Abstract
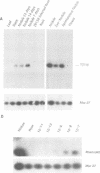
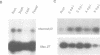
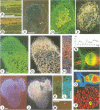
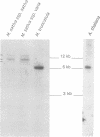
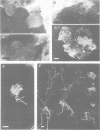
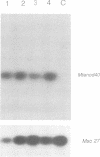
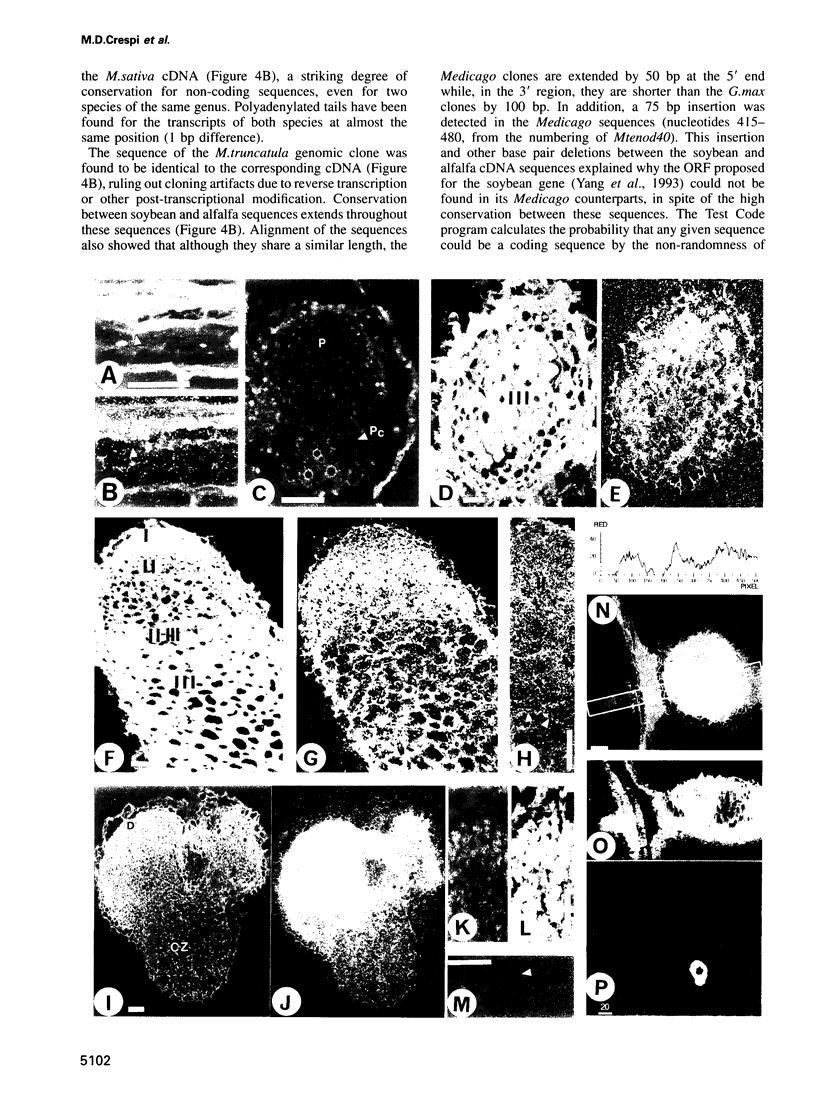
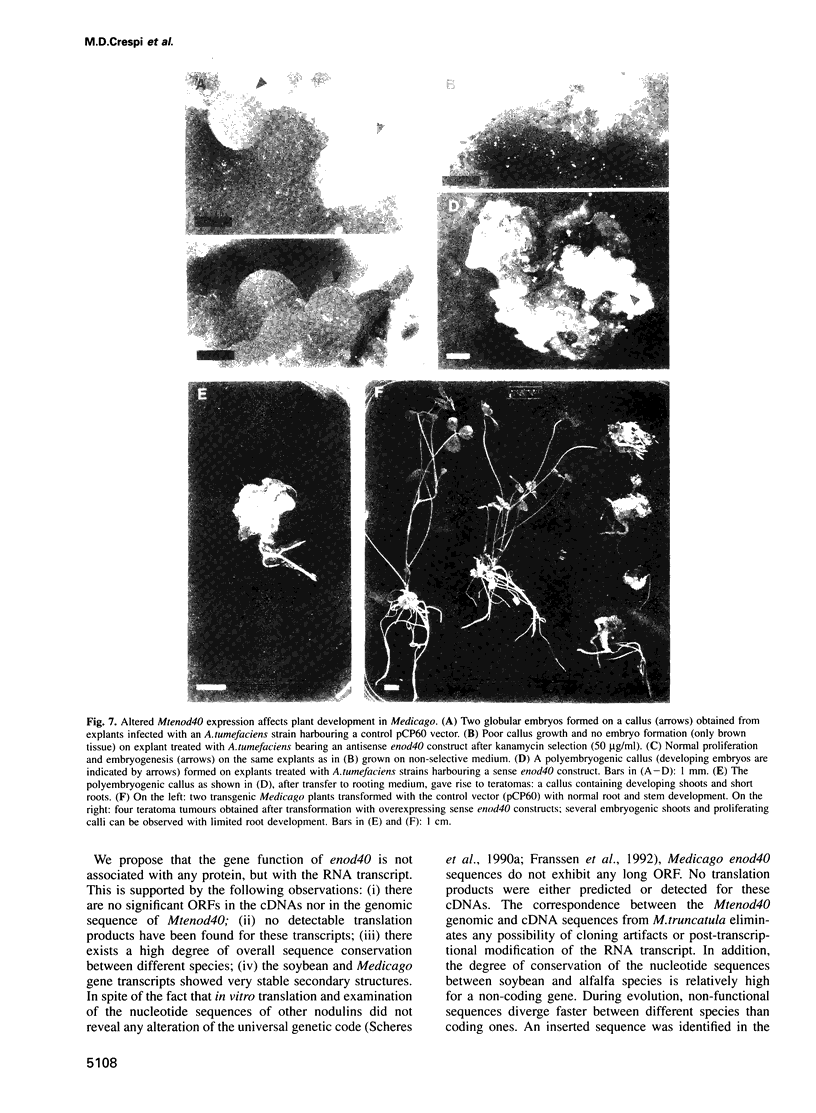
Rhizobium meliloti can interact symbiotically with Medicago plants, thereby inducing root nodules. However, certain Medicago plants can form nodules spontaneously, in the absence of rhizobia. A differential screening was performed using spontaneous nodule versus root cDNAs from Medicago sativa ssp. varia. Transcripts of a differentially expressed clone, Msenod40, were detected in all differentiating cells of nodule primordia and spontaneous nodules, but were absent in fully differentiated cells. Msenod40 showed homology to a soybean early nodulin gene, Gmenod40, although no significant open reading frame (ORF) or coding capacity was found in the Medicago sequence. Furthermore, in the sequences of cDNAs and a genomic clone (Mtenod40) isolated from Medicago truncatula, a species containing a unique copy of this gene, no ORFs were found either. In vitro translation of purified Mtenod40 transcripts did not reveal any protein product. Evaluation of the RNA secondary structure indicated that both msenod40 and Gmenod40 transcripts showed a high degree of stability, a property shared with known non-coding RNAs. The Mtenod40 RNA was localized in the cytoplasm of cells in the nodule primordium. Infection with Agrobacterium tumefaciens strains bearing antisense constructs of Mtenod40 arrested callus growth of Medicago explants, while overexpressing Mtenod40 embryos developed into teratomas. These data suggest that the enod40 genes might have a role in plant development, acting as 'riboregulators', a novel class of untranslated RNAs associated with growth control and differentiation.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Kiss G. B., Bauer P., Poiret M., Pierre M., Savouré A., Kondorosi E., Kondorosi A. Identification of two alfalfa early nodulin genes with homology to members of the pea Enod12 gene family. Plant Mol Biol. 1993 Jan;21(2):375–380. doi: 10.1007/BF00019952. [DOI] [PubMed] [Google Scholar]
- Bauer P., Crespi M. D., Szécsi J., Allison L. A., Schultze M., Ratet P., Kondorosi E., Kondorosi A. Alfalfa Enod12 genes are differentially regulated during nodule development by Nod factors and Rhizobium invasion. Plant Physiol. 1994 Jun;105(2):585–592. doi: 10.1104/pp.105.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Brunkow M. E., Tilghman S. M. Ectopic expression of the H19 gene in mice causes prenatal lethality. Genes Dev. 1991 Jun;5(6):1092–1101. doi: 10.1101/gad.5.6.1092. [DOI] [PubMed] [Google Scholar]
- Cech T. R. RNA. Fishing for fresh catalysts. Nature. 1993 Sep 16;365(6443):204–205. doi: 10.1038/365204a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. B., Long S. R. Morphogenetic Rescue of Rhizobium meliloti Nodulation Mutants by trans-Zeatin Secretion. Plant Cell. 1994 Feb;6(2):215–225. doi: 10.1105/tpc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberledge S., Zaratzian A., Sakonju S. Characterization of two RNAs transcribed from the cis-regulatory region of the abd-A domain within the Drosophila bithorax complex. Proc Natl Acad Sci U S A. 1990 May;87(9):3259–3263. doi: 10.1073/pnas.87.9.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C., de Bruijn F. J. The early nodulin gene SrEnod2 from Sesbania rostrata is inducible by cytokinin. Plant J. 1992 Jan;2(1):117–128. doi: 10.1046/j.1365-313x.1992.t01-51-00999.x. [DOI] [PubMed] [Google Scholar]
- Franssen H. J., Vijn I., Yang W. C., Bisseling T. Developmental aspects of the Rhizobium-legume symbiosis. Plant Mol Biol. 1992 May;19(1):89–107. doi: 10.1007/BF00015608. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Hemerly A. S., Ferreira P., de Almeida Engler J., Van Montagu M., Engler G., Inzé D. cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell. 1993 Dec;5(12):1711–1723. doi: 10.1105/tpc.5.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Bhuvaneswari T. V., Torrey J. G., Bisseling T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Heidstra R., Lados M., Moerman M., Spaink H. P., Promé J. C., van Kammen A., Bisseling T. Lipo-oligosaccharides of Rhizobium induce infection-related early nodulin gene expression in pea root hairs. Plant J. 1993 Oct;4(4):727–733. doi: 10.1046/j.1365-313x.1993.04040727.x. [DOI] [PubMed] [Google Scholar]
- Kouchi H., Hata S. Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet. 1993 Apr;238(1-2):106–119. doi: 10.1007/BF00279537. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Liu Cm., Xu Zh., Chua N. H. Auxin Polar Transport Is Essential for the Establishment of Bilateral Symmetry during Early Plant Embryogenesis. Plant Cell. 1993 Jun;5(6):621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nap J. P., Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990 Nov 16;250(4983):948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Hoffarth V., Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992 Jun 5;256(5062):1416–1419. doi: 10.1126/science.1604315. [DOI] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Van Veen R. J., Van Beelen P., Regensburg-Tuïnk T. J., Schilperoort R. A. Octopine Ti-plasmid deletion mutants of agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid. 1982 Jan;7(1):15–29. doi: 10.1016/0147-619x(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Smith D. K., Olsen G. J., James B. D. Phylogenetic comparative analysis and the secondary structure of ribonuclease P RNA--a review. Gene. 1989 Oct 15;82(1):65–75. doi: 10.1016/0378-1119(89)90031-0. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Brannan C. I., Tilghman S. M. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988 Mar;7(3):673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pay A., Heberle-Bors E., Hirt H. An alfalfa cDNA encodes a protein with homology to translationally controlled human tumor protein. Plant Mol Biol. 1992 Jun;19(3):501–503. doi: 10.1007/BF00023399. [DOI] [PubMed] [Google Scholar]
- Putnoky P., Petrovics G., Kereszt A., Grosskopf E., Ha D. T., Bánfalvi Z., Kondorosi A. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol. 1990 Sep;172(9):5450–5458. doi: 10.1128/jb.172.9.5450-5458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Conboy M. J., Rando T. A., Blau H. M. Tumor suppression by RNA from the 3' untranslated region of alpha-tropomyosin. Cell. 1993 Dec 17;75(6):1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- Sassanfar M., Szostak J. W. An RNA motif that binds ATP. Nature. 1993 Aug 5;364(6437):550–553. doi: 10.1038/364550a0. [DOI] [PubMed] [Google Scholar]
- Scheres B., Van De Wiel C., Zalensky A., Horvath B., Spaink H., Van Eck H., Zwartkruis F., Wolters A. M., Gloudemans T., Van Kammen A. The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell. 1990 Jan 26;60(2):281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- Scheres B., van Engelen F., van der Knaap E., van de Wiel C., van Kammen A., Bisseling T. Sequential induction of nodulin gene expression in the developing pea nodule. Plant Cell. 1990 Aug;2(8):687–700. doi: 10.1105/tpc.2.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M., Quiclet-Sire B., Kondorosi E., Virelizer H., Glushka J. N., Endre G., Géro S. D., Kondorosi A. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P. Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol Biol. 1992 Dec;20(5):977–986. doi: 10.1007/BF00027167. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Vasse J., de Billy F., Camut S., Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990 Aug;172(8):4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijn I., das Nevas L., van Kammen A., Franssen H., Bisseling T. Nod factors and nodulation in plants. Science. 1993 Jun 18;260(5115):1764–1765. doi: 10.1126/science.8511583. [DOI] [PubMed] [Google Scholar]
- Yang W. C., Katinakis P., Hendriks P., Smolders A., de Vries F., Spee J., van Kammen A., Bisseling T., Franssen H. Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 1993 Apr;3(4):573–585. doi: 10.1046/j.1365-313x.1993.03040573.x. [DOI] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- d'Aubenton Carafa Y., Brody E., Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990 Dec 20;216(4):835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]