Summary
Recent studies have revealed that ARID1A is frequently mutated across a wide variety of human cancers and also has bona fide tumor suppressor properties. Consequently, identification of vulnerabilities conferred by ARID1A mutation would have major relevance for human cancer. Here, using a broad screening approach, we identify ARID1B, a related but mutually exclusive homolog of ARID1A in the SWI/SNF chromatin remodeling complex, as the number one gene preferentially required for the survival of ARID1A-mutant cancer cell lines. We show that loss of ARID1B in ARID1A-deficient backgrounds destabilizes SWI/SNF and impairs proliferation. Intriguingly, we also find that ARID1A and ARID1B are frequently co-mutated in cancer, but that ARID1A-deficient cancers retain at least one ARID1B allele. These results suggest that loss of ARID1A and ARID1B alleles cooperatively promotes cancer formation but also results in a unique functional dependence. The results further identify ARID1B as a potential therapeutic target for ARID1A-mutant cancers.
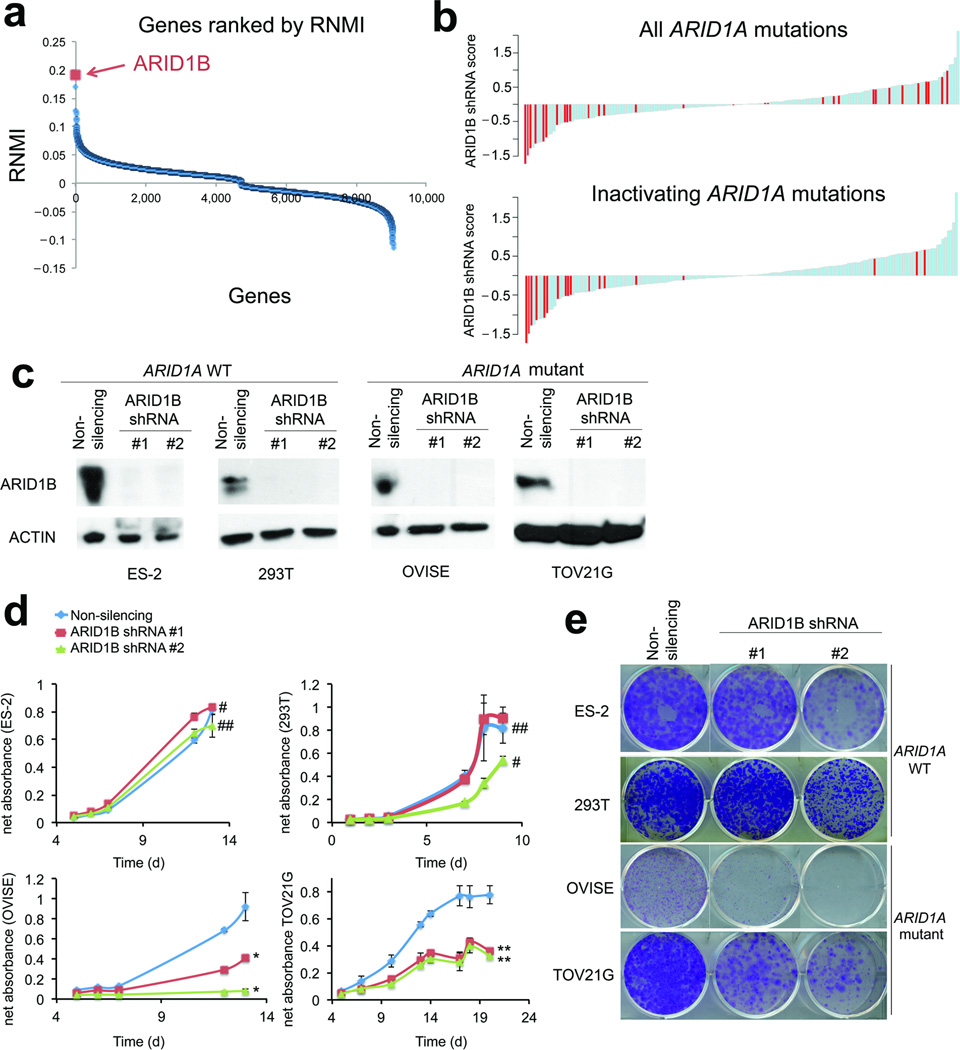
To search for specific dependencies created by ARID1A mutation, we utilized data from Project Achilles, a large-scale project focused on identifying essential genes in a wide panel of cancer cell lines using genome-scale loss-of-function genetics1. We compared 18 ARID1A-mutant and 147 cell lines wildtype for ARID1A (hereafter referred to as wildtype). Of 9,050 genes interrogated, ARID1B scored as the top candidate preferentially required for the growth of ARID1A-mutant cancer cell lines (p=7.366×10−6, FDR<0.001) (Fig. 1a, Fig. S1a). Vulnerability to ARID1B depletion was even more pronounced in the large subset of cell lines that contained inactivating ARID1A mutations (rather than missense mutations) (Fig. 1b), suggesting that ARID1B is specifically essential for cell lines lacking ARID1A (see supplementary discussion). To further evaluate this finding, we examined effects of the individual ARID1B shRNAs. Three of the four ARID1B shRNAs passed the Achilles quality control metrics2. These scored #1 (p=1.211×10−6, FDR<0.001), #4 (p=1.211×10−6, FDR<0.001), and #11 (p=1.816×10−5, FDR=0.090) of the 54,020 shRNAs in the screen. We confirmed ARID1B as a vulnerability by knocking it down in two cell lines that contained ARID1A-inactivating mutations, OVISE and TOV21G, and two ARID1A wildtype lines, ES-2 and 293T (Fig. 1c, Fig. S1b). Proliferation (Fig. 1d) and colony formation (Fig. 1e) were impaired in ARID1A-mutant cells but not in wildtype cells.
Figure 1. ARID1B is a specific vulnerability in ARID1A-mutant cancer cell lines.
(a) Rank list of vulnerabilities identified by screen of Achilles platform cell lines. ARID1B is the #1 gene preferentially essential for the growth of ARID1A-mutant cancer cell lines as compared to wildtype cancer cell lines.
(b) Effects of ARID1B shRNAs across cell lines in the Achilles screen. Negative values indicate growth inhibition while positive values reflect growth enhancement. In the top panel, cell lines with any identified ARID1A mutation compared to the reference genome are indicated in red. In the bottom panel, only those cell lines with clear inactivating mutations in ARID1A are shown in red.
(c) Immunoblots showing the results of two independent shRNAs targeting ARID1B in ES-2 and 293T (ARID1A-wildtype lines) and in OVISE, and TOV21G (ARID1A-mutant lines).
(d) Proliferation of wildtype (ES-2 and 293T) and ARID1A-mutant (OVISE and TOV21G) cell lines in response to two independent ARID1B shRNAs. * p<0.0002 **p<4×10−8 #p>0.05 ##p<0.05 Data are expressed as mean ± S.D.
(e) Colony formation in response to ARID1B knockdown in wildtype and ARID1A-mutant cell lines.
ARID1B and ARID1A are 60% identical, have been reported to have opposing functions in cell cycle arrest, and are mutually exclusive since individual SWI/SNF chromatin remodeling complexes can contain either ARID1A or ARID1B, but not both3. To investigate the relationship between ARID1A and ARID1B in cancer, we asked whether an ARID1B-containing SWI/SNF complex was present in ARID1A-mutant cells. Immunoprecipitation of the SMARCC1 (BAF155) core subunit of the SWI/SNF complex4 resulted in co-precipitation of ARID1B and other SWI/SNF subunits in both wildtype and ARID1A-mutant cells, indicating that intact ARID1B-containing complexes are present (Fig. S2a–b) in both wildtype and ARID1A-mutant cells.
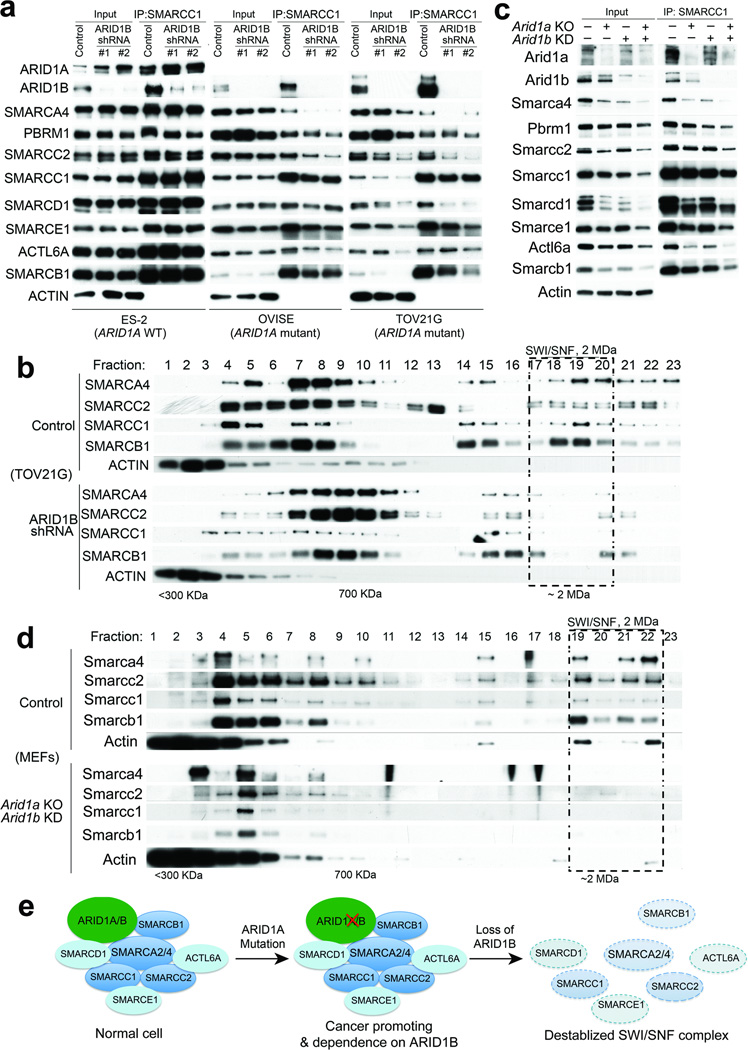
We next sought to determine whether ARID1B loss affects the composition of the SWI/SNF complex in ARID1A-mutant cancer cells. Knockdown of ARID1B in wildtype cell lines had no effect on the expression of other SWI/SNF complex subunits (Fig. 2a, Fig. S2c) or upon their incorporation into the complex (Fig. 2a, Fig. S2c). However, depletion of ARID1B in ARID1A-mutant cells resulted in dissociation of the core catalytic ATPase subunit SMARCA4 (BRG1) and reduced incorporation of several other subunits (Fig. 2a). Protein levels of core subunits such as SMARCA4, SMARCC2, and SMARCB1 were also decreased, particularly in the TOV21G line (Fig. 2a), while the mRNA levels were largely unaffected (Fig. S3), suggesting post-translational loss of these proteins.
Figure 2. ARID1B is required for the maintenance of an intact SWI/SNF complex in ARID1A-mutant cancer cell lines and primary cells.
(a) Immunoprecipitation of the SWI/SNF complex by SMARCC1 from the nuclear extract of ES-2, OVISE, and TOV21G cells upon treatment with control shRNA or two independent ARID1B shRNAs.
(b) Sucrose sedimentation (20−50%) assay of SWI/SNF complex from ARID1A-mutant TOV21G cells treated with either control shRNA (top half) or ARID1B shRNA (bottom half).
(c) Immunoprecipitation of the SWI/SNF complex by Smarcc1 from the nuclear extract of MEFs with indicated treatment.
(d) Sucrose sedimentation (20−50%) assay of the SWI/SNF complex from the nuclear extract of MEFs with indicated treatment: control shRNA treated MEFs (top half) or Arid1a knockout (KO) and Arid1b knockdown (KD) MEFs (bottom half)
(e) Model: Inactivating mutations in ARID1A promote oncogenic transformation but also create specific dependency on ARID1B. Inhibition of ARID1B in ARID1A-mutant cells destabilizes the SWI/SNF complex and results in impaired cell growth.
To further investigate how ARID1B loss affects assembly of the SWI/SNF complex, we performed a sucrose sedimentation assay on cells treated with either control shRNA or ARID1B shRNA. Consistent with the co-immunoprecipitation results, an intact 2 MDa SWI/SNF complex is observed in ARID1A-mutant cells treated with control shRNA (Fig. 2b, full figure in Fig. S4–5) and in wildtype cells treated with either control or ARID1B shRNA (Fig. S6). In contrast, knockdown of ARID1B in ARID1A-mutant cells eliminated the intact SWI/SNF complex (Fig. 2b; additional subunits shown in Fig. S4–5), indicating that in human ARID1A-mutant cancer cell lines, the presence of ARID1B is essential for formation or stabilization of an intact SWI/SNF complex. Despite ARID1A/ARID1B and PBRM1 having been reported to exist in mutually exclusive versions of the SWI/SNF complex5, our findings are consistent with a more recent publication, which found that these subunits can co-exist6, as PBRM1 association with smaller complexes was substantially affected by the combined loss of ARID1A/B (Fig S4–5). As the SWI/SNF complex binds up to one-third of all genes7 and several members of the SWI/SNF complex are essential in mouse development8–10 and for survival of many cell lineages11,12, loss of an intact SWI/SNF complex would be predicted to be incompatible with cell viability.
In order to further validate the identification of ARID1B as a vulnerability in ARID1A-mutant human cancer, we sought to investigate whether inactivation of Arid1a creates a dependence upon Arid1b using primary MEFs conditional for Arid1a 13. Deletion of Arid1a or knockdown of Arid1b individually had only moderate effects on proliferation, while combined loss led to substantial impairment (Fig. S7a). We similarly observed that loss of Arid1a or Arid1b alone had only modest effects on the composition of the complex (Fig. 2c), while loss of both led to dissociation and degradation of Smarca4 and substantial reductions in stability and incorporation of many other SWI/SNF subunits (Fig. 2d). Again, the reduced protein levels were not due to changes in transcription (Fig. S7b). Finally, sucrose sedimentation assay showed that loss of Arid1a and Arid1b in MEFs eliminated the intact SWI/SNF complex (Fig. 2d and Fig. S7c).
Collectively, these findings demonstrate a synthetic lethal relationship between this mutually exclusive pair of SWI/SNF subunits. Notably, however, ARID1B has also been reported mutated in human cancers3,14, and has been found to be mutant in some of the same types of cancer as ARID1A, such as neuroblastoma14. Since we found ARID1B knockdown to impair the growth of ARID1A-mutant cell lines, we initially hypothesized that mutations in ARID1A and ARID1B would be mutually exclusive. Surprisingly, we found that ARID1A and ARID1B mutations co-occur in both cancer cell lines and primary tumors. Using data from cell line sequencing 15,16 we found that 38% of 34 ARID1A-mutant lines also contained ARID1B-inactivating mutations16 (Supplementary Table 1, p<1×10−6). Notably, all lines retained at least one allele of either ARID1A or ARID1B, suggesting that retention of at least one ARID1 allele may be essential for survival. This finding also held true in primary cancer samples. We found that of the 297 ARID1A-mutant primary cancer samples cataloged in the cBio Portal for Cancer Genomics17,18, 30 (10.1%) also contained ARID1B mutations (p=1.07×10−7), significantly higher than the 3% rate in ARID1A-wildtype tumors.
The co-occurrence of ARID1A and ARID1B mutations raises the possibility that the synthetic lethality relationship could be caused simply by the high frequency of ARID1B mutations in ARID1A-mutant cancer cell lines. To evaluate this possibility, we removed all ARID1B-mutant cell lines and conducted a revised class comparison in which four ARID1B-wildtype, ARID1A-mutant cell lines were compared to 49 cell lines wildtype for both ARID1A and ARID1B. ARID1B still scored number four out of 9,000+ genes (p=7.154×10−4), indicating that the synthetic lethality between ARID1A and ARID1B is a result of ARID1A mutation and not co-occurring ARID1B mutations.
In this report, we show that inactivating mutations in ARID1A, frequent across a wide variety of cancers, create a dependency upon ARID1B (Fig. 2e). It is notable that the number one vulnerability in ARID1A-mutant cell lines is another member of the SWI/SNF complex. We previously showed that cancer formation in the absence of the SWI/SNF subunit SMARCB1 does not result from SWI/SNF inactivation but rather that oncogenesis was dependent upon the activity of the residual SWI/SNF complex19. At that time we speculated that, much like the concept of oncogene addiction, targeting the aberrant residual SWI/SNF complex might theoretically be an effective therapeutic approach for SMARCB1-mutant cancers. Our present study, which surveyed 9,050 genes, reveals the role of the residual complex in the growth of ARID1A-deficient cancers and also identifies a specific subunit as a relative vulnerability. This principle may have broad applicability to SWI/SNF-mutant cancers as Oike et al recently showed that SMARCA2, a paralog of SMARCA4, was essential in SMARCA4-mutant cancers20.
Together, our findings may suggest that partial loss of ARID1 function via mutation of ARID1A alleles or, less frequently, ARID1B alleles can drive cancer growth but at the same time create a specific vulnerability compared to non-mutant cells. This suggests ARID1B as a potential therapeutic target for cancers that contain inactivating ARID1A mutations. Recent examples have demonstrated the feasibility and efficacy of targeting chromatin regulators such as BRD421,22 as well as other non-enzymatic proteins such as BCL-223 and molecules previously found difficult to target such as RAS24. ARID1B could potentially be targetable through its E3 ubiquitin ligase interaction25. Additionally, novel approaches using small stabilized peptides have recently been shown capable of disrupting association of EZH2 with its chromatin remodeling complex26. Analogous approaches may now be considered for targeting ARID1B.
Online Methods
Achilles Analysis
To find genes that are preferentially essential in mutant cell lines, we used the GenePattern module PARIS (http://www.broadinstitute.org/cancer/software/genepattern) using the default parameters except quality, which was changed to final. The gene-level Achilles dataset v2.4 was used as a data file (www.broadinstitute.org/achilles) (file name: Achilles_QC_v2.4_rnai.Gs.gct). The classifier files were generated using the gene mutation status from the Cancer Cell Line Encyclopedia (www.broadinstitute.org/ccle). Cell lines without hybrid capture sequencing data were removed from the analysis. The mutational status of ARID1A was annotated for 165 of the 216 cell lines in the Achilles dataset, and as a result, these 165 cell lines were used in the class comparisons.
Cell Culture
TOV21G (CRL 11730), ES-2 (CRL-1987), and 293T (CRL-3216) cell lines were purchased from ATCC. OVISE cells were obtained from William Hahn’s laboratory. Mouse Embryonic Fibroblasts (MEFs) were generated as described previously27. Cells were transduced with shRNAs and selected with puromycin for 48–72 hours before seeding for MTT or colony formation assays. MTT assays were conducted with a Cell Proliferation Kit (Roche). Colony formation assays were conducted by staining cells for 20 minutes with crystal violet staining solution (0.05% Crystal Violet, 1% Formaldehyde, 1% PBS, 1% methanol).
shRNA-mediated knockdown of ARID1B
ARID1B shRNAs were obtained from the RNA interference (RNAi) screening facility at the Dana-Farber Cancer Institute and were lentivirally transduced into OVISE, TOV21G, ES-2, and 293T cells. ARID1B and non-silencing control shRNAs are in the pLKO.1 lentiviral expression vector backbone. Target sequences for shRNAs are available upon request.
Density Sedimentation Analysis
Nuclear extract (1 mg) was diluted in 300 μl of 0% sucrose RIPA buffer and carefully overlaid onto a 12 ml 20%–50% sucrose (in RIPA buffer) gradient prepared in a 14ml 14×95 mm polyallomer centrifuge tube (Cat. #331374, Beckman Coulter). Tubes were placed in an SW-40 Ti swing bucket rotor and centrifuged at 4°C for 16 hours at 40,000 rpm. Fractions (0.5 ml) were collected and used in gel electrophoresis and subsequent Western blotting analyses.
Immunoblots and co-immunoprecipitation experiments
Whole cell extracts for immunoblotting were prepared by incubating cells on ice in 1% NP-40 lysis buffer (50 mM Tris-HCL pH 7.4, 5mM EDTA, 12% Glycerol, 50mM NaCl, 1% NP-40) plus protease inhibitors (Complete, Mini, EDTA-free. Roche: 11836170001) for 30 minutes. Supernatants were collected following a brief spin (10 min.) at 17900 r.c.f. to separate cellular debris in a 4° C centrifuge. Protein concentrations were determined using the Bradford reagent (Biorad). SDS-polyacrylamide gel electrophoresis was used to separate proteins, which were subsequently transferred to PVDF membranes (Millipore). ARID1B antibody (Abcam: ab54761) was used to detect efficient knockdown.
Nuclear extracts for immunoprecipitation were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific: 78833). Nuclear extracts were diluted with RIPA buffer (1 mg/ml, with protease inhibitors and DTT). Each IP was incubated with indicated antibodies overnight at 4° C. Protein G Dynabeads (Life Technologies: 10004D) were added and incubated at 4° C for 3 hrs. Beads were then washed three times with RIPA buffer and resuspended in reducing SDS gel loading buffer. Antibodies used in the immunoprecipitation and immunoblots are: SMARCC1/BAF155 (Santa Cruz: 9746); ARID1A (Bethyl Laboratories: A301-041A); PBRM1 (Bethyl Laboratories: A301-591A); SMARCA4 (Santa Cruz: sc17796); SMARCC2/BAF170 (Bethyl Laboratories: A301-039A); SMARCD1/BAF60A (Bethyl Laboratories: A301-595A); SMARCE1/BAF57 (Bethyl Laboratories: A300-810A); ACTL6A/BAF53A (Bethyl Laboratories: A301-391A); ACTIN (Cell Signaling Technology: 5125).
RNA purification and RT-qPCR
Total RNA was extracted using Trizol reagent (Invitrogen) following the manufacturer’s instructions. 2 µg of total RNA was reverse-transcribed into first-strand cDNA using oligo(dT)20 primers and the SuperScript III Reverse Transcriptase (Invitrogen). RT-qPCR was performed on the ViiA 7 Real-Time PCR System (Life Technologies) using SYBR Select Master Mix (Life Technologies). Reactions were performed in triplicate, and gene expression was normalized to GAPDH. Error bars represent SD of mean expression.
Cell Line Sequencing
Cell lines were sequenced as previously described15. Cell line sequencing data and the data from the Cancer Cell Line Encyclopedia16 were used to identify cell lines with co-occurring mutations of ARID1A and ARID1B.
Statistical Significance of Mutation Overlap
To evaluate the statistical significance of the overlap of ARID1A and ARID1B mutations, the probability of observing at least n12 cell lines with both mutations was estimated under the null hypothesis that these two mutations are independent. For that, given n1 cell lines with ARID1A and n2 cell lines with ARID1B mutations and n12 cell lines with both mutations the following simulation was run: n1 cell lines were randomly picked with the probability for each cell line being selected set relative to its mutation rate and assigned mutation ‘A’ to these cell lines. Next, n2 cell lines were similarly selected and assigned mutation ‘B’ to those cell lines and then the number of cell lines with both mutations ‘A’ and ‘B’ was counted. This process was repeated many times to estimate the probability of observing n12 cell lines or more with both mutations.
For primary cancer samples, a contingency table was formed consisting of the counts for all the four possibilities of ARID1A or ARID1B mutation status. The Fisher’s exact test was used to calculate the statistical significance of the overlap of ARID1A and ARID1B mutations.
Supplementary Material
Acknowledgements
We thank P. Lu for technical assistance with the set up of the sucrose sedimentation assay. X. Wang was supported by a post-doctoral fellowship from David Abraham Foundation and Rally Foundation, and a research grant from St. Baldrick’s Foundation. B. Wilson was supported by a Childhood Cancer Research Grant from the Pablove Foundation. This work was supported by R01CA172152 (C. Roberts) and R01CA113794 (C. Roberts), and a U01 NCI Mouse Models of Cancer Consortium Award (C. Roberts). The Garrett B. Smith Foundation, Miles for Mary, and the Cure AT/RT Now foundation (C. Roberts) provided additional support.
Footnotes
Contributions
C. Roberts directed the study. K. Helming and X. Wang designed and performed experiments. J. Haswell and H. Manchester performed experiments. C. Roberts, K. Helming, X. Wang, B. Wilson, J. Haswell, and H. Manchester analyzed and interpreted the data. K. Helming, B. Wilson, F. Vazquez, and A. Aguirre performed analysis of Project Achilles data. G. Kryukov, M. Ghandi, and L. Garraway provided and analyzed sequencing data. Z. Wang provided Arid1a-conditional mice, intellectual contribution, and useful discussion. Y.Kim established Arid1a-conditional mouse strain. W. Hahn directs the Achilles Project, provided reagents, helped interpret Achilles data, and edited the manuscript. C. Roberts, K. Helming and X.Wang wrote the manuscript.
References
- 1.Cheung HW, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12372–12377. doi: 10.1073/pnas.1109363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao DD, et al. ATARiS: computational quantification of gene suppression phenotypes from multisample RNAi screens. Genome research. 2013;23:665–678. doi: 10.1101/gr.143586.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discov. 2013;3:35–43. doi: 10.1158/2159-8290.CD-12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Molecular cell. 1999;3:247–253. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 5.Nie Z, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Molecular and cellular biology. 2000;20:8879–8888. doi: 10.1128/mcb.20.23.8879-8888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryme J, Asp P, Bohm S, Cavellan E, Farrants AK. Variations in the composition of mammalian SWI/SNF chromatin remodelling complexes. Journal of cellular biochemistry. 2009;108:565–576. doi: 10.1002/jcb.22288. [DOI] [PubMed] [Google Scholar]
- 7.Tolstorukov MY, et al. Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10165–10170. doi: 10.1073/pnas.1302209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klochendler-Yeivin A, et al. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO reports. 2000;1:500–506. doi: 10.1093/embo-reports/kvd129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bultman S, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Molecular cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim JK, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Molecular and cellular biology. 2001;21:7787–7795. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gresh L, et al. The SWI/SNF chromatin-remodeling complex subunit SNF5 is essential for hepatocyte differentiation. The EMBO journal. 2005;24:3313–3324. doi: 10.1038/sj.emboj.7600802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts CW, Leroux MM, Fleming MD, Orkin SH. Highly penetrant, rapid tumorigenesis through conditional inversion of the tumor suppressor gene Snf5. Cancer cell. 2002;2:415–425. doi: 10.1016/s1535-6108(02)00185-x. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, et al. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sausen M, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2012 doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baca SC, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. Oncogenesis caused by loss of the SNF5 tumor suppressor is dependent on activity of BRG1, the ATPase of the SWI/SNF chromatin remodeling complex. Cancer research. 2009;69:8094–8101. doi: 10.1158/0008-5472.CAN-09-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oike T, et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer research. 2013;73:5508–5518. doi: 10.1158/0008-5472.CAN-12-4593. [DOI] [PubMed] [Google Scholar]
- 21.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 24.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XS, Trojer P, Matsumura T, Treisman JE, Tanese N. Mammalian SWI/SNF--a subunit BAF250/ARID1 is an E3 ubiquitin ligase that targets histone H2B. Molecular and cellular biology. 2010;30:1673–1688. doi: 10.1128/MCB.00540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim W, et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nature chemical biology. 2013;9:643–650. doi: 10.1038/nchembio.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isakoff MS, et al. Inactivation of the Snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17745–17750. doi: 10.1073/pnas.0509014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.