Abstract
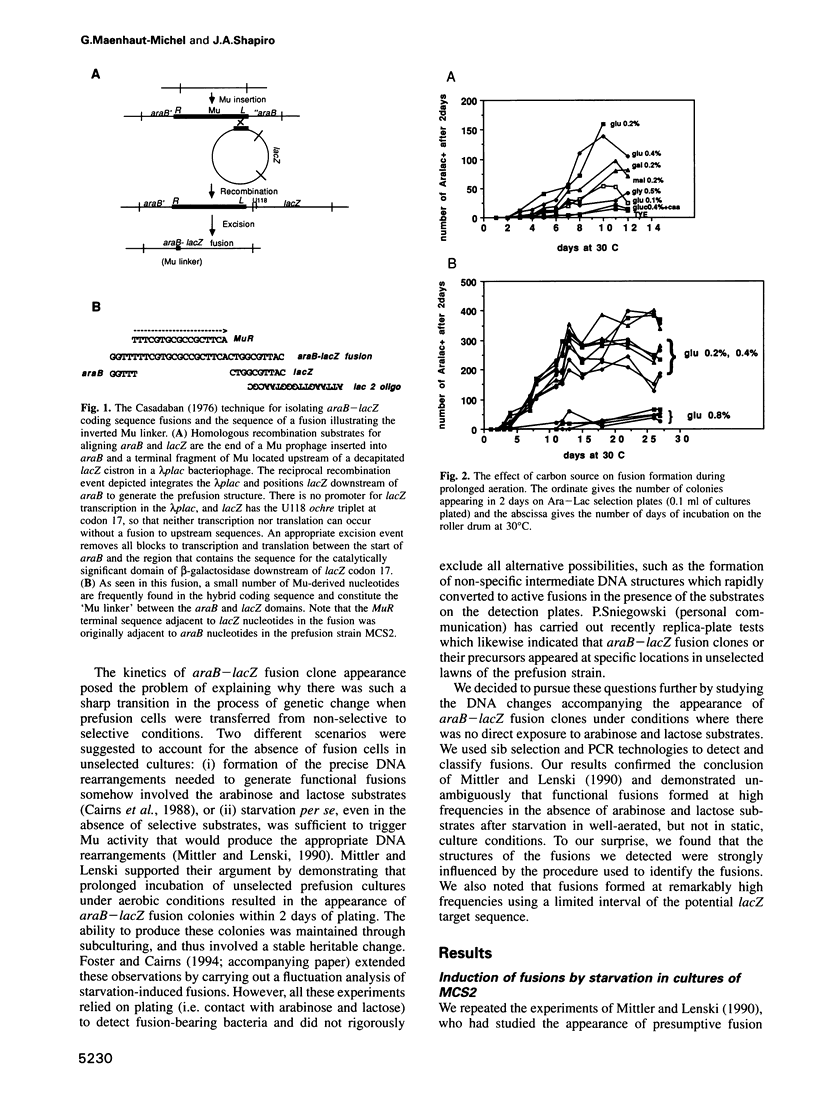
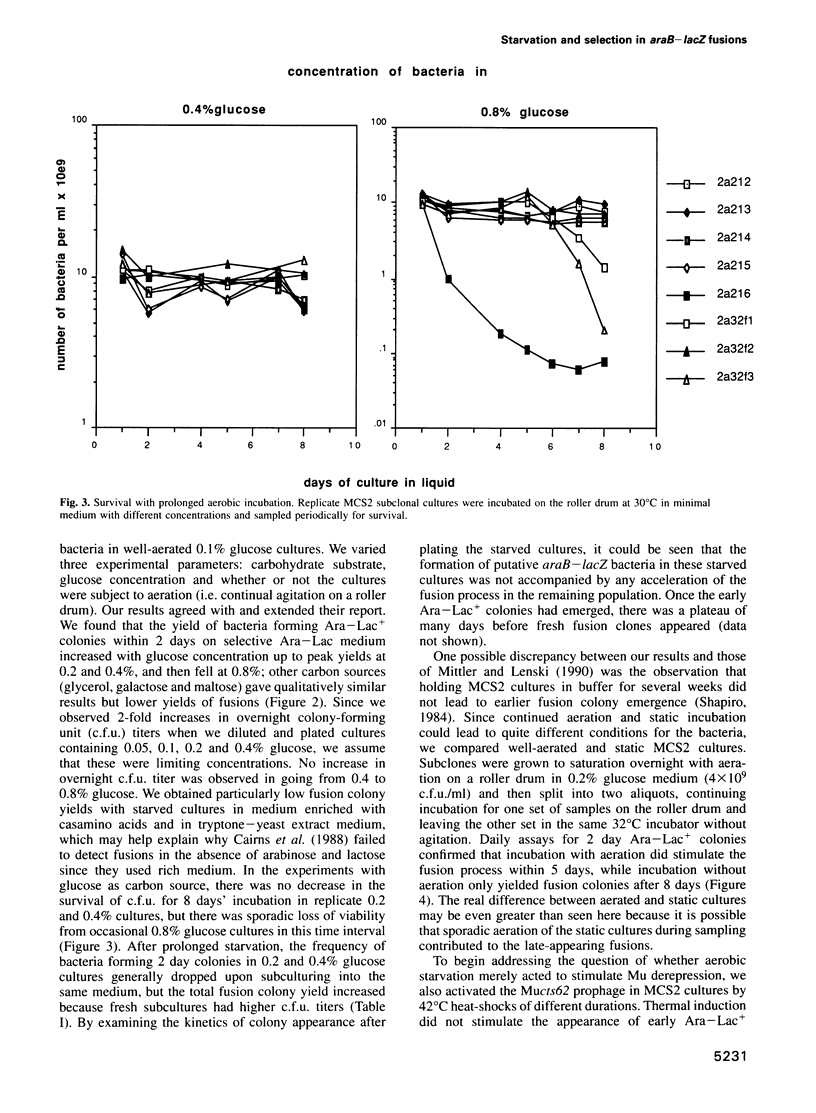
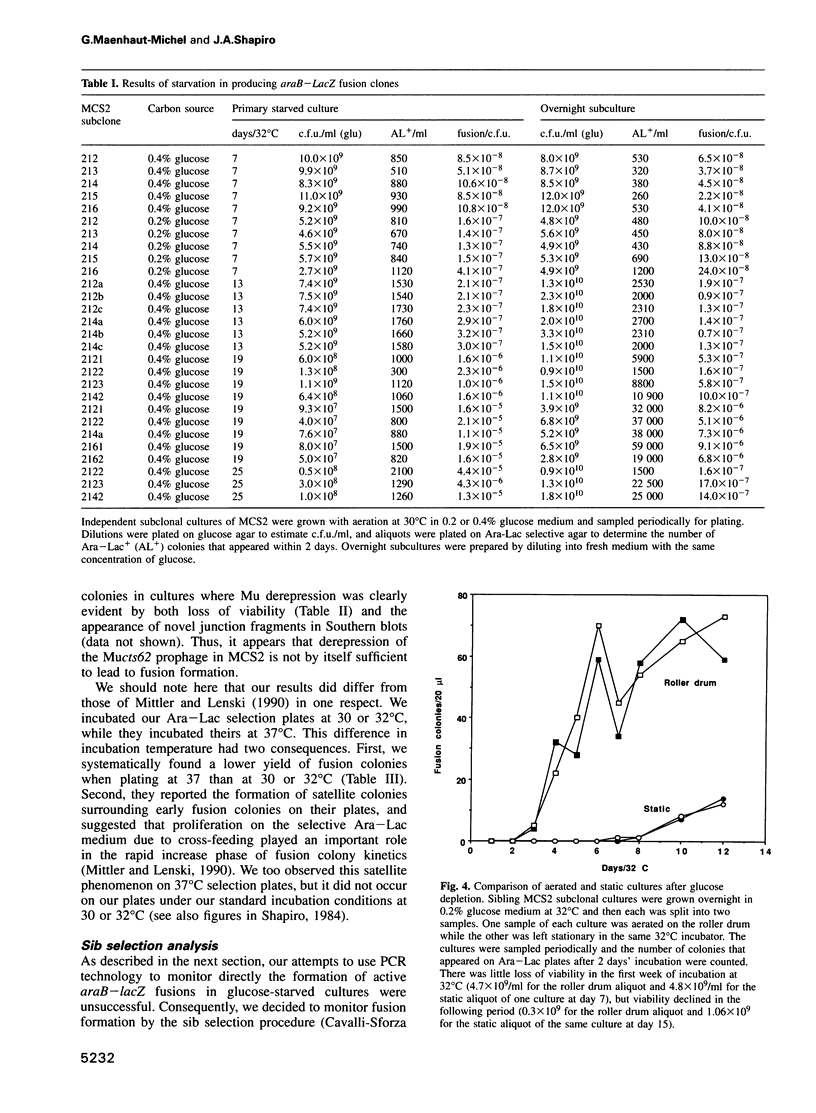
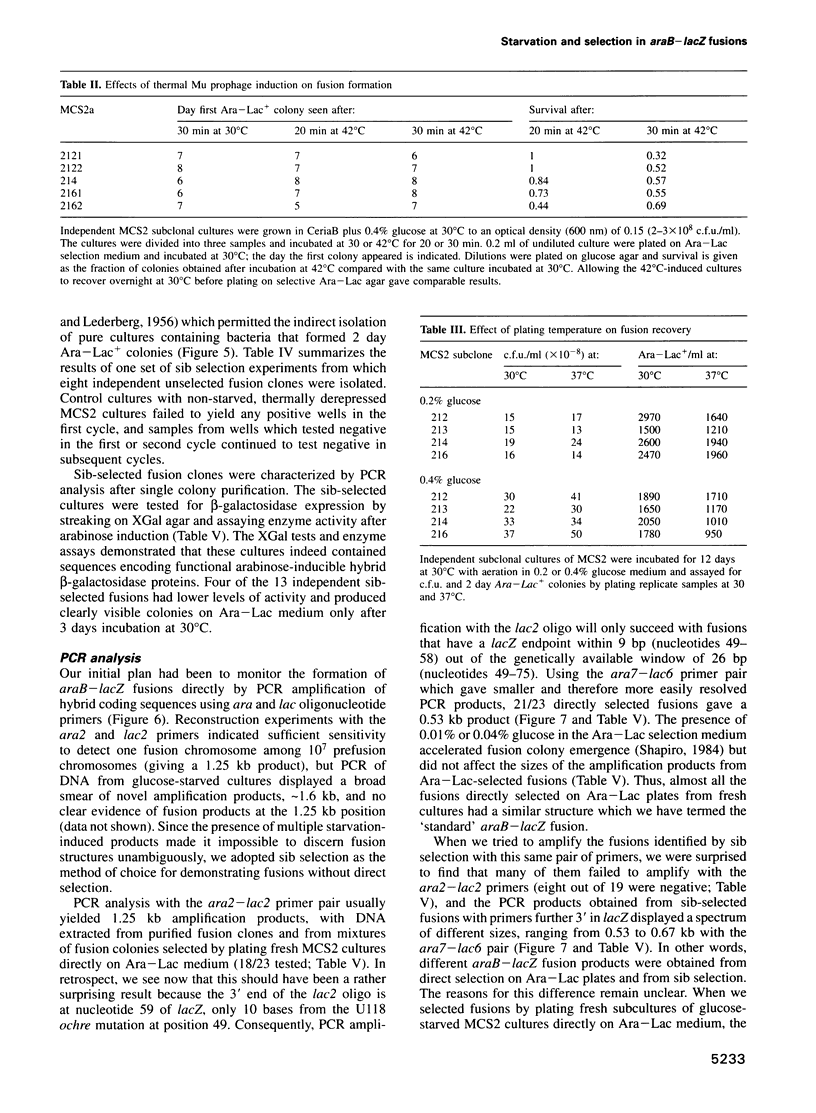
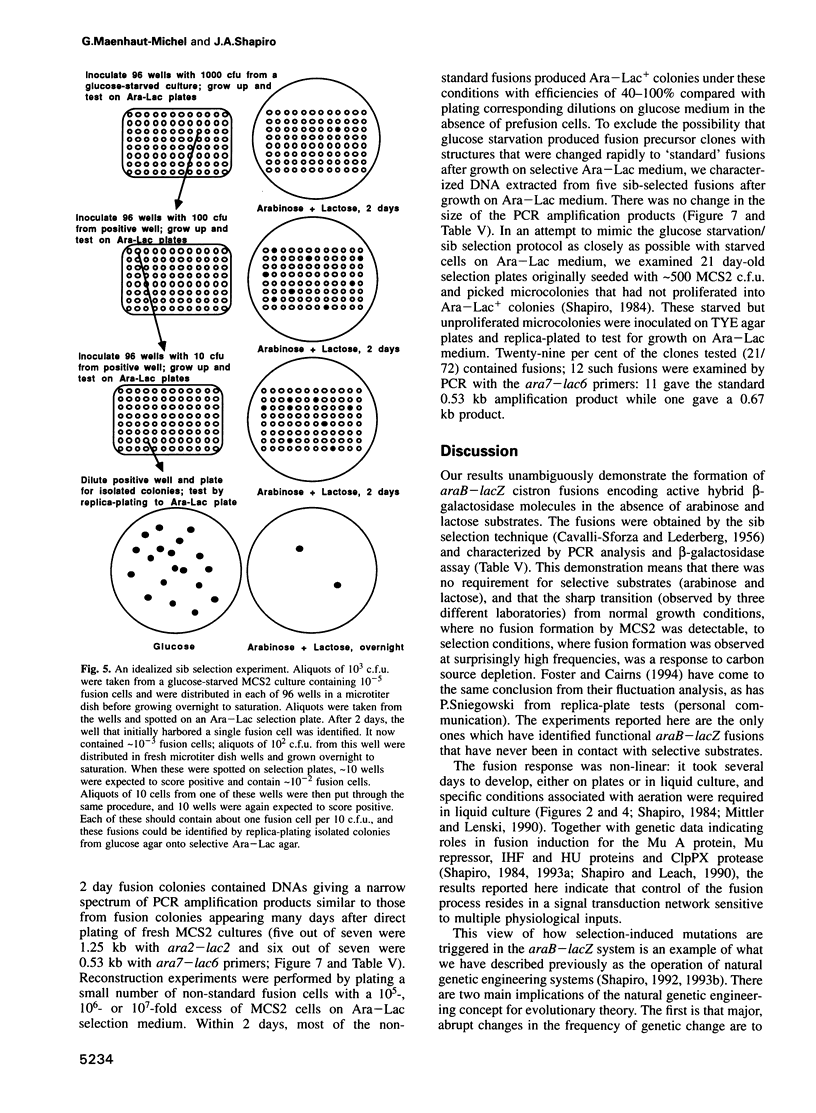
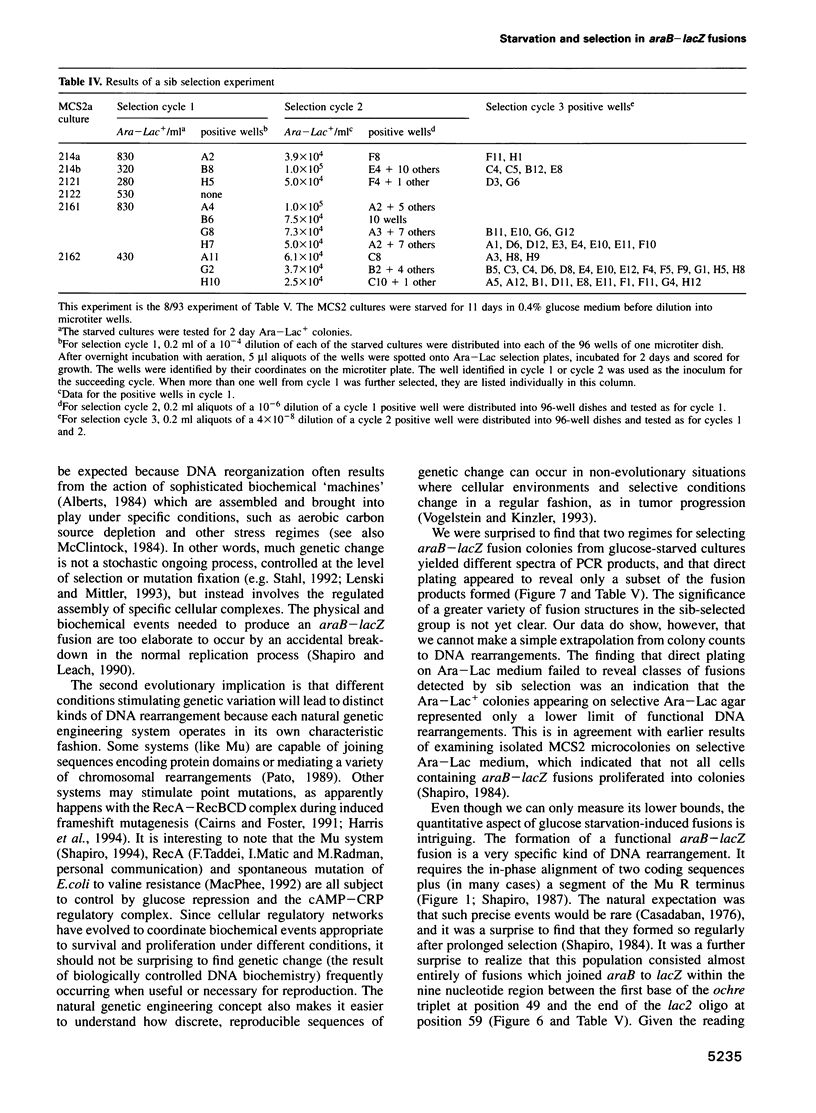
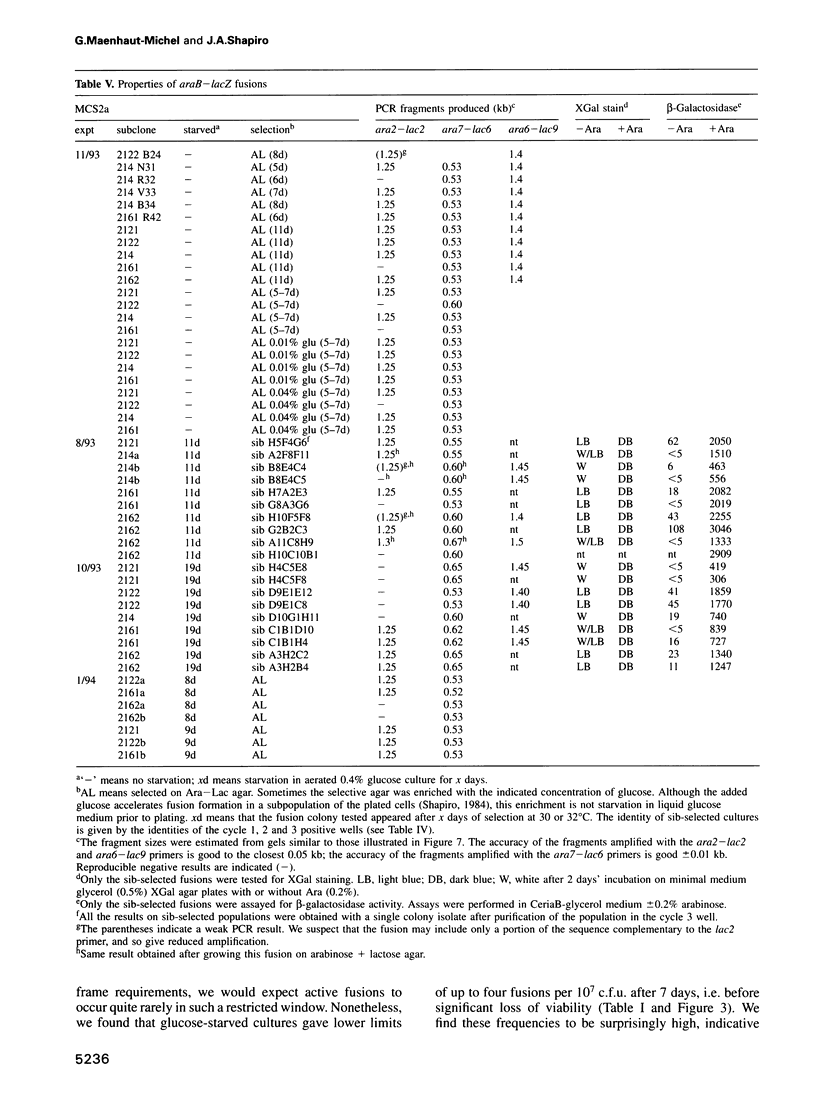
The araB-lacZ fusion system has been a key case in the 'directed mutation' controversy. Fusions did not occur detectably during normal growth but formed readily after prolonged incubation on selective Ara-Lac medium. To distinguish the roles of starvation stress and selective substrates in coding sequence fusions, we applied sib selection and PCR technologies. Sib selection of the prefusion strain, MCS2, starved under aerobic conditions permitted us to isolate active fusion clones which had never been in contact with arabinose or lactose. Hence, a directive role for selective substrates is not essential. Aerobiosis was necessary for fusions to appear in glucose-starved cultures. The difference in fusion formation between normal and starved conditions is best explained by the response of a signal transduction network to physiological stimuli to activate Mu prophage joining of araB and lacZ sequences. PCR analysis revealed that direct plating on selective Ara-Lac agar yielded mostly a single class of 'standard' fusions, while sib selection yielded a broader spectrum of fusion structures. Standard fusions were found to occur within a narrow 9 bp window in lacZ. The high frequency of standard fusions in glucose-starved cultures suggested efficient and/or specific Mu action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M. The DNA enzymology of protein machines. Cold Spring Harb Symp Quant Biol. 1984;49:1–12. doi: 10.1101/sqb.1984.049.01.003. [DOI] [PubMed] [Google Scholar]
- Allgood N. D., Silhavy T. J. Escherichia coli xonA (sbcB) mutants enhance illegitimate recombination. Genetics. 1991 Apr;127(4):671–680. doi: 10.1093/genetics/127.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Retroposons--seeds of evolution. Science. 1991 Feb 15;251(4995):753–753. doi: 10.1126/science.1990437. [DOI] [PubMed] [Google Scholar]
- Cairns J., Foster P. L. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991 Aug;128(4):695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L L, Lederberg J. Isolation of Pre-Adaptive Mutants in Bacteria by Sib Selection. Genetics. 1956 May;41(3):367–381. doi: 10.1093/genetics/41.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr L. K., Strathern J. N., Garfinkel D. J. RNA-mediated recombination in S. cerevisiae. Cell. 1991 Oct 18;67(2):355–364. doi: 10.1016/0092-8674(91)90187-4. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Cairns J. The occurrence of heritable Mu excisions in starving cells of Escherichia coli. EMBO J. 1994 Nov 1;13(21):5240–5244. doi: 10.1002/j.1460-2075.1994.tb06855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Purification, structure, and properties of hybrid beta-galactosidase proteins. J Biol Chem. 1983 Dec 10;258(23):14354–14358. [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Boone T., Wilcox G. DNA sequence of the araBAD promoter in Escherichia coli B/r. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4724–4728. doi: 10.1073/pnas.75.10.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. S., Longerich S., Rosenberg S. M. Recombination in adaptive mutation. Science. 1994 Apr 8;264(5156):258–260. doi: 10.1126/science.8146657. [DOI] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski R. E., Mittler J. E. The directed mutation controversy and neo-Darwinism. Science. 1993 Jan 8;259(5092):188–194. doi: 10.1126/science.7678468. [DOI] [PubMed] [Google Scholar]
- Long M., Langley C. H. Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila. Science. 1993 Apr 2;260(5104):91–95. doi: 10.1126/science.7682012. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Directed mutation: paradigm postponed. Mutat Res. 1993 Jan;285(1):109–116. doi: 10.1016/0027-5107(93)90058-n. [DOI] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984 Nov 16;226(4676):792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Miklos G. L., Campbell H. D. The evolution of protein domains and the organizational complexities of metazoans. Curr Opin Genet Dev. 1992 Dec;2(6):902–906. doi: 10.1016/s0959-437x(05)80113-3. [DOI] [PubMed] [Google Scholar]
- Mittler J. E., Lenski R. E. New data on excisions of Mu from E. coli MCS2 cast doubt on directed mutation hypothesis. Nature. 1990 Mar 8;344(6262):173–175. doi: 10.1038/344173a0. [DOI] [PubMed] [Google Scholar]
- Nieder M., Shapiro J. Physiological function of the Pseudomonas putida PpG6 (Pseudomonas oleovorans) alkane hydroxylase: monoterminal oxidation of alkanes and fatty acids. J Bacteriol. 1975 Apr;122(1):93–98. doi: 10.1128/jb.122.1.93-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. A role for the Clp protease in activating Mu-mediated DNA rearrangements. J Bacteriol. 1993 May;175(9):2625–2631. doi: 10.1128/jb.175.9.2625-2631.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A., Brinkley P. M. Programming of DNA rearrangements involving mu prophages. Cold Spring Harb Symp Quant Biol. 1984;49:313–320. doi: 10.1101/sqb.1984.049.01.037. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Leach D. Action of a transposable element in coding sequence fusions. Genetics. 1990 Oct;126(2):293–299. doi: 10.1093/genetics/126.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Natural genetic engineering in evolution. Genetica. 1992;86(1-3):99–111. doi: 10.1007/BF00133714. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Natural genetic engineering of the bacterial genome. Curr Opin Genet Dev. 1993 Dec;3(6):845–848. doi: 10.1016/0959-437x(93)90003-8. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Observations on the formation of clones containing araB-lacZ cistron fusions. Mol Gen Genet. 1984;194(1-2):79–90. doi: 10.1007/BF00383501. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Pattern and control in bacterial colony development. Sci Prog. 1992;76(301-302):399–424. [PubMed] [Google Scholar]
- Stahl F. W. Unicorns revisited. Genetics. 1992 Dec;132(4):865–867. doi: 10.1093/genetics/132.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Swain A., Coffin J. M. Mechanism of transduction by retroviruses. Science. 1992 Feb 14;255(5046):841–845. doi: 10.1126/science.1371365. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. The multistep nature of cancer. Trends Genet. 1993 Apr;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Wild J. R., Wales M. E. Molecular evolution and genetic engineering of protein domains involving aspartate transcarbamoylase. Annu Rev Microbiol. 1990;44:193–218. doi: 10.1146/annurev.mi.44.100190.001205. [DOI] [PubMed] [Google Scholar]