Abstract
Current theories suggest that mitotic checkpoint proteins are essential for proper cellular response to taxanes, a widely-used family of chemotherapeutic compounds. We recently demonstrated that absence or depletion of protein Daxx increases cellular taxol (paclitaxel) resistance—a common trait of patients diagnosed with several malignancies, including breast cancer. Further investigation of Daxx-mediated taxol response revealed that Daxx is important for the proper timing of mitosis progression and cyclin B stability. Daxx interacts with mitotic checkpoint protein Rassf1 and partially co-localizes with this protein during mitosis. Rassf1/Daxx depletion or expression of Daxx binding domain of Rassf1 elevates cyclin B stability and increases taxol resistance in cells and mouse xenograft models. In breast cancer patients, we observed the inverse correlation between Daxx and clinical response to taxane-based chemotherapy. These data suggest that Daxx and Rassf1 define a mitotic stress checkpoint that enables cells to exit mitosis as micronucleated cells (and eventually die) when encountered with specific mitotic stress stimuli, including taxol. Surprisingly, depletion of Daxx or Rassf1 does not change activity of E3 ubiquitin ligase APC/C in in vitro settings, suggesting necessity of mitotic cellular environment for proper activation of this checkpoint. Daxx and Rassf1 may become useful predictive markers for the proper selection of patients for taxane chemotherapy.
Keywords: Daxx, Rassf1A/C, taxane chemotherapy, mitosis, SAC, APC
Introduction
Cell cycle checkpoints are necessary controls that halt cell cycle progression in response to stresses that may otherwise promote genome instability. Under physiological conditions, the spindle assembly checkpoint (SAC) ensures that during mitosis all kinetochores are stably attached to microtubules before separation of chromosomes during anaphase. Spindle toxins such as taxanes (taxol/paclitaxel and docetaxel/taxotere), nocodazole, and others promote conditions of prolonged mitotic stress by binding tubulin heterodimers and interrupting the polymerization/depolymerization rate of microtubules; they are widely used as cell biology tools and as chemotherapeutic agents. Paclitaxel (taxol) is one of the most common chemotherapeutic agents used to treat human cancers (reviewed in (O'Shaughnessy, 2005)). However, many cancer patients are resistant or become resistant to taxol during drug administration (Crown et al., 2004); (Ravdin et al., 2003); (Bonneterre et al., 1999). Overcoming resistance or incomplete response to these agents would represent a major advantage in the clinical treatment of breast cancer (Henderson et al., 2003); (Aapro, 2001).
Block of microtubule dynamics can result in the potential alteration of mitotic processes, including separation of centrosomes, microtubule attachment to kinetochores (and activation of the SAC) and proper alignment and separation of chromosomes. When cells are exposed to conditions of prolonged mitotic stress in the presence of these toxins, the SAC is eventually inactivated and cells can exit mitosis as micro-nucleated and tetraploid (Mantel et al., 2008); (Wysong et al., 2009). This mitotic exit is primarily dependent upon the ubiquitination and proteolysis of cyclin B and Securin, a process referred to as “mitotic slippage”, or “mitotic catastrophe” (Brito and Rieder, 2006). The proteins and pathways that govern this slippage during prolonged stress conditions, however, are insufficiently characterized. Cells that lack key SAC-related proteins like Mad2 and BubR1, experience enhanced sensitivity to spindle toxins with increased occurrence of micro-nucleated cells (Lee et al., 2004); (Sudo et al., 2004); (Niikura et al., 2007). However, very few instances of enhanced mitotic arrest or “resistance” are ascribed to proteins in response to spindle toxins. To date, only few examples are known when inactivation of mitotic checkpoint proteins leads to reduced sensitivity to taxanes, including BRCA1 (Chabalier et al., 2006) and Mad2 antagonist protein p31comet (Xia et al., 2004). Recently, we reported that the nuclear protein Daxx is involved in taxane sensitivity (Lindsay et al., 2007). Daxx is a highly conserved and developmentally essential nuclear protein (Ishov et al., 2004; Lindsay et al., 2009; Michaelson et al., 1999a). Daxx is involved in numerous cellular processes such as transcriptional regulation (Lindsay et al., 2008), anti-viral immunity (Saffert and Kalejta, 2008), apoptosis (Michaelson, 2000; Salomoni and Khelifi, 2006) and carcinogenesis. We previously showed that, upon taxol treatment, cells with reduced level of Daxx remain in a prolonged mitotic arrest and complete cell division after taxol removal, while wild-type cells exit from taxol-induced mitotic block as micro-nucleated cells incapable for proliferation (Lindsay et al., 2007). Thus, Daxx-deficient cancer cells that are exposed to taxol can survive treatment, but the mechanism remains elusive.
Taxanes affect microtubule stability, but mutations or alterations in tubulin occur very rarely in cancers (Hari et al., 2003a). Functional screens have been directed at finding novel targets affecting sensitivity to taxol and other compounds but it remains unclear whether these targets play a direct role in taxol sensitization or offer prognostic value to clinicians (Swanton et al., 2007). The existence of a mitotic stress checkpoint(s), separate in function from the SAC has been proposed in regard to cells that have been exposed to spindle toxins (Scolnick and Halazonetis, 2000) and guardian proteins or pathways may be in place at particular stages of mitosis where these drugs may target important mitotic functions. Thus, it is essential to identify the proteins and pathways involved in taxol sensitization/resistance because foreknowledge of these targets may prove useful for proper selection of patients for taxane-based chemotherapy. To this end, we sought to further understand the phenomenon of Daxx-dependent taxol resistance related to mitosis and to determine its importance in tumors subjected to this chemotherapy. Here, we describe the cell cycle dependent interaction of Daxx with protein Rassf1 in establishing the proper cellular response to taxol.
Materials and Methods
Biochemical Fractionation
HEp2 cells were separated into nuclear and cytosolic fractions using a biochemical fractionation method described in (Lindsay et al., 2009).
Cell Culture
HEp2 cells and primary MEFs (Ishov et al., 2004) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine and 100 U/mL penicillin and 100 µg/ml streptomycin (Gibco BRL) and grown in a humidified 5% CO2 incubator. Taxol (Paclitaxel; Sigma; 100 µM in DMSO) was used at a final concentration of 10 nM. Thymidine (Sigma) was dissolved in 1N NaOH for 1M stock and used at a final concentration of 2 mM.
Cell cycle synchronization
HEp2 cells were synchronized using a double thymidine block protocol as described in (Lindsay et al., 2007). For taxol studies, 10 nM taxol was added six hours after release of cells from second thymidine block; at this time point, up to 95% of cells were accumulated in G2 phase by FACS analysis.
Colony Formation Assay
Cells exposed to control or 10 nM taxol were originally plated on 3.5 cm dishes (Corning) for treatment. Following exposure, cells were trypsinized and re-plated (in triplicates) at 1:1000 dilution on six well plates (Costar) for colony formation analysis. Five to seven days afterwards, colonies were stained with crystal violet and counted.
Immunofluoresence
Immunofluoresence analysis was completed on cells grown overnight on cover slips in 24 well plates (Costar). Briefly, cells were fixed using 1% formaldehyde or ice-cold methanol (for analysis of microtubules). Following fixation, cells were permeabilized with 0.4% Triton X-100 (formaldehyde fixed cells only). Cells were incubated for 1 h with the following primary antibodies: Daxx rabbit (Santa Cruz), Daxx 5.14 monoclonal (Ishov et al., 2004), PML 14 rabbit (Ishov et al., 1999), Rassf1C mouse polyclonal (UT Southwestern), Rassf1 rabbit (gift from Dr. Dae Sik Lim, Korea Advanced Institute for Science and Technology), and alpha-Tubulin (Sigma). Cells were washed in PBS and incubated with appropriate FITC- or Texas Red-conjugated secondary antibodies (Vector Labs; all diluted 1:300), stained with HOECHST 33342 (Sigma) for DNA visualization and mounted on slides with Fluoromount G (Southern Biotech). Images were analyzed using Leica TCS SP5 confocal microscope.
Immunohistochemistry
For this study, twenty two women were identified with locally advanced HER-2 non-amplified breast cancer who were treated with standard taxane/anthracycline based neoadjuvant chemotherapy at H. Lee Moffitt Cancer Center. Initial core biopsy was performed for the diagnosis and all patients underwent neoadjuvant chemotherapy followed by definitive surgery with either lumpectomy or mastectomy and axillary lymph node dissection. Two patients had invasive lobular carcinoma, one patient papillary carcinoma, while the remainder had invasive ductal carcinoma. Four tumors were hormone receptor negative and eighteen tumors were hormone receptor positive. Tissue blocks were obtained from initial biopsy (prior to neoadjuvant chemotherapy) to perform immunohistochemical staining for Daxx. Slides were de-paraffinized with xylene and re-hydrated through decreasing concentrations of ethanol to water, including an intermediary step to quench endogenous peroxidase activity (3% hydrogen peroxide in methanol). For heat-induced antigen retrieval, sections were heated in a water bath at 95°C while submerged in Trilogy buffer (Cell Marque, Hot Springs, AR) for 25 minutes and afterwards incubated with a universal protein blocker Sniper (Biocare Medical, Walnut Creek, CA) for 15 min, RT. Monoclonal mouse anti-Daxx 5.14 was added o/n at RT. Mach 2 goat anti-mouse-horse radish peroxidase-conjugated (Biocare Medical, Walnut Creek, CA) was then added for 30 min, RT. Detection of Daxx was achieved by incubating slides in 3’3’ diaminobenzidine (Biocare Medical, Walnut Creek, CA) for 15 min, RT. Slides were counterstained with hematoxylin (Vector Laboratories Inc., Burlingame, CA) for 10 sec and mounted with Cytoseal XYL (Richard-Allen Scientific, Kalamazoo, MI). Slides were analyzed using Leica DM2000 microscope and pictures were taken using Leica DFC480 CCD camera with Leica FireCam 1.7.1 software. For each specimen, at least one thousand cells were examined for Daxx expression, and the number of cells with an evident signal were recorded and categorized by the intensity of staining (0 for undetectable, 5 for highest) of Daxx multiplied by the percent of staining cells (Daxx score).
Time lapse microscopy
Time lapse imaging of cells was performed according to (Meraldi et al., 2004). Briefly, control and Daxx-depleted HEp2 cells were stably transfected with GFP-histone H2B (gift of Dr. Duane Compton, Dartmouth) and analyzed by Leica TCS SP5 confocal microscope equipped with environmental chamber; images were taken every 2 min. Mitotic stages were determined by three hallmark events including 1) first indication of chromatin condensation marked as late G2/prophase transition (T=0); 2) invagination of the nucleus marking the prophase/pro-metaphase transition; and 3) beginning of chromosome segregation marking the metaphase/anaphase transition. Three experiments were completed for each shRNA group with an average of 20–30 cells per experiment.
Yeast two-hybrid screen
mDaxx wt was cloned in pGBDC1-Trp1 and transformed in PJ69-4A MATa and tested for self-activation. Screening for Daxx interaction partners was done using pre-transformedi (Y187 yeast strain, MATa) cDNA library from 11 E embryos (library in pACT2 Leu2, Clontech # MY4012AH). Sequence analysis of two strong interaction clones from –His-Leu-Trp plates revealed homology with amino acids 5-270 and 30-270 of mouse RAS associated domain family 1 splice form C (Rassf1C). Retransformation and betta-gal assay confirmed the specificity of this interaction in yeast.
In vitro pull down assay
Daxx constructs were cloned into pGEX-2T or pGEX-4T3 (Invitrogen). Rassf1 contruct were generated in pQE-30 (Qiagen) or pMAL-c2x vectors (New England BioLabs). Constructs were then transformed into a Rosetta strain of E. coli. Protein expression was induced using 50 µM IPTG (Fisher) (pGEX-4T3 constructs), 0.1 mM IPTG (pQE-30 constructs) or 0.3 mM IPTG (pMAL constructs) at room temperature for 3 hrs (pGEX and pMAL) or 18° C overnight (pQE-30). Cells were lysed using buffer consisting of 0.1% Triton X-100, 200 µM phenyl-methyl-sulfonyl fluoride (PMSF) (Calbiochem), 1 µg/mL aprotinin (Sigma), 1 µM leupeptin (Sigma), 1 µM pepstatin (Sigma) and 10 mM 2–mercapto-ethanol (Sigma) in TBS. A GST- and 6X-His pull-down kit (Pierce Biotechnology) was then used to determine binding capability as per the manufacturer’s instructions. Maltose Binding Protein (MBP) and derivative fusion proteins were purified following instructions for pMAL™ protein fusion and purification system (New England BioLabs). Protein samples were analyzed on 4–20% SDS-PAGE gels (Biorad).
Mouse Xenografts
HEp2 xenografts were generated in Nu/Nu mice by subcutaneous injection of 5 × 106 HEp2 cells containing a 1:1 mixture of matrigel (BD Bioscience)/DMEM suspension. Tumors were monitored daily and grown to a volume of approximately 150 mm3 (day 7–9 after cell injection) before drug treatment. Vehicle (1 part of 1:1 solution of 50% EtOH/Cremaphor EL (Sigma) to 10 parts of PBS) or 20 mg/kg taxol (LC Laboratories, Woburn, MA; Paclitaxel stock = 25 mg/mL dissolved in EtOH/Cremaphor EL) was injected intraperitonealy (IP) every second day for a total of five injections. Up to fifteen animals were used for each experimental group. Tumor volume was measured by calipers and calculated on a daily basis using the formula V= (1/6) πa(b)2 where (a) and (b) are the measured length and width (millimeters) of the tumor, respectively. Increases or reductions in tumor size were determined according to the relative initial tumor size beginning on day one of injection. Experiment was terminated at day 15 of first drug injection or when tumor volume reaches 1000 mm3.
shRNA
HEp2 cells were transduced with recombinant lentivirus supernatants encoding hairpin siRNA for hDaxx, hRassf1A and control expression constructs in the presence of 4 ug/ml polybrene. The lentiviral expression system was provided by Peter M. Chumakov (Lerner Research Institute, Cleveland (Sablina et al., 2005). This lentiviral system comprises a targeting envelope expression vector pCMV-VSV-G, a generic packaging expression vector pCMV-deltaR8.2 and the expression cassette for custom siRNA pLSL-GFP that contains a minimal histone H4 promoter that drives transcription of a GFP gene allowing fluorescent cell sorting. Candidate siRNAs for Daxx and Rassf1A were designed according to the Dharmacon siDESIGN algorithm (http://www.dharmacon.com/sidesign/). Anti-Daxx siRNA 1 was targeted against base pairs 1552-1570 of hDaxx (CTACAGATCTCCAATGAAA); anti-Daxx siRNA 2 was targeted against base pairs 100-118 of hDaxx (GATGAAGCAGCTGCTCAGC) anti-Rassf1A si1: targets 282-300 bp of of AF132675 (hRassf1A) (TGCGCGCATTGCAAGTTCA); control siRNA was directed against base pairs 1262-1284 of SETDB1 (TCCTCTTTCTTATCCTCGTATGT).
Western blot analysis
Protein samples were separated by 4–20% SDS-PAGE (Biorad), transferred to nitrocellulose membranes (Watman) and blocked with 3% non-fat milk/PBS, 0.1% Tween (PBS-T). Primary antibodies to Daxx 677 rabbit (in house produced), Rassf1A (ab23950, Abcam), actin (A 5316, Sigma), Maltose Binding Protein (E8032S, New England BioLabs), Glutathione-S-transferase (G 1160, Sigma), His-G (46-1008, Invitrogen) cyclin B1 (SC-245, Santa Cruz), Cdc20 (SC-8358, Santa Cruz), (Cdc27 SC-9972, Santa Cruz), Mad2 (SC-47747, Santa Cruz), GFP (Living Colors A.v. peptide Antibody: 632377, Clontech), Rassf1 (gift of Dr. Gerd Pfeifer) or HP1-alpha (gift of Dr. Frank Rauscher) were diluted in 3% milk/PBST and incubated overnight at 4°C. Membranes were then washed 3X with PBST for 1 hr at RT with appropriate secondary antibody (Chemicon; all 1:2500). Membranes were then washed with PBST and exposed using ECL reagent (Amersham). Densitometry analysis of cyclin B and actin western blots was performed using the Quantity One software from Bio-Rad (Hercules, CA, USA).
APC assay
Cellular pellets were resuspended in lysis buffer (20mM Tris-HCl, pH 7.2, 2mM DTT, 0.25mM EDTA, 5mM KCl, 5mM MgCl2) on ice and subjected to 1500psi N2 in a nitrogen disruption chamber. The lysate was spun for 15min at 15, 000g. Supernatants were divided into single use aliquots and flash frozen in N2. For assays, extracts, on ice, were supplemented with an energy regenerating system (30U/ml rabbit creatine phosphokinase type I, 7.5mM creatine phosphate, 1mM ATP, 1mM MgCl2, 0.1mM EGTA), non-destructible cyclin B, and cycloheximide. Proteins were then added in a final volume of 14ml. 35S-labeled substrate (1ml) was added; aliquots were made and shifted to 30°C. Samples were quenched at the indicated times by the addition of sample buffer, resolved by SDS-PAGE and imaged using a Typhoon phosphorimager (GE Healthcare).
Results
Duration of Mitotic Stages are Affected in the Absence of Daxx
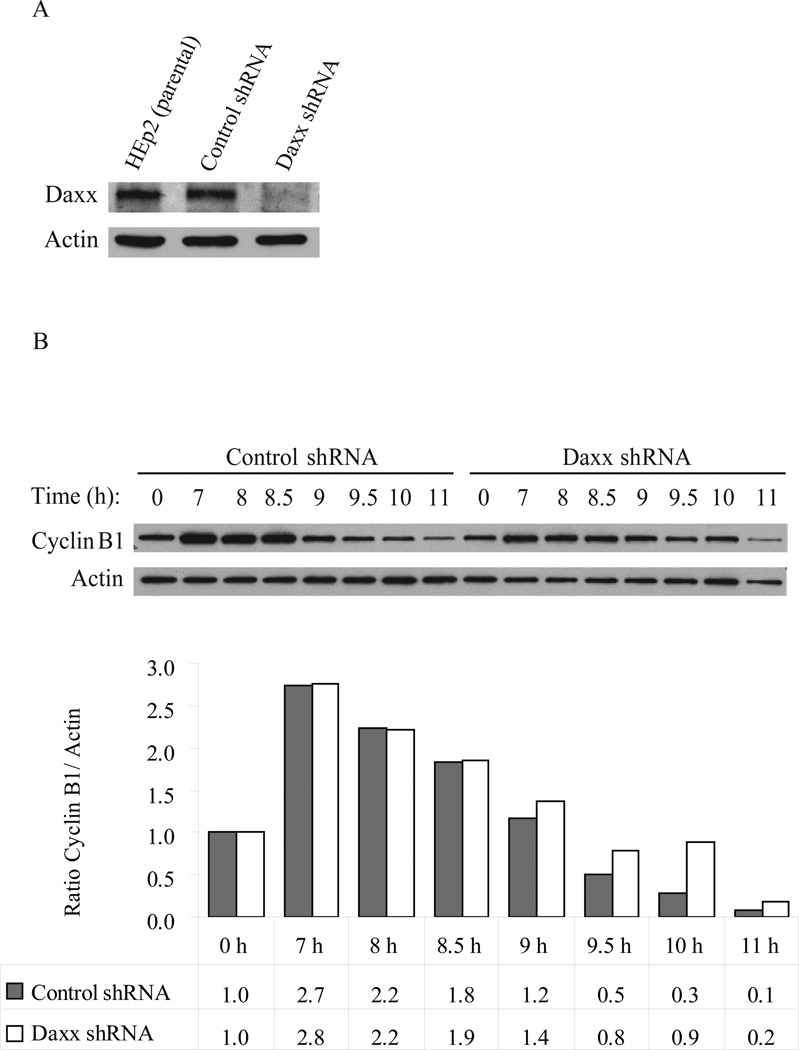
Resistance to taxol was observed in human breast cancer and human larynx carcinoma HEp2 cells with experimentally reduced Daxx (Lindsay et al., 2007). To understand the function of Daxx in taxol response we utilized HEp2 cells expressing control or anti-Daxx shRNAs (Fig. 1A). We used this model cell line since taxane-based therapy is one of treatment options in head and neck cancer (De Mulder, 1999) and given the ability of HEp2 cells to recapitulate the Daxx-dependent taxol response observed in breast cancer cell lines as previously shown (Lindsay et al., 2007). HEp2 cells were synchronized using a double thymidine block and released for cyclin B protein level analysis to monitor G2/M/G1 progression. While control-shRNA cells showed destruction of cyclin B by 9 hrs post-thymidine release, Daxx-depleted cells showed prolonged stabilization of cyclin B at 9.5–11 hrs post-release, suggesting Daxx is required for normal mitosis progression (Figure 1B).
Figure 1. Daxx-dependent stability of cyclin B.
A) Western blot analysis of Daxx depletion in HEp2 cells. B) Control- and Daxx-depleted HEp2 cells were synchronized by a double thymidine block (0 hr) and then released into normal media for progression through mitosis (6–11 hrs). Top: Western blot analysis of cyclin B protein stability. Bottom: Relative quantization of cyclin B protein levels (normalized to actin). Cyclin B protein is stabilized longer in Daxx-depleted cells (at 9.5–11 hrs, post-thymidine release), indicating that Daxx-depleted cells are delayed in mitosis. Data show a representative experiment out of four.
Next, we studied mitotic progression by time-lapse microscopy in control- and Daxx-depleted cells stably transfected with histone H2B-GFP; results are summarized in Table 1. The occurrence of chromatin condensation in Daxx-depleted cells was more rapid, indicating faster progression of prophase compared to control cells (Daxx shRNA cells has average 7.5 min, control shRNA average 10.2 min). Contrary, the average prometaphase/metaphase timing of Daxx-depleted cells (37.6 min) was longer than in control-depleted cells (average 31.2 min). No differences in mitotic progression were observed in control shRNA compared to parental HEp2 cells (data not shown). The combination of these data suggests that depletion of Daxx in human cells results in perturbation of normal mitosis implying that Daxx is necessary for proper mitotic progression.
Table 1. Depletion of Daxx influences mitosis stages.
Control- and Daxx-depleted HEp2 cells were stably transfected with H2B-GFP and analyzed using fluorescence time-lapse video microscopy. Duration of mitotic stages was calculated based on key morphological events associated with chromatin. Average (mean), standard deviation and minimum/maximum values were determined for duration of prophase and pro-metaphase/metaphase.
| Stage | shRNA | Average | Standard Deviation | Min | Max |
|---|---|---|---|---|---|
| Prophase timing | Control | 10.2 min | 2.3 min | 6 min | 18 min |
| Daxx | 7.5 min | 2.64 min | 2 min | 14 min | |
| Prometaphase-anaphase timing | Control | 31.2 min | 7.9 min | 22 min | 58 min |
| Daxx | 37.6 min | 10.36 min | 22 min | 78 min | |
During interphase, Daxx is a predominately nuclear protein (Lindsay et al., 2009) accumulating at ND10/PML nuclear bodies (PML NBs) (Ishov et al., 1999), while conversely, taxol sensitivity is mostly affected by mitotic-related proteins; thus, we analyzing Daxx distribution throughout the cell cycle by immunofluorescent staining. We found Daxx to localize predominately in nuclei at PML NBs during interphase in MEFs (Fig. S1, upper cells in both rows); however, the distribution of Daxx at PML NBs began to change during early pro-metaphase, when the majority of Daxx is still associated with PML NBs, and also begins to localize at spindle-like pattern (Fig S1, upper row). By late pro-metaphase, the majority of Daxx is absent from PML NBs, but remains at a spindle-like pattern (Fig S1, bottom row).
Cell Cycle-Dependent Localization of Daxx and Rassf1
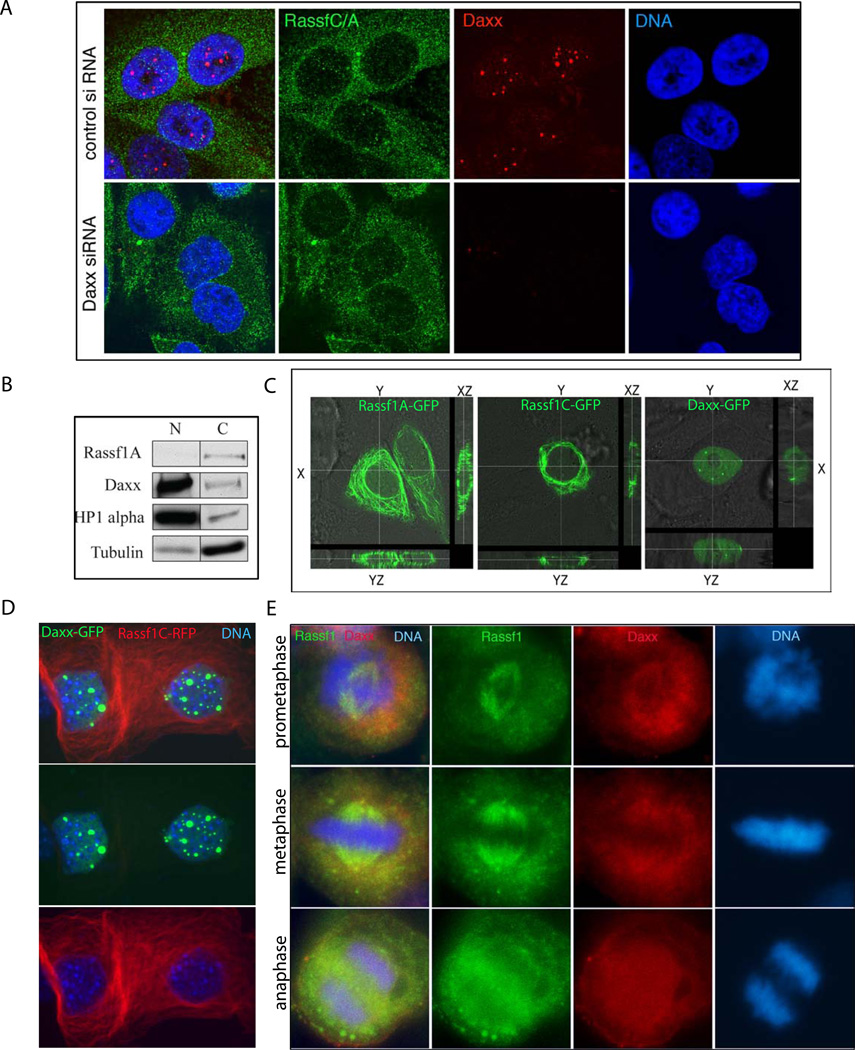
We performed a yeast two-hybrid screen to identify interacting partners that could determine Daxx-mediated mitosis progression and taxol sensitivity. Two clones corresponding to RAS associated domain family 1 splice form C (Rassf1C) were identified using this method, which mapped to aa 5–270 and aa 30–270 of the mouse Rassf1C polypeptide (Fig. S2). Re-transformation and beta-gal assay confirmed interaction of Daxx and Rassf1C clones in yeast (data not shown). Rassf1C and Rassf1A are two major isoforms of tumor suppressor protein Rassf1; Rassf1A has been extensively studied in regard of cell cycle progression, mitosis and apoptosis (reviewed in (Agathanggelou et al., 2005), while Rassf1C biology is much less understood. Previous reports addressed the interaction and function of Daxx and Rassf1. The first study described Rassf1C as a nuclear protein with Daxx-dependent accumulation at PML NBs during interphase (Kitagawa et al., 2006). Upon degradation of Daxx, the authors found Rassf1C to translocate into the cytoplasm where it localized to microtubules. The other report suggests that Daxx interacts with Rassf1A, although the dynamic of localization is much less understood (Song et al., 2008). We found that endogenous Rassf1A and Rassf1C are strictly cytoplasmic, while endogenous Daxx is a nuclear protein associated mostly with PML NBs (Fig. 2A). In addition, the depletion of Daxx did not affect the distribution of Rassf1C/A as was previously reported in (Kitagawa et al., 2006): it has same cytoplasmic distribution upon Daxx depletion (Fig. 2A, bottom row) as in control shRNA expressing cells (Fig. 2A, top row). Biochemical fractionation of HEp2 cells into nuclear and cytosolic fractions confirmed that endogenous Rassf1 was strictly segregated into tubulin-containing (cytosolic) fractions, while endogenous Daxx was predominately associated with HP1-alpha containing nuclear fractions (Fig. 2B) supporting previous data showing predominately nuclear association of Daxx (Lindsay et al., 2009). Confocal imaging of transiently expressed GFP-Rassf1A and GFP-Rassf1C in HEp2 cells also revealed an exclusively cytoplasmic distribution of these proteins, while GFP-Daxx localization was, in contrast, predominately nuclear (Fig. 2C); same results were obtained upon double-transfection of Daxx-GFP and Rassf1C-RFP (Fig. 2D). In summary, Daxx and Rassf1 are compartmentally separated proteins during interphase: Daxx is associated with PML-NBs in the nucleus, and both Rassf1 isoforms are cytoplasmic. These data confirm many previous findings of cytosolic deposition of Rassf1; Rassf1A and Rassf1C has been shown to bind tubulin, and influence microtubule dynamics (reviewed in (Agathanggelou et al., 2005).
Figure 2. Cell cycle dependent localization of Rassf1 C/A and Daxx.
A) Rassf1C/A and Daxx localization during interphase. Control- (top) and Daxx-depleted (bottom) HEp2 cells were immunostained for Rassf1C/A (green) and Daxx (red); DNA (blue) for nuclear visualization. Note nuclear localization of Daxx in PML NBs (top). Cytoplasmic localization of Rassf1C/A is unaltered upon Daxx depletion (bottom). B) Biochemical fractionation of cells shows differential localization of Daxx/Rassf1 proteins. HEp2 cells were separated into nuclear (N) and cytosolic (C) fractions. Daxx is found in HP1-alpha containing nuclear fractions, while Rassf1 is found in tubulin-containing cytoplasmic fractions. C) Distribution of GFP-Rassf1A, GFP-Rassf1C and GFP-Daxx in HEp2 cells. GFP-Rassf1A (left), -Rassf1C (middle) and -Daxx (right) were transiently transfected into HEp2 cells and then analyzed by confocal microscopy. Note that distribution of both Rassf1 isoforms (Rassf1A and Rassf1C) is exclusively cytoplasmic (compare XY, XZ and YZ planes), while, in contrast, the distribution of Daxx is exclusively nuclear. D) Distribution of Daxx-GFP and Rassf1C-RFP upon double transfection in HEp2 cells. Daxx-GFP is nuclear mostly accumulating in domains (PML NBs), while Rassf1C is cytoplasmic. E) Partial co-localization of Daxx and Rassf1 during mitotic stages. HEp2 cells were immunostained with Rassf1C/A ab (green) and Daxx (red).
Being that Daxx and Rassf1 are compartmentally separated proteins during interphase, we sought to understand the dynamics of this interaction in cells by first analyzing the distribution of Daxx and Rassf1 throughout the cell cycle. Previous reports have shown that Rassf1 is a microtubule-associated protein that associates with the spindle apparatus during mitosis ((Dallol et al., 2004; Liu et al., 2003); (Rong et al., 2004) and we reasoned that the interaction between Daxx and Rassf1 may occur primarily at this time. To address this possibility, we immunostained HEp2 cells with Daxx and Rassf1 antibodies; partial co-localization between Daxx and Rassf1 was observed during pro-metaphase and metaphase (Fig. 2E), suggesting that the primary stage of cell cycle for Daxx and Rassf1 co-localization and potential interaction is mitosis and not interphase.
Mapping of Daxx/Rassf1 interaction
As implied from yeast two-hybrid data (Fig. S2), the interaction between Daxx and Rassf1 occurs between the Rassf1C isoform and Daxx. We confirmed this interaction by in vitro pull-down assay (Fig. S3A). We next mapped the regions of interaction between Daxx, Rassf1C and Rassf1A using full length or truncation mutants of these molecules in in vitro pull-down assay.
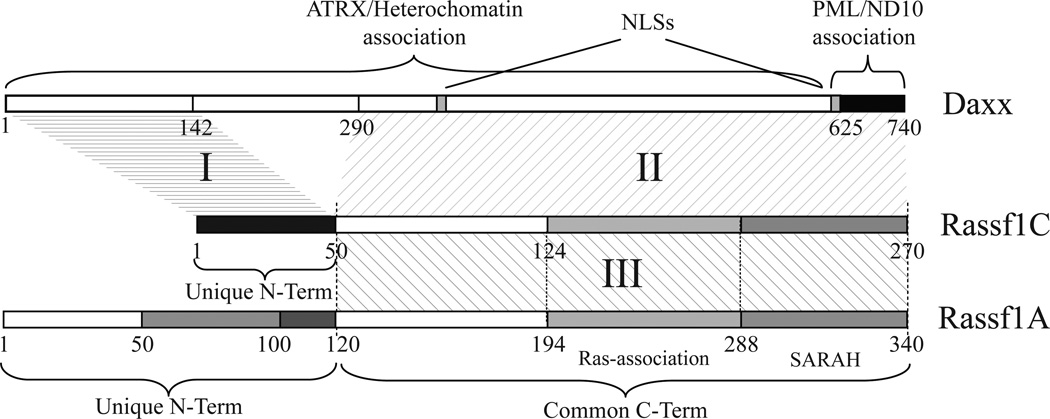
Human Daxx is a 740 aa protein, while Rassf1A and Rassf1C are 340 aa and 270 aa proteins, respectively (Fig. 3). Rassf1A and Rassf1C share a common 220 aa carboxyl-terminal peptide sequence that includes the microtubule binding domain and Ras-association domain, while their amino-terminal regions are unique: 120 aa for Rassf1A and 50 aa for Rassf1C.
Figure 3. Mapping Daxx and Rassf1 regions of interaction.
Diagram depicting human Daxx, Rassf1A and Rassf1C and their mutual regions of interaction summarizing results obtained by in vitro pull down assay (Fig. S3). The fist region of interaction (I) was identified between Daxx first 142 aa and Rassf1C unique N-terminus. The second region of interaction (II) is mapped between Daxx C-terminus (Daxx 290-740) and Rassf1C/A common region. The third region (III) involves the common region of Rassf1A and Rassf1C indicating that the two molecules may homo- or hetero-dimerize via their common region.
Analysis of several truncation mutants of Daxx and Rassf1C reveals two strong regions of interaction between these proteins. The first and minimal Rassf1C-interacting region localizes among the amino terminal 142 aa, while the second one is mapped between aa 290 and 740 of Daxx (I and II in Fig 3 and S3B, correspondingly). Both regions are able to bind independently to Rassf1C. The N-terminus of Daxx (Daxx1-142) binds to the unique 50 aa amino-terminal region of Rassf1C (Fig. S3B top panel “I”). Daxx C-terminal region (Daxx290-740) interacts with Rassf1C full length and with Rassf1C/A common region (Rassf1C51-270) (Fig. S3B central panel, “II”). We did not observe any specific or significant interaction of Daxx290-740 with either of the two unique N-terminal regions of Rassf1C and Rassf1A (Fig. S3B, central panel, “II”). We observed interaction between Rassf1 isoforms (III, Figs. 3 and S3B, bottom panel), suggesting formation of homo- and heterodimers of Rassf1A/C. Thus, by in vitro pull down assay we characterized two main regions of interaction between Rassf1 and Daxx and identified Rassf1A/C dimerization region (I, II, and III in Fig. 3). Region I was extensively mapped by NMR spectroscopy: interaction domain of Daxx forms a left-handed unique four-helix bundle, that binds to the amino-terminal residues of Rassf1C (Escobar-Cabrera et al., 2010).
Daxx and Rassf1-Depleted Cells are Resistant to Taxol Treatment
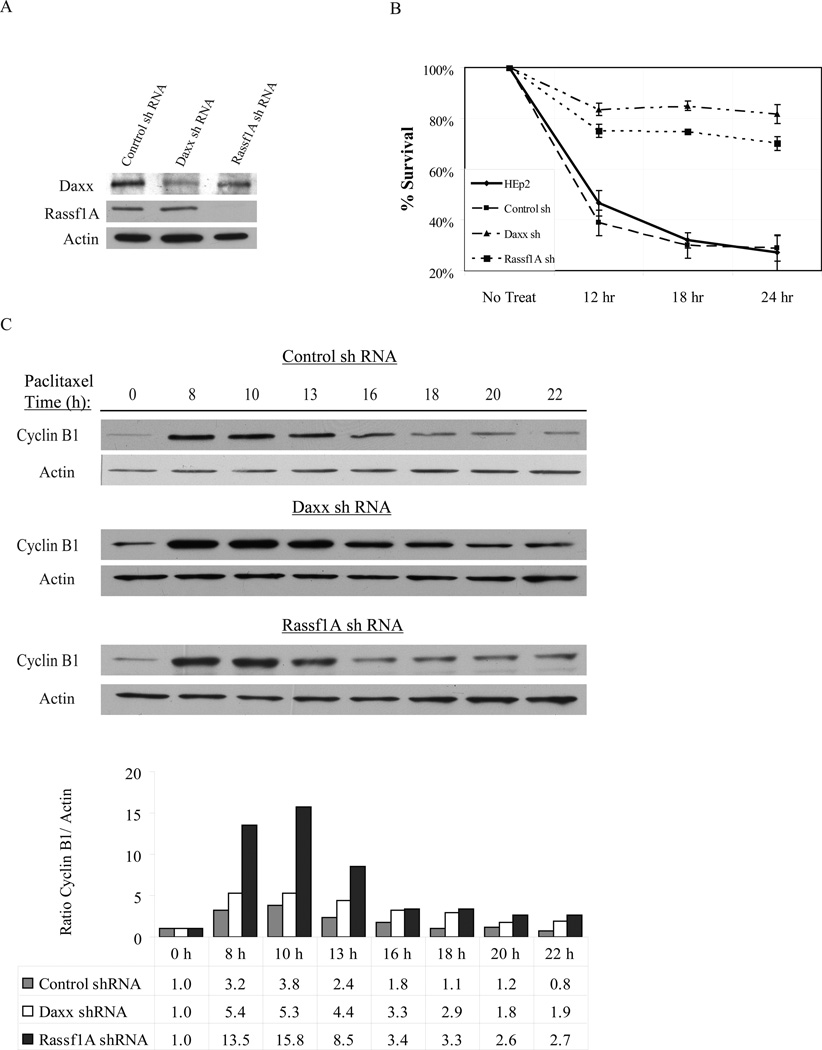
To understand the functionality of the cell cycle-dependent interaction of Daxx and Rassf1 upon taxol treatment, we produced HEp2 cells with stable expression of Rassf1A-shRNA (Fig. 4A). Cells expressing control shRNA, anti-Daxx shRNA, anti-Rassf1A siRNA and parental HEp2 cells were exposed to 10 nM taxol for 12, 18 and 24 hrs and then re-plated for colony formation assay. Daxx- and Rassf1A-depleted cells exhibited a strong taxol-resistant phenotype with the majority of treated cells (75–80%) capable of dividing and forming colonies after removal of taxol (Fig. 4B).
Figure 4. Depletion of Daxx or Rassf1 increases taxol resistance and prolongs Cyclin B stability.
A) Western blot analysis of Daxx and Rassf1A depletion in HEp2 cells. B) Colony formation assay of parental, control- Daxx- and Rassf1A-depleted cells exposed to 10 nM taxol for 12, 18, and 24 hrs after release from double thymidine block. Rapid decrease in survival is seen in parental and control cells, while Daxx- and Rassf1A-depleted cells withstood taxol treatment and produced colonies. C) Western blot analysis of cyclin B protein stability in HEp2 control-, Daxx- and Rassf1A-siRNA cell lines treated with taxol for the indicated amount of time (6–22 hrs). Cells were synchronized using a double thymidine block and released (0 hr) into normal media containing 10 nM taxol. The bottom panel: densitometry analysis of cyclin B normalized by actin; for each cell line the cyclin B/actin ratio at 0 hr set as 1.0. Whereas cyclin B protein levels rapidly decrease by 13 hrs post-thymidine release in control shRNA cells, cyclin B protein levels were stabilized longer in Daxx- and Rassf1A-depleted cells (through 22 hrs, post-thymidine release), indicating Daxx and Rassf1A-depletion prolongs exit from mitosis in response to taxol exposure. Data show a representative experiment out of three.
Cyclin B is Stabilized in Daxx- and Rassf1-Depleted Cells Treated with Taxol
Cells with reduced Daxx display increased resistance to taxol treatment because the majority of cells arrest in mitosis for longer period of time (and thus are able to complete normal division upon taxol wash-out), while control cells exit mitosis towards micro-nucleated cells (and stop proliferation). A similar effect was previously observed upon cell exposure to low or high taxol concentrations (Lindsay et al., 2007). We sought to understand the taxol resistance phenomenon more in depth by analyzing the cyclin B levels in synchronized control, Daxx- and Rassf1A-depleted cells upon 10 nM taxol treatment. Whereas cyclin B protein levels decreased by 13 hrs post-release in control cells, it was stabilized in Daxx- and Rassf1A-depleted cells (Fig. 4C). Stabilized cyclin B upon Daxx or Rassf1A depletion is a biochemical indication of cells arrested in mitosis, while control cells exit mitosis by micronucleation, as confirmed morphologically for Daxx-depleted cells (Lindsay et al., 2007).
Expression Daxx Binding Motif of Rassf1C Increases Taxol Resistance
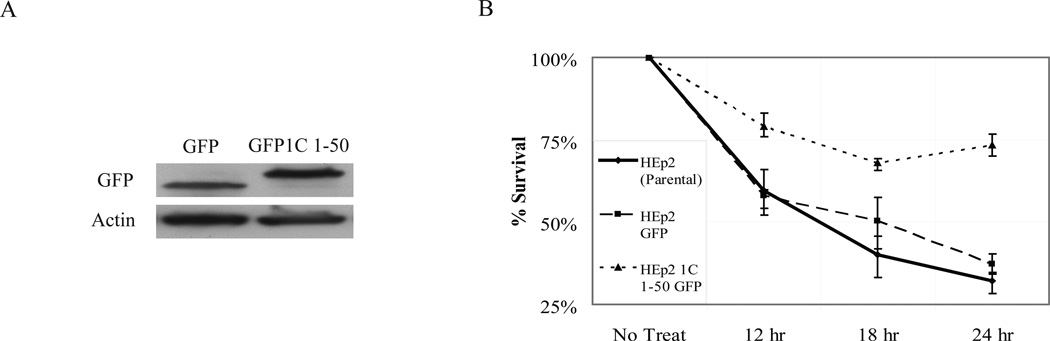
To directly probe the functionality of Daxx-Rassf1 interaction in relation to taxol exposure, we used the interaction mapping information described in Fig. 3 and S3B. We designed a minimal Rassf1C mutant for stable over-expression in cells that 1) retains its Daxx-binding ability and 2) potentially interferes with Daxx-Rassf1C interaction, perturbing taxol sensitization. We expressed GFP-Rassf1C aa 1-50 and GFP in HEp2 cells (Figure 5A for expression) and then treated these stable-expressing cell lines along with parental HEp2 cells with 10 nM taxol and re-plated them for colony formation assay. In the case of the GFP-Rassf1C 1-50 cell line, we observed an overall increase survival after taxol exposure compared to controls (Fig. 5B). This suggested that Rassf1C 1-50-expressing cells could survive taxol treatment due to prevention of mitotic slippage towards micronucleation, brought upon by potential disruption of Daxx-Rassf1C binding.
Figure 5. Expression of Daxx binding motif of Rassf1C leads to increased taxol resistance.
A) Western blot characterization of GFP and GFP-Rassf1C 1-50 aa expressing HEp2 cell lines. B) Expression of Rassf1C 1-50 (but not GFP alone) leads to increased taxol resistance (treatment with 10 nM taxol for indicated time) relative to parental HEp2 cells by colony formation assay.
Analysis of Mitosis-related Proteins upon Daxx depletion
Daxx exhibits transcription repression activity (reviewed in (Lindsay et al., 2008)); thus it could potentially regulate mitotic progression and taxol sensitivity repressing mitotic checkpoint proteins. In this regard, Daxx depletion does not change accumulation of several mitosis-related proteins including Cdc27, Cdc20, and Mad2 (Fig. S4A). No cell cycle specific changes of Daxx protein level were observed either (Fig. S4B). The combination of these data may suggest that Daxx-mediated taxol sensitivity is independent of the previously reported transcription repression activity of Daxx, at least for tested SAC-related proteins, and more likely is dependent upon the function of a Daxx/Rassf1-specific interaction.
Rassf1A or Daxx are not required for activation of the anaphase promotion complex (APC) in vitro
To determine whether Rassf1A or Daxx were required for the activity of mitotic E3 ubiquitine ligase APC/C or release from the spindle checkpoint, we utilized an in vitro system using mitotic extracts, which recapitulates both of these activities (Summers et al., 2008). APC activity was determined by monitoring the destruction of radio-labeled Securin which remained stable throughout all extracts derived from cells (control-, Daxx or Rassf1A-depleted) and confirmed an active spindle checkpoint (Fig S5A). During the incubation, extracts undergo a slow spontaneous release from spindle checkpoint-mediated inhibition. The loss of neither Rassf1A nor Daxx did not delay the kinetics of this release, suggesting that they are not required for direct activation of the APC upon checkpoint silencing. We tested this idea directly, by asking whether the Mad2 antagonist, p31Comet, was able to induce APC activity toward Securin in these extracts. Addition of p31Comet to control as well as Rassf1A or Daxx deficient mitotic extracts (produced from mitotic cells in either nocodazole or taxol block) resulted in equal activation of the APC and subsequent destruction of Securin (Fig. S5B) while addition of recombinant Rassf1 and/or Daxx proteins did not induce activation of the APC (data not shown). Taken together, these results imply that the prolonged mitotic arrest observed upon manipulation of the Rassf1-Daxx complex is not due to an inability to activate the APC, at least in vitro. However, as the mechanism(s) of spindle checkpoint silencing/release are poorly understood, we cannot exclude that this complex participates in an upstream event that is not recapitulated in our in vitro settings.
Daxx and Rassf1-Dependent Tumor Response to Taxol
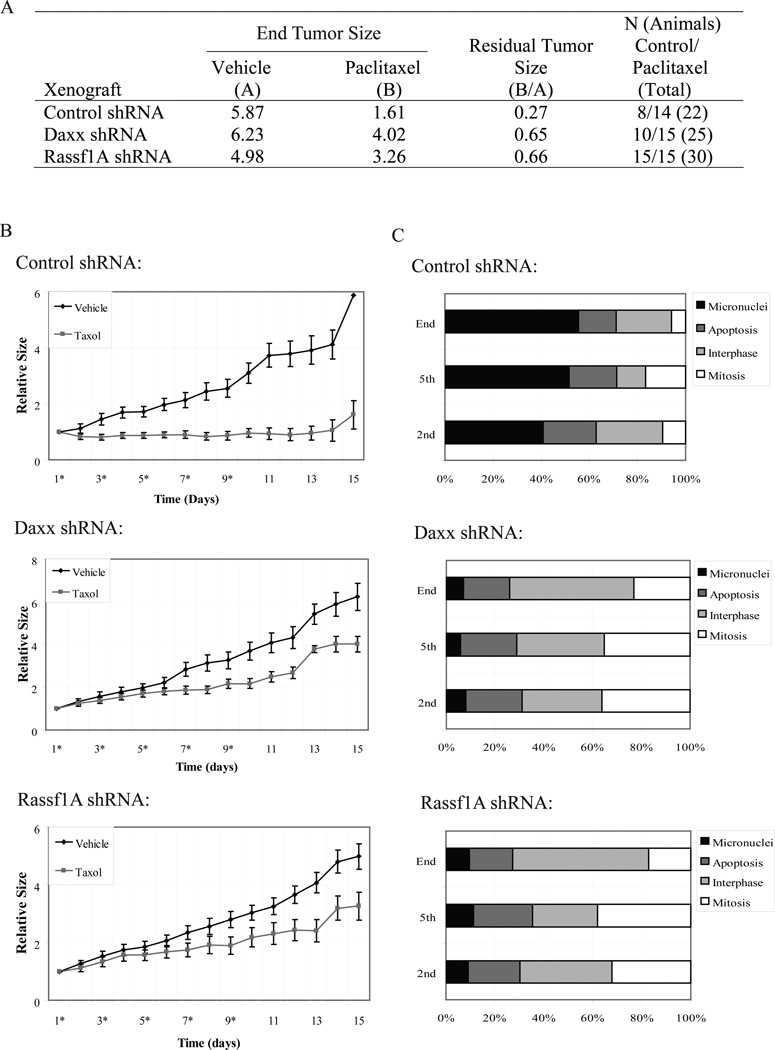
Next, we sought to understand the importance of Daxx and Rassf1A in tumor response to taxol using neoplasm generated by a xenograft system. Taxane-based therapy is one of treatment options in head and neck cancer (De Mulder, 1999); therefore, we assessed the anti-neoplastic activity of taxol exerted on tumors generated from control-, Daxx- or Rassf1A-depleted HEp2 larynx carcinoma cells. By comparing the daily changes in tumor volume (beginning of treatment at approximately 150 mm3 of tumor) between taxol and vehicle treated groups, we could determine regressions in tumor growth. As human tumor xenografts were administered taxol, response of control neoplasms compared to vehicle was markedly sharper due to the sudden drop in volume even after the first injection of drug. The regression trend was observed further after the 2nd-5th drug administration from day 4–11 (Fig. 6B). This drug response, in contrast, was reduced in Daxx or Rassf1A-depleted xenografts as the sizes of taxol-treated tumors in these groups closely followed that of vehicle-administered tumors. The residual tumor size (calculated as a ratio of sizes between taxol-injected to vehicle-injected tumors at the end of experiment) of Daxx- and Rassf1A-depleted tumors after five administrations of taxol averaged to be 0.65 and 0.66, respectively, while the residual size of control-depleted tumors was much smaller at 0.27 (Fig. 6A). We concluded that tumors generated from Daxx- and Rassf1A-depleted cells had reduced response rate to taxol administration compared to control-depleted tumors.
Figure 6. Depletion of Daxx or Rassf1 increases resistance of experimental tumors to taxol.
Control-, Daxx- or Rassf1A-depleted tumors derived from injection of HEp2 cells into Nu/Nu mice were treated with vehicle or 20mg/kg taxol. A) Table summarizing key statistical data generated from tumors exposed to vehicle or taxol regimens. Relative tumor sizes for vehicle (A) or taxol-treated tumors (B) were recorded at the end of treatment and the relative residual tumor volumes were calculated by dividing the values from (B) over (A). B) Graphs charting the changes in relative tumor size of control-, Daxx- or Rassf1A-depleted xenografts exposed to vehicle or taxol. Asterisks at day number (x axis) denote time of injection of vehicle or taxol (days 1, 3, 5, 7, 9). Note discrepancy in tumor growth in vehicle and taxol treated tumors in control shRNA groups, while the rate of growth in Daxx- and Rassf1A-shRNA groups exposed to taxol have reduced response. C) Treatment response at cellular level. Tumor xenografts were extracted 24 hours following the 2nd or 5th taxol injection or at the end of experiment (day 15) and cellular response was analyzed based on appearance of chromatin (stained for DNA) by light microscopy and characterized as 1) interphase, 2) mitotic, 3) micronucleated, and 4) apoptotic as described previously (Lindsay et al., 2007). Control xenografts exhibited an increased number of micronucleated cells (indication of taxol response), while Daxx and Rassf1A-depleted xenografts show increased numbers of mitotic and interphase cells (indication of taxol resistance and continuous proliferation) and correspondingly less micronuclei; number apoptotic cells is similar in all groups.
We reasoned that the differential taxol response of these tumor groups may, in part, be linked to the cellular outcomes as documented previously (Lindsay et al., 2007): taxol-resistant cells arrest in a prolonged mitotic state with sustained cyclin B protein levels and continue cell division upon drug decay, while non-resistant cells exit mitosis forming micronucleated cells incapable of entering next cycle. To address this possibility, we analyzed cellular morphology at tumor xenografts sections. Based on DNA staining, cells were categorized as 1) interphase, 2) mitosis, 3) apoptosis, and 4) micronuclei (Lindsay et al., 2007). In control shRNA xenografts, we observed an increased number of micronucleated cells (indication of taxol response) after 2nd and 5th injections of taxol, elevating to 50% at the end of treatment. In contrast, the number of interphase and mitotic cells (indication of taxol resistance) in Daxx- and Rassf1 depleted xenografts remained high, while micronuclei were low (less than 10% at the end of treatment), suggesting that cells keep cycling (Fig. 6C). Occurrence of apoptotic cells was similar across all xenografts and increased marginally upon taxol exposure. Thus, in current experimental settings, depletion of Daxx and Rassf1 elevated resistance of experimental tumors to taxol treatment, with majority of cells continuously cycling – while tumors derived from control-depleted cells formed micronuclei and thus stop proliferation.
Daxx Levels Have Reverse Correlation with Taxane Chemotherapy Response in Breast Cancer Patients
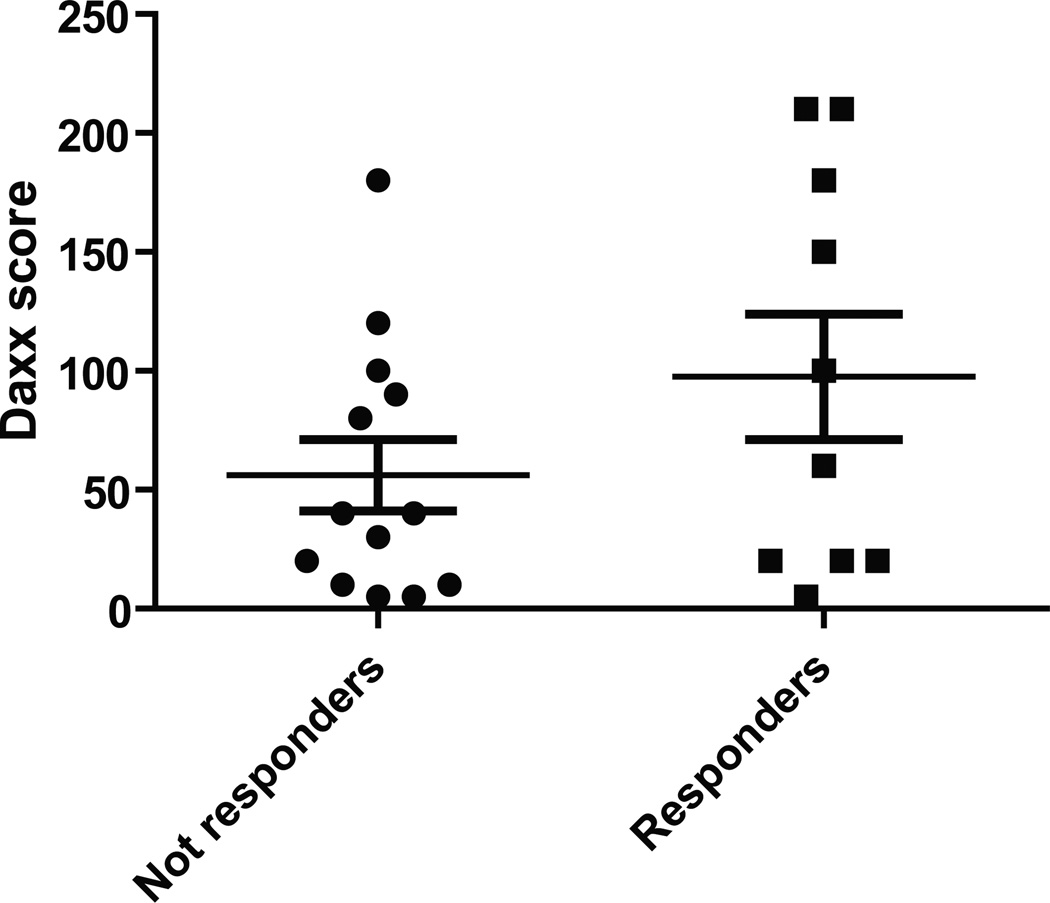
To address the clinical ramifications of Daxx regulation of taxane sensitivity, twenty-two women with locally advanced HER-2 non-amplified breast cancer who were treated with standard taxane and anthracycline based neoadjuvant chemotherapy at H. Lee Moffitt Cancer Center were identified for this study. Patients were classified as either responders or non responders based on the clinical response measured with the longest diameter by physical examination performed by the treating physician at the time of encounter, with responders experiencing >75% reduction. Daxx score was calculated based on the Daxx IHC staining intensity multiplied by the percent of staining cells. Based on the above definition of response, 10 patients were classified as responders and twelve as non-responders. Comparison of pretreatment samples between the responders and non-responders was performed using an independent sample t test. Responders to therapy had a higher mean Daxx score compared to non-responders (Fig. 7; p=0.06). This data suggested that Daxx score could predict the response to neoadjuvant taxane and anthracycline based chemotherapy. The small sample size and the retrospective nature of the study are the main limitation of this finding that will be further validated in a future prospective study.
Figure 7. Daxx levels have reverse correlation with taxane chemotherapy response in breast cancer patients.
Twenty-two women with breast cancer who were treated with standard taxane and anthracycline based neoadjuvant chemotherapy were classified as either responders (up to 75% reduction of tumor size; 10 patients) or non-responders (less than 75% reduction of tumor size; 12 patients). Daxx score was calculated based on the Daxx IHC staining intensity multiplied by the percent of staining cells. Comparison of pretreatment samples between the responders and non-responders was performed using an independent sample t test. Responders to therapy had a higher mean Daxx score compared to non-responders (p=0.06).
Discussion
Taxane chemotherapy is considered among the most responsive treatment options for many cancer patients, either alone or as adjuvant in combination with anthracyclins (O'Shaughnessy, 2005). Nevertheless, large numbers of patients are resistant or become resistant to taxane therapy during treatment. The response rate of docetaxel is ~50% even after the first-line chemotherapy administration and decreases to 20–30% by second- or third-line administration (Bonneterre et al., 1999; Crown et al., 2004). Thus, development of new genomic prognosis factors and in-depth understanding of drug activity on both a cellular and organism levels are needed for optimization of adjuvant therapy and proper patient stratification. Numerous studies have been carried out to determine a genomic profile that could be predictive to taxane treatment (Chang et al., 2003; Chang et al., 2005; Iwao-Koizumi et al., 2005; Mauriac et al., 2005; Miyoshi et al., 2004) while alternative approaches have sought to understand selective resistance to taxanes to decipher mechanisms which regulate responses (Hari et al., 2003a; Hari et al., 2003b); (Wang and Cabral, 2005). Inactivation of mitotic proteins can contribute to the selective response of taxane treatment in vivo (Wassmann and Benezra, 2001). Divergent response to taxol exposure is usually seen in cells deficient of mitotic checkpoint proteins or other regulators of cell division. To date, loss of function of the majority of mitotic proteins, including Mad1, Mad2, Bub1 and BubR1, among others, has shown enhanced response to taxol in cell culture conditions, implying increased output of micronucleation instead of mitotic arrest (Lee et al., 2004); (Carvalho et al., 2003); (Lens et al., 2003). Hence, identification of factors, that increase drug resistance upon inactivation, is largely incomplete or uncharacterized.
To this end, Daxx was verified as a novel regulator of taxol response in cell culture conditions, animal models and primary human tumor specimens. Human breast cancer cells and larynx carcinoma HEp2 cells with experimentally modified levels of Daxx, show reduced responses to taxol as previously described (Lindsay et al., 2007). A mouse xenograft system also recapitulates our initial findings, becoming the first indication that Daxx and its interaction partner Rassf1 could be important for the fate of tumors exposed to taxane based chemotherapy (Fig. 6A, B). We also found that control groups displayed an increased amount of micronucleation upon taxol treatment, which may account for the rapid loss of xenografts tumor volume observed in these regimental settings (Fig. 6C). In contrast, Daxx- and Rassf1A-depleted tumors displayed increased mitotic and interphase index (Fig. 6C), indicating that cells were capable to maintain mitotic block via elevated cyclin B stability (Fig. 4C) and continue proliferation after taxol decay that happens fast in nude mice (Kubota et al., 1997). Thus, mitotic cells from Daxx- and Rassf1A-depleted tumors can potentially reenter G1 after drug decay, providing a working model for how cells or tumors devoid of either of these protein targets can survive chemotherapy treatment and proliferate (Model in Fig S6).
The nature of Daxx function in cells is largely attributed to regulation of apoptosis or transcription. While this functionality is debatable in many circumstances, the prevailing idea of the role of Daxx is that of a modulator or adapter of many cellular functions which are critical to cell vitality (Lindsay et al., 2008). Indeed, Daxx has been found critical and necessary for embryonic development in mice as Daxx−/− embryos exhibit extensive apoptosis and lethality by E11.5 (Michaelson et al., 1999b); (Ishov et al., 2004). Many Daxx-interacting proteins have also been described including the interaction between Daxx and Rassf1C (Kitagawa et al., 2006), (Song et al., 2008). We further characterized this interaction by analyzing the dynamics of cell cycle distribution of Daxx/Rassf1C. Rassf1C (endogenous or transiently expressed, Fig. 2A-D) is exclusively cytoplasmic-associated protein and does not reside at PML bodies during interphase, as previously suggested in (Kitagawa et al., 2006). Moreover, cytolpasmic localization of Rassf1 was unaffected by the presence or absence of Daxx (Fig. 2A). In light of these findings, it is difficult to explain the suggested role of Daxx/Rassf1C interaction in Fas-induced apoptosis (Kitagawa et al., 2006). We demonstrated that Rassf1C is able to bind Daxx by its unique amino-terminal region (I, Fig. 3 and S3B top panel). We also show that Rassf1A and Rassf1C, the two major isoforms of Rassf1 in cells, interact with each other and with Daxx through a common region in their carboxyl-termini by in vitro pull-down assay (III, III, I, Figs. 3 and S3B middle and bottom panel ).
Rassf1A has been implicated as a mitotic regulator and has been shown to interact with several key mitotic-related proteins, including Aurora A (Rong et al., 2007) and Cdc20 (Song et al., 2004), though the latter is debatable (Liu et al., 2007). These findings together with out data indicate that Rassf1A has a pleiotropic effect during mitosis. This may justify the elevated accumulation of Cyclin B observed upon depletion of Rassf1A and taxol exposure (Fig. 4C).
The interaction partners of Rassf1C have until now been limited to Daxx, BetaTrCP, IGFBP-5 (Amaar et al., 2005); (Kitagawa et al., 2006); (Estrabaud et al., 2007) with the cellular functions of Rassf1C, comparatively, being much less studied. Since Rassf1A and Rassf1C have many seemingly unrelated interaction partners, the implications of a Rassf1A-Rassf1C interaction via common protein region suggests a converging point of cellular networks and pathways that may have otherwise been unlinked. It is possible that Daxx, Rassf1A and Rassf1C may form three-party functional mitotic complex where Rassf1C may, at least in some cases, serve as a functional “bridge” between Daxx and Rassf1A as over-expressing a construct with the Daxx-interacting amino-terminal region of Rassf1C (Fig. 5) effectively recapitulates taxol resistance phenotypes seen in Daxx and Rassf1A-depleted cells (Fig. 4B) and xenograft models (Fig. 6).
In addition to function in taxol response, we also report an unexpected role of Daxx in the regulation of mitosis. In the absence of Daxx, the duration of prophase and the prometaphase/metaphase transition is altered (Table 1). Indeed, the stability of cyclin B protein is changed in the absence of Daxx as well (Fig. 1B). This may suggest that the activity of E3 ubiquitin ligase APC is altered in the absence of Daxx. We attempted to study the role of Daxx and Rassf1 as direct regulators of APC using an in vitro assay, but the results were undistinguishable (Fig. S5), suggesting that cellular in vivo mitotic environment is necessary for proper execution of Daxx/Rassf1 function in mitosis. Differential degradation of cyclin B in Daxx-depleted cells could also be due to mis-regulation of cyclin B on a different operational level, namely, the alteration in stability of the APC activator Cdc20 (Nilsson et al., 2008). However, we found no differences in and accumulation of Cdc20 as well as Cdc27 and Mad2 (Fig. S4A). In addition our results indicate that upon Daxx or Rassf1 depletion and taxane exposure both dynamics of degradation and/or accumulation of cyclin B levels are affected. To date, we cannot explain this phenomenon, although it suggests that Daxx or Rassf1-mediated regulation of Cyclin B may occurs at multiple levels. Thus, the detailed mechanism and players underlying the functions of Daxx in mitotic progression remain largely unknown and future studies aimed at unraveling cell cycle-specific roles of Daxx are required.
Evidences presented in this study suggest Daxx and Rassf1 are triggers for cellular taxol sensitivity, that together with the recently reported function of Daxx in the transcription repression of the pro-metastatic tyrosine kinase receptor c-met (Morozov et al., 2008) may further uncover Daxx function in tumor progression. In the future, Daxx and Rassf1 may serve as useful molecular markers for proper selection of cancer patients for taxane chemotherapy. In order to achieve this goal, clinical studies additional to presented here (Fig. 7) will be required examining the status of Daxx and Rassf1 expression in tumors before and after taxane treatment as well as studies in patients with an established history of taxane resistance. Daxx expression vary in breast cancer cell lines (Lindsay et al., 2007), but the mechanism of Daxx down-regulation has largely been unstudied. Rassf1A expression in cancer cell lines, conversely, has been extensively covered and shown to be down-regulated in a majority of cases (Agathanggelou et al., 2005). Our studies have established new roles for Daxx in cell cycle progression—the importance of which may be intensified because of its function as a trigger for taxol sensitization in combination with Rassf1—which adds to our understanding of mechanisms linking cell division, chemotherapy response and cancer progression.
Supplementary Material
Figure S1. Detailed visualization of Daxx redistribution during mitosis. MPEFs were immunostained with anti-Daxx ab’s (green) and anti-PML (marker of PML NB’s/ND10; red); DNA: blue. Big arrowheads mark interphase cells, where Daxx co-localizes with PML at PML NBs. Upper row: in early prophase (bottom cell), Daxx still co-localizes with PML (small arrowhead) and also started to appear at mitotic spindle-like pattern (arrow). Lower row: in late prophase (bottom cell), Daxx does not co-localize with PML (small arrowhead) but is visible at mitotic spindle-like pattern (arrow).
Figure S2. Yeast two-hybrid analysis of Daxx/Rassf1C interaction. S. cerevisae strain Y187 was co-transfected with pACT2-Rassf1C clone 1 or clone 2 and with corresponding pGBD plasmids (scheme, right). Yeast were plated on –LEU-TRP (control, upper row) to check for presence of both plasmids or on –LEU-TRP-HIS (interaction, bottom row) to check for interaction between proteins, including Daxx WT DaxxC, and DaxxDeltaC.
Figure S3. Characterization of Daxx and Rassf1 interaction by in vitro pull down experiments. A) In vitro 6xHis pull down assays demonstrate specificity of Daxx-Rassf1C binding. Western blot analysis of 6xHis pull down assay between GST-Daxx and 6xHis-Rassf1C. Immobilized 6xHis-Rassf1C wt was incubated with either GST or GST-Daxx wt. GST-Daxx but not GST alone binds 6XHis-Rassf1C wt. B) Top panel: Interacting region I. Western blot analysis of MBP pull down assay between GST Daxx 1-142 and MBP-Rassf1C 1-50. Immobilized MBP-Rassf1C 1-50 or MBP were incubated with either GST or GST-Daxx 1-142. GST-Daxx 1-142 binds MBP-Rassf1C 1-50, but not MBP. Central panel: Interacting region II. In similar experimental settings, GST-Daxx 1-290 and GST-Daxx 290-740 retain capacity to bind both MBP-Rassf1C full length (MBP-1C) and Rassf1 common region (MBP- C51-270). While Daxx N-terminal region can bind Rassf1C 1-50, the C-terminal one cannot. Binding of the unique region of Rassf1A (MBP-A 1-120) to all the constructs including GST is considered merely non-specific. Bottom panel: Interacting region III. In vitro 6xHis pull down assay demonstrates specificity of Rassf1A-Rassf1C binding. Western blot analysis of 6xHis pull down assay of GST-Rassf1A wt and truncation mutants using immobilized 6xHis-Rassf1C wt. GST-Rassf1A wt/mutants, GST-Daxx 1-142 (positive control) and GST alone were incubated with 6xHis-Rassf1C wt. GST-Rassf1A wt and deletion mutants 120-340 and 194-340 bind to Rassf1C, while GST alone cannot. An asterisk (*) on the left side of the band indicates the expected molecular weight for the fusion constructs. High mobility bands represent degradation products.
Figure S4. A) Analysis of mitotic checkpoint proteins in control- and Daxx-depleted cell lines. Parental HEp2 cells and cells stably expressing control or two independent anti-Daxx shRNAs were analyzed by Western blot for levels of Cdc27, Cdc20, and Mad2; proteins levels are unchanged. B) Cell cycle-dependent expression of Daxx. HEp2 cells were synchronized using a double thymidine block to arrest cells in the G1/S-boundary and then released and allowed to progress through S, G2, M phase (0–11hr post thymidine release, compare with cyclin B dynamics on Fig. 1B). Daxx protein level showed no significant changes during cell cycle.
Figure S5. Rassf1A and Daxx are not required for in vitro activation of the APC. A) Extracts were generated from nocodazole-arrested cells expressing control-, Rassf1A or Daxx-shRNAs and the stability of the APC substrate Securin, labeled with 35S, was monitored by autoradiography. B) Extracts were generated as in (A) from cells treated with 10 µM nocodazole or 10 nM taxol (paclitaxel). The requirement for Rassf1A and Daxx to activate the APC was tested by addition of the Mad2 antagonist p31 Comet.
Figure S6. Model of Daxx/Rassf-dependent taxol response. At pharmacological concentrations, taxol reversibly inhibits microtubule dynamics blocking cells in prometaphase. Cells that are sensitive to taxol activate mitotic block only transiently, followed by cyclin B proteolysis, micronuclei formation, block of proliferation and cell death, while Daxx/Rassf1 negative cells have a more prolonged prometaphase block due to elevated cyclin B stability; they continue proliferation after drug decay/withdrawal and microtubule dynamics restoration--thus surviving chemotherapy.
Acknowledgments
We want to thank Dr. Gerd Pfeifer, Beckman Research Institute, for the generous gift of anti-Rassf1 antibodies and Dr. Frank Rauscher, The Wistar Institute, for HP1 antibodies. This work was supported by NIH/NCI R01 CA127378-01A1 for CRL, SG, VMM and AMI, by the Canadian Cancer Society for EE and LPM. NMR spectroscopy support was provided by the Canadian Institutes for Health Research (CIHR), the Canadian Foundation for Innovation (CFI), the British Columbia Knowledge Development Fund (BCKDF), the UBC Blusson Fund, and the Michael Smith Foundation for Health Research (MSFHR).
References
- Aapro MS. Neoadjuvant therapy in breast cancer: can we define its role? Oncologist. 2001;6(Suppl 3):36–39. doi: 10.1634/theoncologist.6-suppl_3-36. [DOI] [PubMed] [Google Scholar]
- Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005;65:3497–3508. doi: 10.1158/0008-5472.CAN-04-4088. [DOI] [PubMed] [Google Scholar]
- Amaar YG, Baylink DJ, Mohan S. Ras-association domain family 1 protein, RASSF1C, is an IGFBP-5 binding partner and a potential regulator of osteoblast cell proliferation. J Bone Miner Res. 2005;20:1430–1439. doi: 10.1359/JBMR.050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneterre J, Spielman M, Guastalla JP, Marty M, Viens P, Chollet P, et al. Efficacy and safety of docetaxel (Taxotere) in heavily pretreated advanced breast cancer patients: the French compassionate use programme experience. Eur J Cancer. 1999;35:1431–1439. doi: 10.1016/s0959-8049(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle. 2006;5:1001–1007. doi: 10.4161/cc.5.9.2726. [DOI] [PubMed] [Google Scholar]
- Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Elledge R, et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet. 2003;362:362–369. doi: 10.1016/S0140-6736(03)14023-8. [DOI] [PubMed] [Google Scholar]
- Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Tham YL, et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J Clin Oncol. 2005;23:1169–1177. doi: 10.1200/JCO.2005.03.156. [DOI] [PubMed] [Google Scholar]
- Crown J, O'Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist. 2004;9(Suppl 2):24–32. doi: 10.1634/theoncologist.9-suppl_2-24. [DOI] [PubMed] [Google Scholar]
- Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, et al. RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004;64:4112–4116. doi: 10.1158/0008-5472.CAN-04-0267. [DOI] [PubMed] [Google Scholar]
- De Mulder PH. The chemotherapy of head and neck cancer. Acta Otorhinolaryngol Belg. 1999;53:247–252. [PubMed] [Google Scholar]
- Escobar-Cabrera E, Lau DK, Giovinazzi S, Ishov AM, McIntosh LP. Structural Characterization of the DAXX N-Terminal Helical Bundle Domain and Its Complex with Rassf1C. Structure. 2010;18:1642–1653. doi: 10.1016/j.str.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Estrabaud E, Lassot I, Blot G, Le Rouzic E, Tanchou V, Quemeneur E, et al. RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of beta-catenin by interacting with betaTrCP. Cancer Res. 2007;67:1054–1061. doi: 10.1158/0008-5472.CAN-06-2530. [DOI] [PubMed] [Google Scholar]
- Hari M, Wang Y, Veeraraghavan S, Cabral F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol Cancer Ther. 2003a;2:597–605. [PubMed] [Google Scholar]
- Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III beta-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton. 2003b;56:45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov AM, Vladimirova OV, Maul GG. Heterochromatin and ND10 are cell-cycle regulated and phosphorylation-dependent alternate nuclear sites of the transcription repressor Daxx and SWI/SNF protein ATRX. J Cell Sci. 2004;117:3807–3820. doi: 10.1242/jcs.01230. [DOI] [PubMed] [Google Scholar]
- Iwao-Koizumi K, Matoba R, Ueno N, Kim SJ, Ando A, Miyoshi Y, et al. Prediction of docetaxel response in human breast cancer by gene expression profiling. J Clin Oncol. 2005;23:422–431. doi: 10.1200/JCO.2005.09.078. [DOI] [PubMed] [Google Scholar]
- Kitagawa D, Kajiho H, Negishi T, Ura S, Watanabe T, Wada T, et al. Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation. EMBO J. 2006;25:3286–3297. doi: 10.1038/sj.emboj.7601212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Matsuzaki SW, Hoshiya Y, Watanabe M, Kitajima M, Asanuma F, et al. Antitumor activity of paclitaxel against human breast carcinoma xenografts serially transplanted into nude mice. J Surg Oncol. 1997;64:115–121. doi: 10.1002/(sici)1096-9098(199702)64:2<115::aid-jso5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lee EA, Keutmann MK, Dowling ML, Harris E, Chan G, Kao GD. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther. 2004;3:661–669. [PubMed] [Google Scholar]
- Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, et al. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. Embo J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay CR, Giovinazzi S, Ishov AM. Daxx is a predominately nuclear protein that does not translocate to the cytoplasm in response to cell stress. Cell Cycle. 2009;8 doi: 10.4161/cc.8.10.8379. [DOI] [PubMed] [Google Scholar]
- Lindsay CR, Morozov VM, Ishov AM. PML NBs (ND10) and Daxx: from nuclear structure to protein function. Front Biosci. 2008;13:7132–7142. doi: 10.2741/3216. [DOI] [PubMed] [Google Scholar]
- Lindsay CR, Scholz A, Morozov VM, Ishov AM. Daxx shortens mitotic arrest caused by paclitaxel. Cell Cycle. 2007;6:1200–1204. doi: 10.4161/cc.6.10.4244. [DOI] [PubMed] [Google Scholar]
- Liu L, Baier K, Dammann R, Pfeifer GP. The tumor suppressor RASSF1A does not interact with Cdc20, an activator of the anaphase-promoting complex. Cell Cycle. 2007;6:1663–1665. doi: 10.4161/cc.6.13.4435. [DOI] [PubMed] [Google Scholar]
- Liu L, Tommasi S, Lee DH, Dammann R, Pfeifer GP. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene. 2003;22:8125–8136. doi: 10.1038/sj.onc.1206984. [DOI] [PubMed] [Google Scholar]
- Mantel C, Guo Y, Lee MR, Han MK, Rhorabough S, Kim KS, et al. Cells enter a unique intermediate 4N stage, not 4N-G1, after aborted mitosis. Cell Cycle. 2008;7:484–492. doi: 10.4161/cc.7.4.5316. [DOI] [PubMed] [Google Scholar]
- Mauriac L, Debled M, MacGrogan G. When will more useful predictive factors be ready for use? Breast. 2005;14:617–623. doi: 10.1016/j.breast.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Michaelson JS. The Daxx enigma. Apoptosis. 2000;5:217–220. doi: 10.1023/a:1009696227420. [DOI] [PubMed] [Google Scholar]
- Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 1999a;13:1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development [In Process Citation] Genes Dev. 1999b;13:1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Kim SJ, Akazawa K, Kamigaki S, Ueda S, Yanagisawa T, et al. Down-regulation of intratumoral aromatase messenger RNA levels by docetaxel in human breast cancers. Clin Cancer Res. 2004;10:8163–8169. doi: 10.1158/1078-0432.CCR-04-1310. [DOI] [PubMed] [Google Scholar]
- Morozov VM, Massoll NA, Vladimirova OV, Maul GG, Ishov AM. Regulation of c-met expression by transcription repressor Daxx. Oncogene. 2008;27:2177–2186. doi: 10.1038/sj.onc.1210865. [DOI] [PubMed] [Google Scholar]
- Niikura Y, Dixit A, Scott R, Perkins G, Kitagawa K. BUB1 mediation of caspase-independent mitotic death determines cell fate. J Cell Biol. 2007;178:283–296. doi: 10.1083/jcb.200702134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- Ravdin P, Erban J, Overmoyer B. Phase III comparison of docetaxel and paclitaxel in patients with metastatic breast cancer. Eur J Cancer. 2003;32(suppl1) [Google Scholar]
- Rong R, Jiang LY, Sheikh MS, Huang Y. Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene. 2007;26:7700–7708. doi: 10.1038/sj.onc.1210575. [DOI] [PubMed] [Google Scholar]
- Rong R, Jin W, Zhang J, Sheikh MS, Huang Y. Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest. Oncogene. 2004;23:8216–8230. doi: 10.1038/sj.onc.1207901. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffert RT, Kalejta RF. Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both? Future Virol. 2008;3:265–277. doi: 10.2217/17460794.3.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol. 2006;16:97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Scolnick DM, Halazonetis TD. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406:430–435. doi: 10.1038/35019108. [DOI] [PubMed] [Google Scholar]
- Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol. 2004;6:129–137. doi: 10.1038/ncb1091. [DOI] [PubMed] [Google Scholar]
- Song MS, Song SJ, Kim SY, Oh HJ, Lim DS. The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex. EMBO J. 2008;27:1863–1874. doi: 10.1038/emboj.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- Summers MK, Pan B, Mukhyala K, Jackson PK. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell. 2008;31:544–556. doi: 10.1016/j.molcel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cabral F. Paclitaxel resistance in cells with reduced beta-tubulin. Biochim Biophys Acta. 2005;1744:245–255. doi: 10.1016/j.bbamcr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr Opin Genet Dev. 2001;11:83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- Wysong DR, Chakravarty A, Hoar K, Ecsedy JA. The inhibition of Aurora A abrogates the mitotic delay induced by microtubule perturbing agents. Cell Cycle. 2009;8:876–888. doi: 10.4161/cc.8.6.7897. [DOI] [PubMed] [Google Scholar]
- Xia G, Luo X, Habu T, Rizo J, Matsumoto T, Yu H. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. Embo J. 2004;23:3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Detailed visualization of Daxx redistribution during mitosis. MPEFs were immunostained with anti-Daxx ab’s (green) and anti-PML (marker of PML NB’s/ND10; red); DNA: blue. Big arrowheads mark interphase cells, where Daxx co-localizes with PML at PML NBs. Upper row: in early prophase (bottom cell), Daxx still co-localizes with PML (small arrowhead) and also started to appear at mitotic spindle-like pattern (arrow). Lower row: in late prophase (bottom cell), Daxx does not co-localize with PML (small arrowhead) but is visible at mitotic spindle-like pattern (arrow).
Figure S2. Yeast two-hybrid analysis of Daxx/Rassf1C interaction. S. cerevisae strain Y187 was co-transfected with pACT2-Rassf1C clone 1 or clone 2 and with corresponding pGBD plasmids (scheme, right). Yeast were plated on –LEU-TRP (control, upper row) to check for presence of both plasmids or on –LEU-TRP-HIS (interaction, bottom row) to check for interaction between proteins, including Daxx WT DaxxC, and DaxxDeltaC.
Figure S3. Characterization of Daxx and Rassf1 interaction by in vitro pull down experiments. A) In vitro 6xHis pull down assays demonstrate specificity of Daxx-Rassf1C binding. Western blot analysis of 6xHis pull down assay between GST-Daxx and 6xHis-Rassf1C. Immobilized 6xHis-Rassf1C wt was incubated with either GST or GST-Daxx wt. GST-Daxx but not GST alone binds 6XHis-Rassf1C wt. B) Top panel: Interacting region I. Western blot analysis of MBP pull down assay between GST Daxx 1-142 and MBP-Rassf1C 1-50. Immobilized MBP-Rassf1C 1-50 or MBP were incubated with either GST or GST-Daxx 1-142. GST-Daxx 1-142 binds MBP-Rassf1C 1-50, but not MBP. Central panel: Interacting region II. In similar experimental settings, GST-Daxx 1-290 and GST-Daxx 290-740 retain capacity to bind both MBP-Rassf1C full length (MBP-1C) and Rassf1 common region (MBP- C51-270). While Daxx N-terminal region can bind Rassf1C 1-50, the C-terminal one cannot. Binding of the unique region of Rassf1A (MBP-A 1-120) to all the constructs including GST is considered merely non-specific. Bottom panel: Interacting region III. In vitro 6xHis pull down assay demonstrates specificity of Rassf1A-Rassf1C binding. Western blot analysis of 6xHis pull down assay of GST-Rassf1A wt and truncation mutants using immobilized 6xHis-Rassf1C wt. GST-Rassf1A wt/mutants, GST-Daxx 1-142 (positive control) and GST alone were incubated with 6xHis-Rassf1C wt. GST-Rassf1A wt and deletion mutants 120-340 and 194-340 bind to Rassf1C, while GST alone cannot. An asterisk (*) on the left side of the band indicates the expected molecular weight for the fusion constructs. High mobility bands represent degradation products.
Figure S4. A) Analysis of mitotic checkpoint proteins in control- and Daxx-depleted cell lines. Parental HEp2 cells and cells stably expressing control or two independent anti-Daxx shRNAs were analyzed by Western blot for levels of Cdc27, Cdc20, and Mad2; proteins levels are unchanged. B) Cell cycle-dependent expression of Daxx. HEp2 cells were synchronized using a double thymidine block to arrest cells in the G1/S-boundary and then released and allowed to progress through S, G2, M phase (0–11hr post thymidine release, compare with cyclin B dynamics on Fig. 1B). Daxx protein level showed no significant changes during cell cycle.
Figure S5. Rassf1A and Daxx are not required for in vitro activation of the APC. A) Extracts were generated from nocodazole-arrested cells expressing control-, Rassf1A or Daxx-shRNAs and the stability of the APC substrate Securin, labeled with 35S, was monitored by autoradiography. B) Extracts were generated as in (A) from cells treated with 10 µM nocodazole or 10 nM taxol (paclitaxel). The requirement for Rassf1A and Daxx to activate the APC was tested by addition of the Mad2 antagonist p31 Comet.
Figure S6. Model of Daxx/Rassf-dependent taxol response. At pharmacological concentrations, taxol reversibly inhibits microtubule dynamics blocking cells in prometaphase. Cells that are sensitive to taxol activate mitotic block only transiently, followed by cyclin B proteolysis, micronuclei formation, block of proliferation and cell death, while Daxx/Rassf1 negative cells have a more prolonged prometaphase block due to elevated cyclin B stability; they continue proliferation after drug decay/withdrawal and microtubule dynamics restoration--thus surviving chemotherapy.