Abstract
Transcutaneous electrical nerve stimulation (TENS) is a treatment for pain that involves placement of electrical stimulation through the skin for pain relief. Previous work from our laboratory shows that repeated application of TENS produces analgesic tolerance by the fourth day and a concomitant cross-tolerance at spinal opioid receptors. Prior pharmacological studies show that blockade of cholecystokinin (CCK) receptors systemically and spinally prevents the development of analgesic tolerance to repeated doses of opioid agonists. We therefore hypothesized that systemic and intrathecal blockade of CCK receptors would prevent the development of analgesic tolerance to TENS, and cross-tolerance at spinal opioid receptors. In animals with knee joint inflammation (3% kaolin/carrageenan), high (100 Hz) or low frequency (4 Hz) TENS was applied daily and the mechanical withdrawal thresholds of the muscle and paw were examined. We tested thresholds before and after inflammation, and before and after TENS. Animals treated systemically, prior to TENS, with the CCK antagonist, proglumide, did not develop tolerance to repeated application of TENS on the fourth day. Spinal blockade of CCK-A or CCK-B receptors blocked the development of tolerance to high and low frequency TENS, respectively. In the same animals we show that spinal blockade of CCK-A receptors prevents cross-tolerance at spinal delta-opioid receptors that normally occurs with high frequency TENS; and blockade of CCK-B receptors prevents cross-tolerance at spinal mu-opioid receptors that normally occurs with low frequency TENS. Thus, we conclude that blockade of CCK receptors prevents the development of analgesic tolerance to repeated application of TENS in a frequency-dependent manner.
Keywords: Transcutaneous electric nerve stimulation, Hyperalgesia, Cholecystokinin receptors
1. Introduction
Transcutaneous electrical nerve stimulation (TENS) is the application of surface electrodes applied to the skin for pain relief [2]. Numerous clinical studies attempted to determine the effectiveness of TENS treatment for people with a variety of pain conditions. Systematic reviews and meta-analysis show that TENS is effective for osteoarthritis, rheumatoid arthritis, postoperative pain, and chronic musculoskeletal pain [7,43,44]. Early literature supports a role for opioids in TENS. Specifically, in human subjects systemic blockade of opioid receptors with naloxone prevents low, but not high, frequency TENS analgesia [47]. In animals, higher concentrations of systemic naloxone also prevent the effects of high frequency TENS analgesia [24,64]. In parallel, opioid peptides are released in the cerebrospinal fluid and plasma in humans [22,30,46] in response to either low or high frequency TENS in a frequency-dependent manner. Previous studies from our laboratory extend those studies by showing the ability of opioid receptor antagonists administered directly to the rostroventromedial medulla (RVM) or the spinal cord prevents the anti-hyperalgesic effects of both high and low frequency TENS [1,31]. Furthermore, repeated daily application of high or low frequency TENS produces tolerance at spinal delta-and mu-opioid receptors, respectively [8]. Clinically, development of opioid tolerance is an important and limiting factor to long-term opioid treatment. Since TENS uses opioid receptors and its repeated application produces analgesic tolerance it follows that chronic use of TENS in the clinical setting can be limited as well.
Cholecystokinin (CCK), a neuropeptide widely distributed in the mammalian nervous system [4], reverses analgesic effects of opioids, and co-administration of CCK antagonist and morphine prevents the development of opioid tolerance [33]. Similarly, CCK agonists block the analgesic effect of electroacupuncture and CCK receptor antagonists potentiate analgesic effects of electroacupuncture [11,37]. Electroacupuncture also increases CCK release in spinal cord perfusate and this release is frequency-dependent with higher frequencies demonstrating greater release than lower frequencies [9,10]. Further, tolerance to the analgesic effect of electroacupuncture and morphine is reversed by spinal administration of CCK-8 antiserum, implying an increased endogenous release of CCK-8 may be the likely cause for the development of opioid tolerance [6,27]. It can be inferred that the analgesic and tolerance effects of opioids and electrical stimulation are influenced by the release of endogenous CCK.
These data lead us to conclude that the development of tolerance to TENS is secondary to the development of tolerance at the opioid receptor. Thus, we hypothesized that administration of CCK receptors antagonists, which prevent the development of tolerance to opioids, should similarly prevent tolerance to TENS. We tested this hypothesis by determining whether systemic or intrathecal administration CCK receptor antagonists prevents development of tolerance to high or low frequency TENS.
2. Methods
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the guidelines of National Institute of Health and the International Association for the Study of Pain on use of laboratory animals. Adult male Sprague–Dawley rats (n = 108, 225–250 g, Harlan, Indianapolis, IN) were used for this study.
2.1. Induction of inflammation
Immediately after baseline behavioral measurements, rats were anesthetized with 5% isoflurane and maintained with 1–2% isoflurane and knee joint inflammation was induced by an intra-articular injection of a mixture of 3% carrageenan and 3% kaolin (0.1 mL in sterile saline, pH 7.2) into the left knee joint [50]. After induction of knee inflammation, the rats were returned to their cages and allowed to recover for 24 h. Within 24 h, animals exhibited signs of inflammation such as edematous and warm knee joints and also behavioral signs such as guarding and decreased weight bearing on the inflamed limb [19].
2.2. Behavioral testing
The paw withdrawal threshold (secondary hyperalgesia, cutaneous) and the knee joint withdrawal threshold (primary hyperalgesia, joint) were tested for all groups of rats. These two measures were chosen since both remain decreased throughout the testing period and assess both primary and secondary hyperalgesia. Both measurements were performed before and 24 h after induction of inflammation, and immediately before and after TENS stimulation on each day. The investigator was blinded for all drug treatments and TENS application.
Rats were tested for paw withdrawal threshold with von Frey filaments applied to the paw. Initially, the animals were maintained in their cages in the behavior testing room for acclimation for 30 min. Then, the animals were placed in transparent Lucite cubicles over a wire mesh and acclimated for another 30 min before testing. A series of filaments with increasing bending forces (9.4–495.8 mN) were applied on plantar surface of the hind paw until the rat withdrew from the stimulus [19]. Each filament was applied twice. The lowest force at which the rat withdrew its paw from one of two applications was recorded as the paw withdrawal threshold for mechanical stimuli. A decrease in bending force compared to the baseline was interpreted as cutaneous hyperalgesia. This testing method has shown significant statistical test–retest reliability [49].
Rats were also tested for knee joint withdrawal threshold using a pair of forceps (tweezer) applied to the knee joint as previously described [14,56]. The forceps were equipped with two strain gauges to measure force. Animals were acclimated for two consecutive days before to start the experiment. They were kept inside a restrainer (glove) three times per day with 1-h interval between each 5 min training session. During the acclimation period, animals had their hindpaws mobilized (flexion and extension of knee joint) by the experimenter. To measure knee joint withdrawal thresholds, animals were placed in the restrainer (glove), and the experimenter compressed the knee joint with the tip of the forceps while the hind limb was extended. Compression was continued until the animal withdrew the leg. The maximum force applied at withdrawal was recorded as the knee joint withdrawal threshold. Three trials five minutes apart at each time period were performed and averaged to obtain one reading per time period. A decrease in withdrawal threshold of the inflamed knee joint was interpreted as primary hyperalgesia.
2.3. Application of TENS
Rats were anesthetized with isoflurane, initially with 5% isoflurane and maintained with 1–2% isoflurane for 20 min of TENS [48]. EMPI Select TENS units (with an asymmetrical biphasic square wave) and a pair of circular electrodes were used. Under isoflurane anesthesia, the knee joint was shaved and a half-inch round pregelled surface electrodes were applied to the medial and lateral aspects of the inflamed knee joint in the groups receiving TENS or sham TENS. Animals were observed continuously during TENS to ensure adequate anesthesia and to ensure that the electrodes remained in contact with the skin.
Low (4 Hz) or high (100 Hz) frequency TENS was administered keeping other parameters constant, i.e. pulse duration (100 µs), sensory intensity and 20 min for stimulation. This strategy allowed a comparison of frequency differences without confounding differences in pulse duration or amplitude. Sensory intensity was determined by increasing the intensity until a muscle contraction was visibly observed and then reducing the intensity to just below this level. The parameters were selected to model those used clinically and which produced full inhibition of both primary and secondary hyperalgesia [48,56]. The sham TENS group was anesthetized with 1–2% isoflurane and electrodes were placed on their shaved knee joint but did not receive TENS treatment. Importantly, three rats always were anesthetized with the same vaporizer; at least one rat receiving sham TENS treatment and one rat receiving active TENS treatment were anesthetized at the same time. This procedure ensures that there always were animals in the sham TENS treatment groups that received the same dose of anesthesia.
2.4. Intrathecal catheter placement
Chronic intrathecal (i.t.) catheters (32-gauge polyurethane; 10 cm length; Recathco, Allison Parl, PA) were used to administer drugs to the lumbar space of the spinal cord in rats. Rats were anesthetized with isoflurane and a 23-gauge hypodermic needle was inserted into the intervertebral space between L5 and L6 vertebrae. A 32-gauge polyurethane catheter was inserted through the needle and advanced cranially 3.5–4.0 cm, placing the catheter at the level of the lumbar enlargement of the spinal cord. The external extremity of the catheter was secured to the soft deep tissue with 4–0 silk sutures. The free extremity of the catheter was inserted into PE 10 tubing, glued, and tunneled out through the skin at the cervical region. All animals were allowed to recover for 3–5 days before behavior testing. Drugs were administered in a 10 µL volume. At the end of the experiment, 10 µL of lidocaine was injected (i.t.). If the rats developed paralysis of the hind limbs it was inferred that the catheters were correctly placed in the intrathecal space. The catheter placement was further confirmed by intrathecal administration of methylene blue (10 µL, i.t.) and detecting the location of the dye on the dorsal spinal cord in the lumbar enlargement.
2.5. Drugs
The CCK receptor antagonists (proglumide, lorglumide, and itriglumide) were obtained from Sigma (St. Louis, MO) and diluted in sterile saline (pH 7.2). The mu-opioid receptor agonist DAMGO was obtained from Sigma (St. Louis, MO) and diluted in sterile saline (pH 7.2). The delta-opioid receptor agonist SNC-80 was obtained from Alexis Biochemicals (San Diego, CA). A stock solution of 600 nmol/10 µL of SNC-80 was prepared by diluting into DMSO. The solution was then bubbled with carbon dioxide to aid dissolution. Final concentrations were included 24% DMSO. Concentrations were used as follows: proglumide (20 mg/kg, i.p.), lorglumide (10 µg/10 µL, i.t.), itriglumide (1 µg/10 µL, i.t.), DAMGO (2.34, 7.01, and 23.4 mmol/10 µL, i.t.) and SNC-80 (20, 60, and 120 nmol/10 µL, i.t.). These concentrations were based on previous studies that show effective blockade of CCK effects with these doses [1,33,45].
2.6. Experimental design
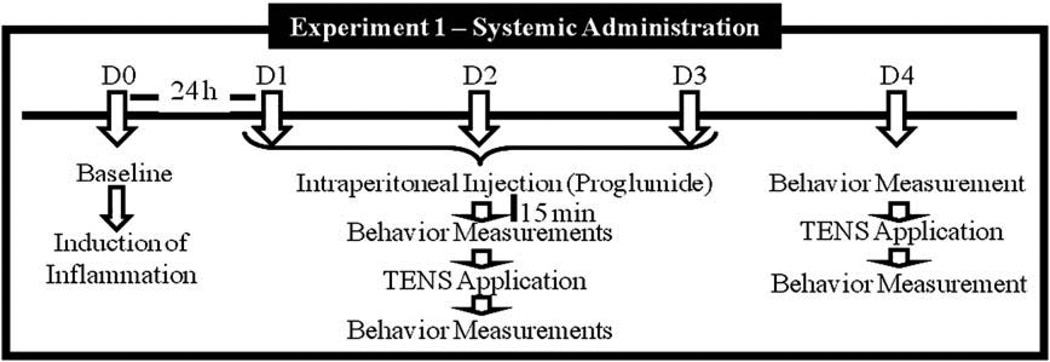
Experiment 1 tested the hypothesis that systemic administration of a non-selective antagonist to CCK receptors, proglumide (20 mg/kg, i.p.), would prevent the development of tolerance to TENS. The following groups were done: (1) high frequency TENS + proglumide (n = 6); (2) low frequency TENS + proglumide (n = 6); (3) sham TENS + proglumide (n = 6); (4) high frequency TENS + vehicle (n = 6); (5) low frequency TENS + vehicle (n = 6); (6) sham TENS + vehicle (n = 6). Fig. 1 depicts the experimental design 1.
Fig. 1.
Experimental timeline for the systemic administration of drugs.
Baseline paw and joint withdrawal thresholds were measured bilaterally prior to the induction of the knee joint inflammation on Day 0. Twenty-four hours after the knee joint inflammation (Day 1), paw and joint withdrawal thresholds were reassessed. Rats were intraperitonealy (i.p.) injected with proglumide or vehicle 30 min before application of TENS. Thirty minutes later, the animals were lightly anesthetized with 1–2% isoflurane for placement of electrodes. TENS was applied for 20 min, and withdrawal thresholds were assessed immediately after TENS and recovery from anesthetic (10–15 min after removal of TENS). The same procedure (assessment of mechanical withdrawal thresholds, pretreatment with proglumide, TENS application, and reassessment of thresholds) was repeated for an additional two consecutive days (Days 2 and 3). On Day 4, no pretreatment with proglumide or vehicle was done; the rats were assessed for mechanical withdrawal thresholds before and after 20 min TENS. Thus, the rats received three consecutive days of TENS treatment in combination with either proglumide or vehicle, and on the last day they received active or sham TENS with no pretreatment. We have previously shown that rats develop tolerance to TENS by the fourth day [4]. All experiments were done with the experimenter blinded to TENS applications and drugs.
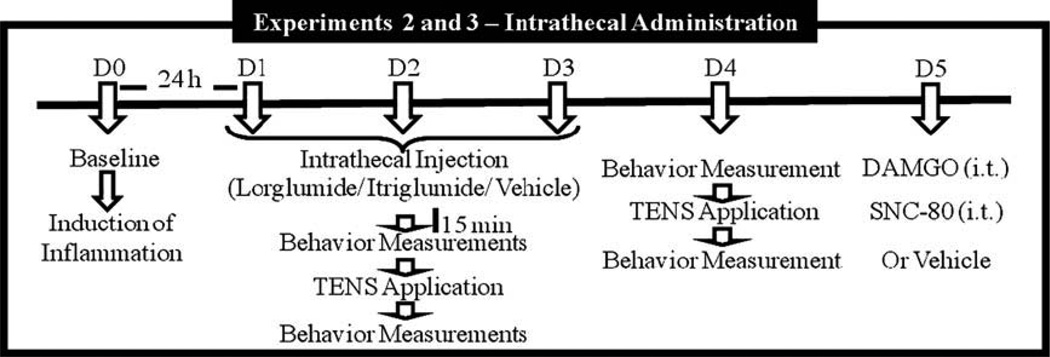
Experiment 2 tested the hypothesis that intrathecal administration of antagonist to different CCK receptors would prevent the development of tolerance to TENS. For this, two drugs were used: lorglumide (selective antagonist to CCK-A receptor, 10 µg/10 µL, i.t.) and itriglumide (selective antagonist to CCK-B receptor, 1 µg/ 10 µL, i.t.). This experiment was divided into parts A and B. Experiment 2A: (1) high frequency TENS + lorglumide (n = 6); (2) low frequency TENS + lorglumide (n = 6); (3) sham TENS + lorglumide (n = 6); (4) high frequency TENS + vehicle (n = 6); (5) low frequency TENS + vehicle (n = 6); (6) sham TENS + vehicle (n = 6). Experiment 2B: (1) high frequency TENS + itriglumide (n = 6); (2) low frequency TENS + itriglumide (n = 6); (3) sham TENS + itriglumide (n = 6); (4) high frequency TENS + vehicle (n = 6); (5) low frequency TENS + vehicle (n = 6); (6) sham TENS + vehicle (n = 6). Fig. 2 depicts the experimental design 2. These experiments directly tested the role of spinal CCK receptors. Animals were implanted with intrathecal catheters 5–7 days prior to injection of the knee joint with 3 % kaolin and carrageenan.
Fig. 2.
Experimental timeline for the intrathecal administration of drugs.
Baseline paw and joint withdrawal thresholds were measured bilaterally prior to the induction of knee joint inflammation on Day 0. Twenty-four hours after the knee joint inflammation (Day 1), paw and joint withdrawal thresholds were reassessed. Rats were intrathecally (i.t.) injected with lorglumide, itriglumide or vehicle 15 min before TENS. Fifteen minutes after drug injection, the animals were lightly anesthetized with 1–2% isoflurane for placement of electrodes. TENS was applied for 20 min, and withdrawal thresholds were assessed immediately after TENS and recovery from anesthetic (10–15 min after removal of TENS). The same procedure (assessment of mechanical withdrawal thresholds, pretreatment with proglumide, TENS application, and reassessment of thresholds) was repeated for an additional two consecutive days (Days 2 and 3). On Day 4, no pretreatment with either lorglumide, itriglumide or vehicle was done; the rats were assessed for mechanical withdrawal thresholds before and after 20 min TENS. Thus, the rats received three consecutive days of TENS treatment in combination with either lorglumide/itriglumide or vehicle, and on the last day they received active or sham TENS with no pretreatment.
Experiment 3 is an extension of the Experiment 2 and established that the loss of tolerance to TENS is concomitant with a loss of tolerance to spinal mu- and delta-opioid receptor agonists. These experiments were restricted to a single effective dose of a single CCK receptor antagonist. Fig. 2 shows the experimental design 3. The CCK receptor antagonists (lorglumide or itriglumide) were administered intrathecally. On Day 5, the withdrawal threshold to mechanical stimuli were redetermined and the rats injected intrathecally in a cumulative manner at 15 min intervals with increasing doses of the mu-opioid receptor agonist DAMGO (2.34, 7.01, and 23.4 mmol/10 µL, i.t.) or the delta-opioid receptor antagonist SNC-80 (20, 60, and 120 nmol/10 µL, i.t.). These doses of DAMGO and SNC-80 reduce inflammatory hyperalgesia [8].
2.7. Statistical analysis
Mechanical withdrawal threshold of the paw was tested for differences between groups of treatment with non-parametric Kruskal– Wallis test, and joint withdrawal threshold with ANOVA for dependent samples at each time point (before inflammation, before TENS, and after TENS, on each day of treatment). ANOVA compared differences across time and between groups for paw and joint withdrawal thresholds. Post-hoc testing between groups was performed with a signed rank test (paw) or a Tukey’s test (joint), as appropriate. Parametric T-test and non-parametric Wilcoxon Matched Pairs test were used to analyze changes in the paw and joint withdrawal thresholds across time, respectively. P value <0.05 was considered significant.
3. Results
3.1. Effect of daily treatment with TENS
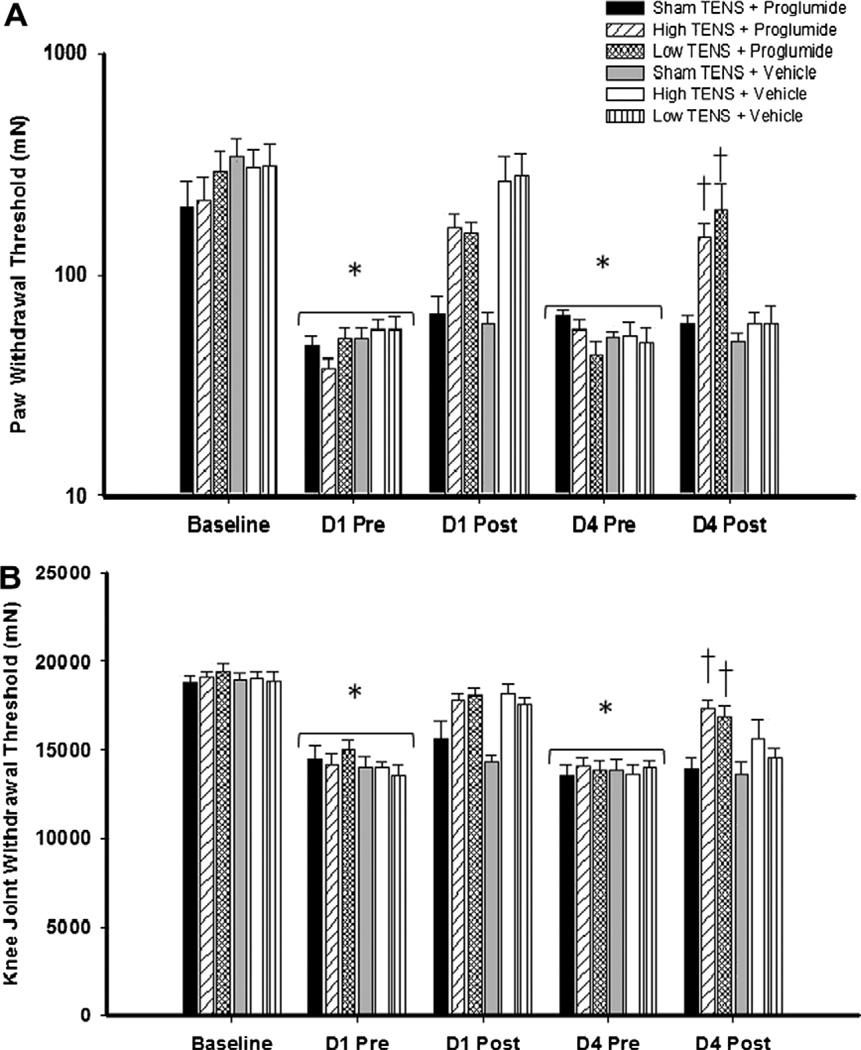
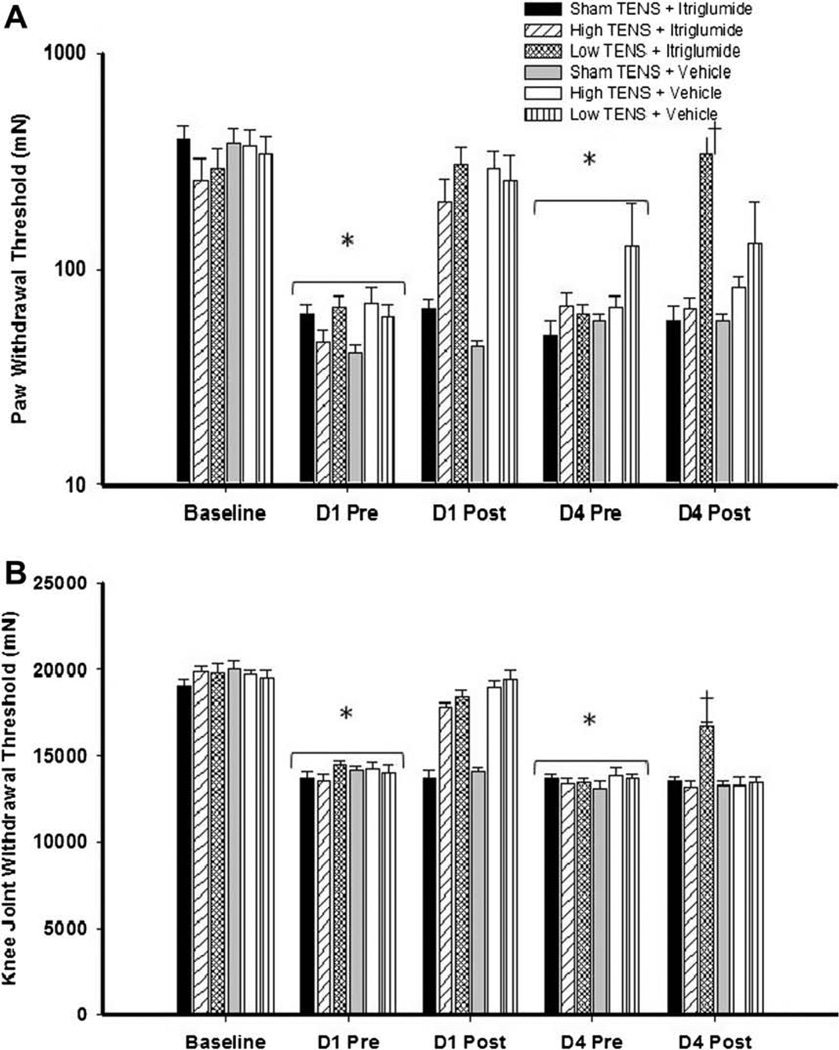
Twenty-four hours following induction of inflammation, the mechanical withdrawal threshold of the paw (Figs. 3A, 4A, and 5A) and the knee joint (Figs. 3B, 4B, and 5B) decreased significantly for all groups of animals, and no difference was observed between groups at this time point. In the vehicle group, both high and low frequency TENS significantly increased the mechanical withdrawal threshold of the paw and the knee joint immediately after TENS treatment when compared with sham TENS on Day 1 (Figs. 3–5). Significant increases in withdrawal threshold of the paw and joint also occurred on Day 2 and Day 3 compared to the group that received sham TENS (data not shown). However, on the fourth day of treatment, the vehicle control group showed no increase in withdrawal threshold of the paw or joint after TENS when compared to sham treatment of to values prior to TENS (Figs. 3–5).
Fig. 3.
Systemic blockade of CCK receptors prevents the development of tolerance to high and low frequency TENS. Bar graph representing mechanical withdrawal threshold of the knee joint (A) and paw (B) from animals receiving TENS treatment combined with proglumide or vehicle. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (baseline), before (pre), and after application of TENS (post). Data are represented as means ± SEM. P value <0.05 was considered statistically significant. *Significantly different from baseline; †significantly different from sham TENS, and vehicle controls on D4.
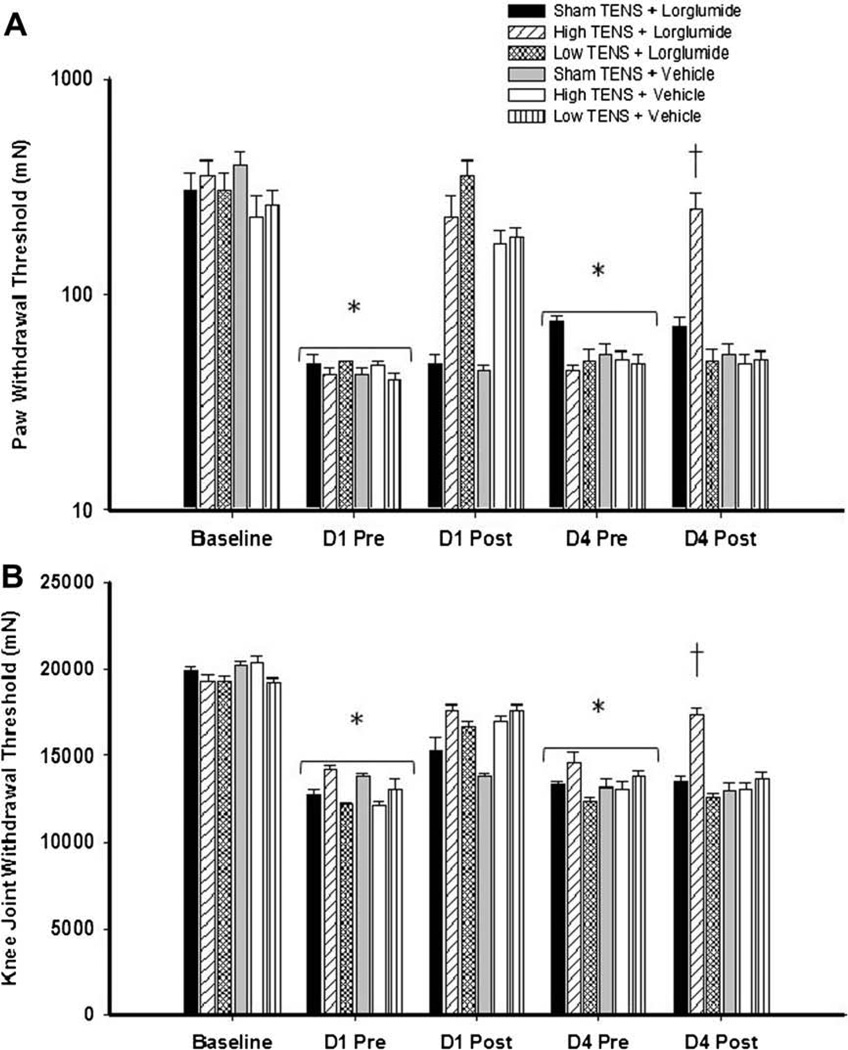
Fig. 4.
Intrathecal blockade of CCK-A receptors prevents the development of tolerance to high frequency TENS, but not low frequency TENS. Bar graph representing mechanical withdrawal threshold of the knee joint (A) and paw (B) from animals receiving TENS treatment combined with lorglumide or vehicle. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (baseline), before (pre), and after application of TENS (post). Data are represented as means ± SEM. P value <0.05 was considered statistically significant. *Significantly different from baseline time; †significantly different from sham TENS and vehicle controls on D4.
Fig. 5.
Intrathecal blockade of CCK-B receptors prevents the development of tolerance to low frequency TENS, but not high frequency TENS. Bar graph representing mechanical withdrawal threshold of the knee joint (A) and paw (B) from animals receiving TENS treatment combined with itriglumide or vehicle. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (baseline), before (pre), and after application of TENS (post). Data are represented as means ± SEM. P value <0.05 was considered statistically significant. *Significantly different from baseline time; †significantly different from sham TENS and vehicle controls on D4.
3.2. Prevention of tolerance to TENS by blocking CCK receptors
On Day 4, animals treated with non-selective CCK antagonist (proglumide) daily, 30 min before either high or low frequency, showed higher paw (bilateral, P < 0.001) and knee joint (bilateral, P < 0.001) withdrawal thresholds when compared to those from vehicle treated groups. No differences were observed between the vehicle treated and proglumide treated sham TENS groups, indicating that 20 mg/kg (i.p.) proglumide only has no effect on the reduced withdrawal threshold produced by joint inflammation (Fig. 3A and B).
For the animals intrathecally administered with the two CCK antagonists, the tolerance effect to TENS was prevented in a receptor-dependent manner. On Day 4, in animals treated with selective CCK-A antagonist (lorglumide, 10 µg/10 µL) daily 15 min before TENS, paw and joint withdrawal thresholds for the group that received high frequency TENS were significantly higher than those pretreated with vehicle and high frequency TENS (Fig. 4A and B). Whereas, rats treated with selective CCK-B antagonist (itriglumide, 1 µg/10 µL) daily 15 min before TENS, paw and joint withdrawal thresholds for the group that received low frequency TENS were significantly higher than those pretreated with vehicle and low frequency TENS (Fig. 5A and B).
3.3. Prevention of cross-tolerance to mu- and delta-opioid agonists
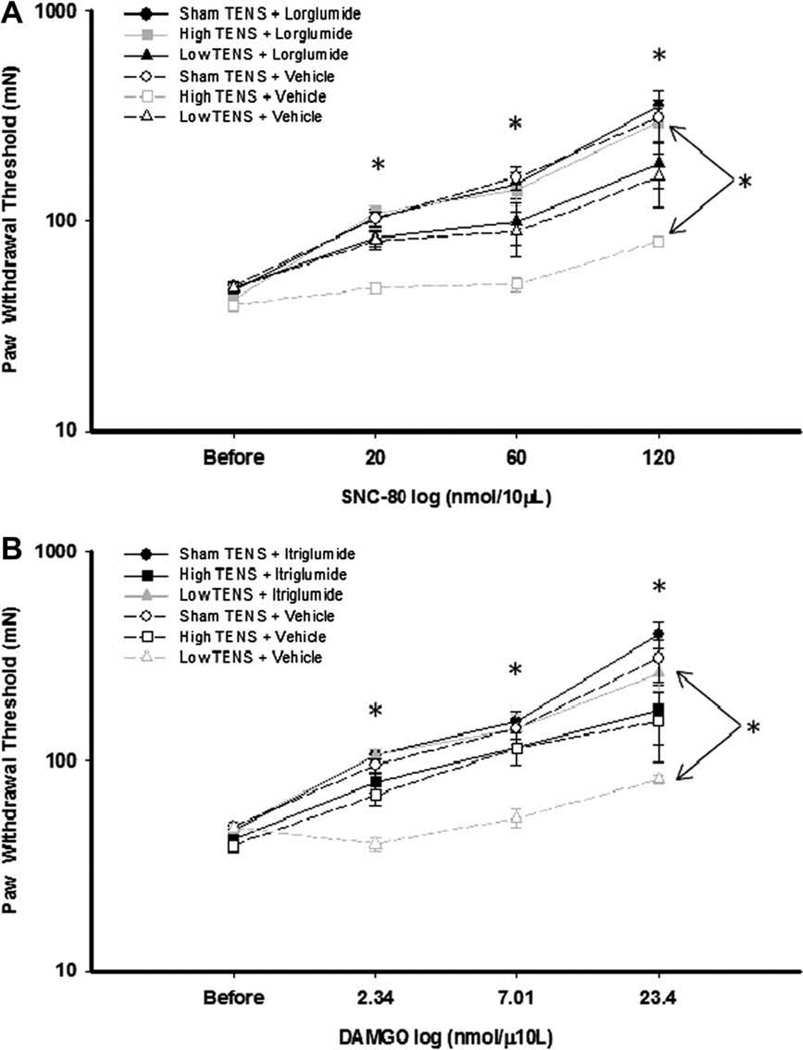
In rats treated with vehicle and sham TENS, there was a dose-dependent increase in paw withdrawal threshold following intrathecal administration of DAMGO (2.4, 7, and 23.4 mmol/10 µL), showing an analgesic effect of DAMGO (Fig. 6B); while the group treated with vehicle and low frequency TENS DAMGO did not change the paw withdrawal threshold suggesting analgesic tolerance. The group that received low frequency TENS with lorglumide showed a dose-dependent increase in paw withdrawal threshold following DAMGO (P < 0.001). This analgesic response to DAMGO was significantly greater than the group that received low frequency TENS and vehicle. In fact, the group receiving low frequency TENS and vehicle did not show an analgesic response to DAMGO. These data indicate that repeated low frequency TENS produces tolerance at mu-opioid receptors in the spinal cord, and that this tolerance is prevented by blockade of CCK-A receptors in the spinal cord.
Fig. 6.
Dose–response curves for SNC-80 and DAMGO. Line graphs representing paw withdrawal threshold after intrathecal injection of increasing doses of SNC-80 (A) and DAMGO (B) in groups receiving TENS treatment combined with CCK receptor antagonists or vehicle. After administration of SCN-80, groups treated with high TENS and vehicle, the withdrawal thresholds were lower than those treated with high TENS and lorglumide*. Following administration of DAMGO, groups treated with low TENS and vehicle, the withdrawal thresholds were lower than those treated with low TENS and itriglumide*.
Paw withdrawal threshold was significantly increased for the group treated with vehicle and sham TENS following intrathecal administration of SNC-80 (20, 60, and 120 nmol/µL) suggesting an antinociceptive effect of SNC-80 (Fig. 6B); while the group treated with vehicle and high frequency TENS SNC-80 did not change the paw withdrawal threshold suggesting analgesic tolerance. The group that received high frequency TENS with itriglumide showed a dose-dependent increase in paw withdrawal threshold following SNC-80 (P < 0.001). This analgesic effect of SNC-80 was significantly greater than the group that received high frequency TENS and vehicle. These data indicate that repeated high frequency TENS produces tolerance at delta-opioid receptors in the spinal cord, and that this tolerance is prevented by blockade of CCK-B receptors in the spinal cord.
4. Discussion
4.1. Development of tolerance
Similar to previous studies from our laboratory [8,26], the current study shows that repeated daily application of either high or low frequency TENS results in a decreased analgesia by the fourth day of treatment when TENS (vehicle and TENS groups), which is interpreted as analgesic tolerance. The current study shows development of analgesic tolerance to TENS in rats with joint inflammation, which is in agreement with previous studies showing the development of opioid tolerance after induction of inflammation [36,39], and our prior studies in rats with joint inflammation and repeated TENS [8,26]. We also show the development of tolerance to TENS for measures of both primary (knee joint) and secondary (cutaneous) mechanical hyperalgesia after joint inflammation occurs within 4 days of treatment. Previously, development of tolerance to morphine occurs within 4 days in animals with CFA-induced paw inflammation, a similar time-course to the current study [35,39]. Analgesic tolerance was enhanced after inflammation compared to non-inflamed [35,39]. Thus, tolerance to morphine after joint inflammation is enhanced and develops faster and with a similar time-frame to that observed for repeated application of TENS.
The current study shows that there was a cross-tolerance to opioid agonists delivered intrathecally after four days of repeated TENS application suggesting that TENS produces analgesic tolerance through release of endogenous opioids spinally, as shown in our prior studies [8,26]. As with TENS, electroacupuncture applied at either high or low frequency repeatedly results in decreased analgesia [23,28], and cross-tolerance at spinal opioid receptors [10]. Previous studies from our laboratory confirm a role for spinal opioid receptors in TENS. Specifically, high frequency TENS analgesia is prevented by blockade of delta-opioid receptors while low frequency TENS analgesia is prevented by blockade of mu-opioid receptors [49]. Similarly, we show that blockade of mu- and delta-opioid receptors in the brainstem, i.e. the rostroventromedial medulla (RVM), also prevents the analgesia produced by low and high frequency TENS [31]. The cross-tolerance to mu- and delta-opioid receptors with repeated application of low or high frequency TENS in the current and prior studies [8,26] is in agreement with these prior studies on mechanisms of TENS [10,21]. Thus, TENS utilizes central opioid receptors to produce analgesia in a frequency- dependent manner, and analgesic tolerance develops after repeated use of TENS due to chronic activation of opioid receptors.
4.2. Blockade of tolerance
The current study extends prior studies by showing the development of analgesic tolerance to TENS is prevented by pretreatment with the CCK receptor antagonist proglumide systemically. These data are in agreement with studies showing that treatment with the CCK antagonist proglumide prevents the development of analgesic tolerance to exogenously administered opioid agonists acting at either delta- or mu- opioid receptors [33,53]. Interestingly, intrathecal blockade of CCK-A receptors prevents the development of tolerance to high frequency TENS, but not low frequency TENS. And, conversely, blocking CCK-B receptors prevents the development of tolerance to low frequency TENS, but not high frequency TENS. These data suggest that there is a specific interaction between (1) low frequency TENS (mu-opioid receptors) and CCK-B receptors, and (2) high frequency TENS (delta-opioid receptors) and CCK-A receptors in the central nervous system.
The differential role of CCK antagonists on low and high frequency TENS analgesia was surprising in light of prior studies. Prior studies suggest that blockade of CCK-B, but not CCK-A, receptors centrally prevents tolerance to mu-opioid receptor agonists such as morphine [15,57,62]. The CCK-B receptor antagonists administered systemically, or supraspinally to the periaqueductal gray or rostral ventromedial medulla, attenuates tolerance to the mu-opioid agonist morphine [54,65,66]. Further the activation of PAG by CCK is prevented by blockade of CCK-B, but not CCK-A receptors [67]. Although there is a lack of information on tolerance at delta- opioid receptors and interactions with CCK, our results show the development of tolerance to either delta-opioid agonist or high frequency TENS is mediated by CCK-A receptors. Interestingly, analgesia produced by an intrathecally administered delta-opioid agonist, DPDPE, was not antagonized by activation of CCK receptors with CCK-8 [59]. While CCK-A receptors are located principally in the peripheral nervous system of rodents, they have also been detected in a number of discrete regions of the brain, e.g. area postrema, nucleus tractus solitaries and interpeduncular [25,41,63]. Our novel data showing a role for CCK-A receptors in delta-opioid induced tolerance could be a result of an upregulation of CCK-A receptors after tissue injury, or an as yet untested interaction between delta-opioid and CCK-A receptors.
Administration of CCK receptor antagonists not only prevents tolerance but also enhances antinociception to opioid agonists in acute pain tests and the formalin test [3,42]. Additionally, proglumide potentiates morphine-induced analgesia in healthy human subjects [5,34,40], and in animals [60]. Irrefutably, CCK antagonists synergize with opioid agonists, both delta and mu [15,16,19,29,51,52,58]. On the other hand, it is well established that cholecystokinin antagonizes antinociception mediated by endogenous as well as exogenous opioids [55,58,60]. Thus, during TENS synergistic interaction would be presented as an increase in analgesic effect of TENS on each day. However, our data argue against the synergistic interaction between CCK and TENS since TENS effects on Day 1, when there is no tolerance, are similar between groups treated with vehicle and those treated with CCK antagonists. Similarly, in rats with hindpaw inflammation induced by carrageenan, the antinociception to combining morphine and CCK antagonists does not occur [51]. However, in rats with visceral inflammation, the antinociception to morphine is enhanced with a CCK-B antagonist, but not a CCK-A antagonist given either spinally or supraspinally [17,18]. Further, CCK antagonists administered alone in the current study, also produced no analgesia suggesting that there is no endogenous release of CCK after joint inflammation. This is in agreement with prior study showing that CCK antagonists alone show no antinociceptive effect [61]. Thus, in animals with inflammation, the antinociceptive effects of endogenous, as well as exogenous opioids, do not result in an enhanced antinociception when combined with CCK antagonists.
The fact that administration of TENS with a CCK antagonists prevents the development of tolerance suggests there is an increased release of CCK during the TENS treatment. As above, however, it is unlikely that there is release of CCK in response to the inflammation as CCK antagonists have no effect in animals without TENS. In support of this, there is increased CCK release in the spinal cord in response to exogenously administered opioids acting at mu- or delta-opioid receptors [21], and the development of antinociceptive tolerance to morphine is associated with an upregulation of CCK [13,68,69]. Further, chronic administration of morphine increases the level of endogenous CCK in the central nervous system, including spinal cord [38]. Together these data suggest that there is increased CCK release during TENS, that repeated TENS results in increase CCK over multiple treatments, and thus, CCK interferes with the analgesic effects of TENS making it less effective with repeated application.
4.3. Mechanisms of tolerance
Multiple mechanisms are involved in the development of opioid tolerance and include uncoupling of the opioid receptors from their second messengers to the adaptive changes in parallel antagonistic or facilitatory pain transmission systems [12]. The diversity of possible adaptive mechanisms implies tolerance to opioids could be mechanistically different under different conditions of drug administrations. Indeed, prior data show that tolerance to different mu-opioid agonists utilize different mechanisms to produce tolerance [20,66]. Our previous studies show that tolerance to repeated application of high and low frequency TENS can be prevented by blockade of systemic NMDA receptors during the TENS application, and this prevents the cross-tolerance at spinal opioid receptors [36]. Further, combining high and low frequency TENS delays the onset of tolerance by one week [14]. Together with the findings that CCK receptor activation prevents tolerance to repeated TENS, these data suggest there are multiple parallel systems that contribute to tolerance. These include increased release and activation of CCK receptors and activation of NMDA glutamate receptors. It is likely, that there will be different mechanisms of tolerance to high and low frequency TENS application, based on the differential activation of delta- and mu-opioid receptors, respectively. Indeed, the current study shows differential activation of CCK-A and CCK-B contributes to the tolerance that results from repeated application of high and low frequency TENS.
4.4. Clinical relevance
Clinically, TENS is typically administered for several consecutive days or months, mainly in cases of chronic pain, likely resulting in tolerance to its analgesic effects. Administration of CCK receptor antagonists at low doses may serve as an adjunct to enhance the efficacy of TENS. Clinically, pharmaceutical agents that block CCK receptors enhance morphine analgesia, reduce opioid requirements, and reduce opioid side effects, suggesting that CCK receptor antagonist prevents tolerance-like effects of opioids in human subjects [32]. As many health practitioners are reluctant to use opioids to treat chronic benign pain, TENS is a viable alternative that would release endogenous opioids to produce analgesia. If a drug such as proglumide maximizes and maintains the analgesia created by TENS, the long-term efficacy of TENS may be enhanced.
Acknowledgments
The authors thank PAIRD (Programa de Auxílio à Integração de Docentes e Técnicos Administrativos Recém Doutores às Atividades de Pesquisa) from the Federal University of Sergipe for the support.
Footnotes
Supported by a competitive grant from EMPI Inc. and NIH R01 AR052316.
Conflict of interest
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
References
- 1.Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, Leeper BJ, Moeller L, Mutch S, O’Dell K, Ross J, Radhakrishnan R, Sluka KA. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–187. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 2.American Physical Therapy Association (APTA) Guide to physical therapy practice. On what concepts is the guide based? Phys Ther. 2001;81:19–25. [Google Scholar]
- 3.Baber NS, Dourish CT, Hill DR. The role of CCK caerulein, and CCK antagonists in nociception. Pain. 1989;39:307–328. doi: 10.1016/0304-3959(89)90045-6. [DOI] [PubMed] [Google Scholar]
- 4.Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981;212:51–57. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein ZP, Yucht S, Battista E, Lema M, Spaulding MB. Proglumide as a morphine adjunct in cancer pain management. J Pain Symptom Manage. 1998;15:314–320. doi: 10.1016/s0885-3924(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 6.Bian JT, Sun MZ, Han JS. Reversal of electroacupuncture tolerance by CCK-8 antiserum: an electrophysiological study on pain-related neurons in nucleus parafascicularis of the rat. Int J Neurosci. 1993;72:15–29. doi: 10.3109/00207459308991620. [DOI] [PubMed] [Google Scholar]
- 7.Brosseau L, Yonge KA, Robinson V, Marchand S, Judd M, Wells G, Tugwell P. Transcutaneous electrical nerve stimulation (TENS) for the treatment of rheumatoid arthritis in the hand (Cochrane Review) The Cochrane library. 2008;(Issue 4) doi: 10.1002/14651858.CD004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen XH, Han JS. All three types of opioid receptors in the spinal cord are important for 2/15 Hz electroacupuncture analgesia. Eur J Pharmacol. 1992;211:203–210. doi: 10.1016/0014-2999(92)90530-h. [DOI] [PubMed] [Google Scholar]
- 10.Chen XH, Han JS. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: another cross-tolerance study. Behav Brain Res. 1992;47:143–149. doi: 10.1016/s0166-4328(05)80120-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen XH, Han JS, Huang LT. CCK receptor antagonist L-365, 260 potentiated electroacupuncture analgesia in Wistar rats but not in audiogenic epileptic rats. Chin Med J (Engl) 1994;107:113–118. [PubMed] [Google Scholar]
- 12.Cox BM. Opioid receptor-g protein interactions: acute and chronic effects of opioids. In: Herz A, editor. Handbook of experimental pharmacology. vol 104. New York: Springer; 1993. pp. 145–886. [Google Scholar]
- 13.de Araujo Lucas G, Alster P, Brodin E, Wiesenfeld-Hallin Z. Differential release of cholecystokinin by morphine in rat spinal cord. Neurosci Lett. 1998;245:13–16. doi: 10.1016/s0304-3940(98)00163-3. [DOI] [PubMed] [Google Scholar]
- 14.DeSantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89:754–760. doi: 10.1016/j.apmr.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dourish CT, O’Neill MF, Coughlan J, Kitchener SJ, Hawley D, Iversen SD. The selective CCK-B receptor antagonist L-365, 260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur J Pharmacol. 1990;176:35–44. doi: 10.1016/0014-2999(90)90129-t. [DOI] [PubMed] [Google Scholar]
- 16.Faris PL, Komisaruk BR, Watkins LR, Mayer DJ. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983;219:310–312. doi: 10.1126/science.6294831. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich AE, Gebhart GF. Effects of spinal cholecystokinin receptor antagonists on morphine antinociception in a model of visceral pain in the rat. J Pharmacol Exp Ther. 2000;292:538–544. [PubMed] [Google Scholar]
- 18.Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;104:93–101. doi: 10.1016/s0304-3959(02)00469-4. [DOI] [PubMed] [Google Scholar]
- 19.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 20.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce micro-opioid receptor–arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafsson H, Afrah AW, Stiller CO. Morphine-induced in vivo release of spinal cholecystokinin is mediated by delta-opioid receptors – effect of peripheral axotomy. J Neurochem. 2001;78:55–63. doi: 10.1046/j.1471-4159.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 22.Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L. Effect of low- and high-frequency TENS on met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47:295–298. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- 23.Han JS, Li SJ, Tang J. Tolerance to electroacupuncture and its cross tolerance to morphine. Neuropharmacology. 1981;20:593–596. doi: 10.1016/0028-3908(81)90213-6. [DOI] [PubMed] [Google Scholar]
- 24.Han JS, Xie GX, Ding ZX, Fan SG. High and low frequency electroacupuncture analgesia are mediated by different opioid peptides. Pain. 1984;(Suppl. 2):543. [Google Scholar]
- 25.Hill DR, Shaw TM, Woodruff GN. Species differences in the localization of ‘peripheral’ type cholecystokinin receptors in rodent brain. Neurosci Lett. 1987;79:286–289. doi: 10.1016/0304-3940(87)90445-9. [DOI] [PubMed] [Google Scholar]
- 26.Hingne PM, Sluka KA. Blockade of NMDA receptors prevents analgesic tolerance to repeated transcutaneous electrical nerve stimulation (TENS) in rats. J Pain. 2008;9:217–225. doi: 10.1016/j.jpain.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C, Hu ZP, Jiang SZ, Li HT, Han JS, Wan Y. CCK(B) receptor antagonist L365, 260 potentiates the efficacy to and reverses chronic tolerance to electroacupuncture-induced analgesia in mice. Brain Res Bull. 2007;71:447–451. doi: 10.1016/j.brainresbull.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Long H, Shi YS, Han JS, Wan Y. Nocistatin potentiates electroacupuncture antinociceptive effects and reverses chronic tolerance to electroacupuncture in mice. Neurosci Lett. 2003;350:93–96. doi: 10.1016/s0304-3940(03)00863-2. [DOI] [PubMed] [Google Scholar]
- 29.Hughes J, Hunter JC, Woodruff GN. Neurochemical actions of CCK underlying the therapeutic potential of CCK-B antagonists. Neuropeptides. 1991;19:85–89. doi: 10.1016/0143-4179(91)90087-y. [DOI] [PubMed] [Google Scholar]
- 30.Hughes GS, Lichstein PR, Whitlock D, Harker C. Response of plasma beta-endorphins to transcutaneous electrical nerve stimulation in healthy subjects. Phys Ther. 1984;64:1062–1066. doi: 10.1093/ptj/64.7.1062. [DOI] [PubMed] [Google Scholar]
- 31.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 32.Kalso E. Improving opioid effectiveness: from ideas to evidence. Eur J Pain. 2004;9:131–135. doi: 10.1016/j.ejpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Kellstein DE, Mayer DJ. Spinal co-administration of cholecystokinin antagonists with morphine prevents the development of opioid tolerance. Pain. 1991;47:221–229. doi: 10.1016/0304-3959(91)90208-F. [DOI] [PubMed] [Google Scholar]
- 34.Lavigne GJ, Hargreaves KM, Schmidt EA, Dionne RA. Proglumide potentiates morphine analgesia for acute postsurgical pain. Clin Pharmacol Ther. 1989;45:666–673. doi: 10.1038/clpt.1989.88. [DOI] [PubMed] [Google Scholar]
- 35.Li JY, Wong CH, Huang KS, Liang KW, Lin MY, Tan PP, Chen JC. Morphine tolerance in arthritic rats and serotonergic system. Life Sci. 1999;64:111–116. doi: 10.1016/s0024-3205(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 36.Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Liu NJ, Bao H, Li N, Yu YX, Han JS. Cholecystokinin octapeptide reverses the inhibitory effect induced by electroacupuncture on C-fiber evoked discharges. Int J Neurosci. 1996;86:241–247. doi: 10.3109/00207459608986714. [DOI] [PubMed] [Google Scholar]
- 38.Lucas GA, Hoffmann O, Alster P, Wiesenfeld-Hallin Z. Extracellular cholecystokinin levels in the rat spinal cord following chronic morphine exposure: an in vivo microdialysis study. Brain Res. 1999;82:179–186. doi: 10.1016/s0006-8993(99)01068-9. [DOI] [PubMed] [Google Scholar]
- 39.Luis-Delgado OE, Barrot M, Rodeau JL, Schott G, Benbouzid M, Poisbeau P, Freund-Mercier MJ, Lasbennes F. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J Pain. 2006;7:32–39. doi: 10.1016/j.jpain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 40.McCleane GJ. The cholecystokinin antagonist proglumide enhances the analgesic efficacy of morphine in humans with chronic benign pain. Anesth Analg. 1998;87:1117–1120. [PubMed] [Google Scholar]
- 41.Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- 42.Noble F, Blommaert A, Fournié-Zaluski MC, Roques BP. A selective CCKB receptor antagonist potentiates, mu-, but not delta-opioid receptor-mediated antinociception in the formalin test. Eur J Pharmacol. 1995;273:145–151. doi: 10.1016/0014-2999(94)00688-4. [DOI] [PubMed] [Google Scholar]
- 43.Osiri M, Welch V, Brosseau L, Shea B, McGowan J, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation for knee osteoarthritis (Cochrane Review) The Cochrane library. 2008;(Issue 4) doi: 10.1002/14651858.CD002823. [DOI] [PubMed] [Google Scholar]
- 44.Pelland L, Brosseau L, Casimiro L, Robinson VA, Tugwell P, Wells G. Electrical stimulation for the treatment of rheumatoid arthritis (Cochrane Review) The Cochrane library. 2008;(Issue 4) doi: 10.1002/14651858.CD003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezayat M, Tabarrai E, Parvini S, Zarrindast MR, Pirali M. Effects of CCK antagonists on GABA mechanism-induced antinociception in the formalin test. Eur Neuropsychopharmacol. 1999;9:9–14. doi: 10.1016/s0924-977x(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 46.Salar G, Job I, Mingrino S, Bosio A, Trabucchi M. Effect of transcutaneous electrotherapy on CSF beta-endorphin content in patients without pain problems. Pain. 1981;10:169–172. doi: 10.1016/0304-3959(81)90192-5. [DOI] [PubMed] [Google Scholar]
- 47.Sjölund BH, Eriksson MB. The influence of naloxone on analgesia produced by peripheral conditioning stimulation. Brain Res. 1979;173:295–301. doi: 10.1016/0006-8993(79)90629-2. [DOI] [PubMed] [Google Scholar]
- 48.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77:97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 49.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 50.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 51.Stanfa L, Dickenson A, Xu XJ, Wiesenfeld-Hallin Z. Cholecystokinin and morphine analgesia: variations on a theme. Trends Pharmacol Sci. 1994;15:65–66. doi: 10.1016/0165-6147(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 52.Suh HH, Tseng LF. Differential effects of sulfated cholecystokinin octapeptide and proglumide injected intrathecally on antinociception induced by beta-endorphin and morphine administered intra cerebroventricularly in mice. Eur J Pharmacol. 1990;179:329–338. doi: 10.1016/0014-2999(90)90173-4. [DOI] [PubMed] [Google Scholar]
- 53.Tortorici V, Nogueira L, Aponte Y, Vanegas H. Involvement of cholecystokinin in the opioid tolerance induced by dipyrone (metamizol) microinjections into the periaqueductal gray matter of rats. Pain. 2004;112:113–120. doi: 10.1016/j.pain.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Tortorici V, Nogueira L, Salas R, Vanegas H. Involvement of local cholecystokinin in the tolerance induced by morphine microinjections into the periaqueductal gray of rats. Pain. 2003;102:9–16. doi: 10.1016/s0304-3959(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 55.Valverde O, Maldonado R, Fournie-Zaluski MC, Roques BP. Cholecystokinin B antagonists strongly potentiate antinociception mediated by endogenous enkephalins. J Pharmacol Exp Ther. 1994;270:77–88. [PubMed] [Google Scholar]
- 56.Vance CG, Radhakrishnan R, Skyba DA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87:44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- 57.Vanderah TW, Lai J, Yamamura HI, Porreca F. Antisense oligodeoxynucleotide to the CCKB receptor produces naltrindole- and [Leu5]enkephalin antiserum-sensitive enhancement of morphine antinociception. Neuroreport. 1994;5:2601–2605. doi: 10.1097/00001756-199412000-00049. [DOI] [PubMed] [Google Scholar]
- 58.Verge VMK, Wiesenfeld-Hallin Z, Hokfelt T. Cholecystokinin in mammalian primary sensory neurons and spinal cord: in situ hybridization studies in rat and monkey. Eur J Neurosci. 1993;5:240–250. doi: 10.1111/j.1460-9568.1993.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang XJ, Han JS. Modification by cholecystokinin octapeptide of the binding of mu-, delta-, and kappa-opioid receptors. J Neurochem. 1990;55:1379–1382. doi: 10.1111/j.1471-4159.1990.tb03149.x. [DOI] [PubMed] [Google Scholar]
- 60.Watkins LR, Kinscheck IB, Kaufman EF, Miller J, Frenk H, Mayer DJ. Cholecystokinin antagonists selectively potentiate analgesia induced by endogenous opiates. Brain Res. 1985;327:181–190. doi: 10.1016/0006-8993(85)91512-4. [DOI] [PubMed] [Google Scholar]
- 61.Wiesenfeld-Hallin Z, Xu XJ. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Regul Pept. 1996;65:23–28. doi: 10.1016/0167-0115(96)00068-7. [DOI] [PubMed] [Google Scholar]
- 62.Wiesenfeld-Hallin Z, Xu XJ, Hughes J, Horwell DC, Hökfelt T. PD134308, a selective antagonist of cholecystokinin type B receptor, enhances the analgesic effect of morphine and synergistically interacts with intrathecal galanin to depress spinal nociceptive reflexes. Proc Natl Acad Sci USA. 1990;87:7105–7109. doi: 10.1073/pnas.87.18.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodruff GN, Hughes J. Cholecystokinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:469–501. doi: 10.1146/annurev.pa.31.040191.002345. [DOI] [PubMed] [Google Scholar]
- 64.Woolf CJ, Mitchell D, Barrett GD. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980;8:237–252. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]
- 65.Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- 67.Yang YM, Chung JM, Rhim H. Cellular action of cholecystokinin-8S-mediated excitatory effects in the rat periaqueductal gray. Life Sci. 2006;79:1702–1711. doi: 10.1016/j.lfs.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y, Sun YH, Zhang ZW, Han JS. Accelerated expression of cholecystokinin gene in the brain of rats rendered tolerant to morphine. Neuroreport. 1992;3:1121–1123. doi: 10.1097/00001756-199212000-00022. [DOI] [PubMed] [Google Scholar]
- 69.Zhou Y, Sun YH, Shen JM, Han JS. Increased release of immunoreactive CCK-8 by electroacupuncture and enhancement of electroacupuncture analgesia by CCK-B antagonist in rat spinal cord. Neuropeptides. 1993;24:139–144. doi: 10.1016/0143-4179(93)90077-n. [DOI] [PubMed] [Google Scholar]