taiwan. The association between hypnotics and SRBD may reflect, at least in part, the issue of under-diagnosis or hidden SRBD in depressed patients with comorbid insomnia who require a high dosage of hypnotics. Our findings show that a history of poor response to hypnotics in depression could be a useful predictor of underlying SRBD.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by Taipei Veterans General Hospital (V101B-019, V101D-001-1, and V102D-001-2). The views expressed in the manuscript are those of the authors and not necessarily those of the Taipei Veterans General Hospital. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
The authors express their appreciation to Dr. Lei Zhang for his clinical input on this manuscript.
SUPPLEMENTAL MATERIAL
Defined daily dose (DDD) of hypnotics at baseline among patients without SRBD (no SRBD), with early-diagnosed SRBD (diagnosis of SRBD before enrollment), and with late-diagnosed SRBD (development of SRBD diagnosis during follow-up). Both groups with SRBD had a significantly higher DDD of hypnotics than those without SRBD (*P < 0.05).
Table S1.
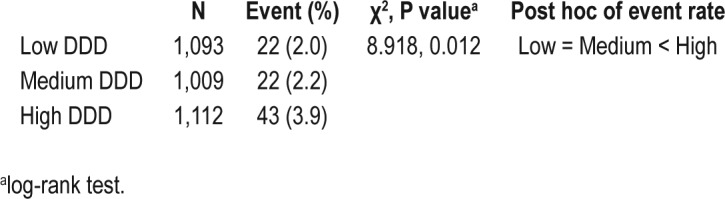
Use of high dosages of hypnotics in MDD predicts the diagnosis of sleep related breathing disorders (SRBD) during follow-up
Table S2.
Baseline characteristics and long-term outcome measurements between MDD with and without SRBD
REFERENCES
- 1.Krakow B, Ulibarri VA, Romero EA. Patients with treatment-resistant insomnia taking nightly prescription medications for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010;12(4) doi: 10.4088/PCC.09m00873bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong JC, Gress JL, San Pedro-Salcedo MG, Manber R. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67:135–41. doi: 10.1016/j.jpsychores.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vozoris NT, Leung RS. Sedative medication use: prevalence, risk factors, and associations with body mass index using population-level data. Sleep. 2011;34:869–74. doi: 10.5665/SLEEP.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8:17–25. [PMC free article] [PubMed] [Google Scholar]
- 5.Aloia MS, Arnedt JT, Smith L, Skrekas J, Stanchina M, Millman RP. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005;6:115–21. doi: 10.1016/j.sleep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz DJ, Karatinos G. For individuals with obstructive sleep apnea, institution of CPAP therapy is associated with an amelioration of symptoms of depression which is sustained long term. J Clin Sleep Med. 2007;3:631–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Roest AM, Carney RM, Stein PK, Freedland KE, Meyer H, Steinmeyer BC, et al. Obstructive sleep apnea/hypopnea syndrome and poor response to sertraline in patients with coronary heart disease. J Clin Psychiatry. 2012;73:31–6. doi: 10.4088/JCP.10m06455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller P, Iyer M, Gold AR. Treatment resistant adolescent depression with upper airway resistance syndrome treated with rapid palatal expansion: a case report. J Med Case Rep. 2012;6:415. doi: 10.1186/1752-1947-6-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Insurance Research Database T. 2007.
- 10.Li CT, Bai YM, Huang YL, Chen YS, Chen TJ, Cheng JY, et al. Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: cohort study. Br J Psychiatry. 2012;200:45–51. doi: 10.1192/bjp.bp.110.086983. [DOI] [PubMed] [Google Scholar]
- 11.Chou KT, Huang CC, Chen YM, Su KC, Shiao GM, Lee YC, et al. Sleep apnea and risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. Am J Med. 2012;125:374–80. doi: 10.1016/j.amjmed.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Shiao TH, Liu CJ, Luo JC, Su KC, Chen YM, Chen TJ, et al. Sleep apnea and risk of peptic ulcer bleeding: a nationwide population-based study. Am J Med. 2013;126:249–55. doi: 10.1016/j.amjmed.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Li CT, Bai YM, Tu PC, Lee YC, Huang YL, Chen TJ, et al. Major depressive disorder and stroke risks: a 9-year follow-up population-based, matched cohort study. PLoS One. 2012;7:e46818. doi: 10.1371/journal.pone.0046818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 15.Little A. Treatment-resistant depression. Am Fam Physician. 2009;80:167–72. [PubMed] [Google Scholar]
- 16.Bschor T. Therapy-resistant depression. Expert Rev Neurother. 10:77–86. doi: 10.1586/ern.09.137. [DOI] [PubMed] [Google Scholar]
- 17.Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:963–71. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- 18.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 19.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C. Benzodiazepines, breathing, and sleep. Am J Med. 1990;88:25S–28S. doi: 10.1016/0002-9343(90)90282-i. [DOI] [PubMed] [Google Scholar]
- 21.Schneider H, Grote L, Peter JH, Cassel W, Guilleminault C. The effect of triazolam and flunitrazepam--two benzodiazepines with different half-lives--on breathing during sleep. Chest. 1996;109:909–15. doi: 10.1378/chest.109.4.909. [DOI] [PubMed] [Google Scholar]
- 22.Krakow B, Ulibarri VA, Romero E. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: a retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198:734–41. doi: 10.1097/NMD.0b013e3181f4aca1. [DOI] [PubMed] [Google Scholar]
- 23.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 24.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustberg L, Reynolds CF. Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4:253–62. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- 29.Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46:445–53. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 30.Sunderajan P, Gaynes BN, Wisniewski SR, Miyahara S, Fava M, Akingbala F, et al. Insomnia in patients with depression: a STAR*D report. CNS Spectr. 2010;15:394–404. doi: 10.1017/s1092852900029266. [DOI] [PubMed] [Google Scholar]
- 31.Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50:347–56. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Defined daily dose (DDD) of hypnotics at baseline among patients without SRBD (no SRBD), with early-diagnosed SRBD (diagnosis of SRBD before enrollment), and with late-diagnosed SRBD (development of SRBD diagnosis during follow-up). Both groups with SRBD had a significantly higher DDD of hypnotics than those without SRBD (*P < 0.05).