Abstract
Prenatal exposure to bisphenol A (BPA) may be associated with adverse health effects in the developing fetus; however, little is known about predictors of BPA exposure during pregnancy. We examined BPA exposure in 491 pregnant women from the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort and explored the role of living in the United States on significant dietary predictors of BPA exposure. Women provided urine samples up to two times during pregnancy (n=866 total samples). We computed the intraclass correlation coefficient (ICC) to evaluate variability in concentrations between collections and used generalized estimating equation (GEE) models to assess predictors of exposure. Geometric mean (GSD) BPA concentrations were 0.9 (2.8) µg/L and 1.0 (2.6) µg/L at the first and second prenatal visit, respectively. We observed greater within- than between-woman variability in urinary BPA concentrations (ICC=0.22). GEE models suggest that women who lived in the United States their entire life had 38% (CI:−0.1, 89.3) higher urinary BPA concentrations compared with other immigrant women. Additionally, women who consumed ≥3 sodas per day or hamburgers three times a week or more had 58% (CI:18.0, 112.1) and 20% (CI: −0.2, 45.2) higher urinary BPA concentrations, respectively, compared with women who consumed no sodas or hamburgers. A higher percentage of women who lived their entire life in the United States reported increased consumption of sodas and hamburgers compared with other immigrant women. Independent of other factors, BPA urinary concentrations were slightly higher when the sample was collected later in the day. As in previous studies, high within-woman variability in urinary BPA concentrations confirms that several samples are needed to properly characterize exposure during pregnancy. Results also suggest that some factors could be modified to minimize exposures during pregnancy in our study participants (e.g., reducing soda and hamburger intake) and that factors associated with acculturation might increase BPA concentrations.
Keywords: Bisphenol A, prenatal, pregnancy, Mexican-American
Introduction1
Bisphenol A (BPA) is a high-volume production chemical primarily used in the manufacture of polycarbonate plastics and epoxy resins. It is present in many consumer products including plastic food containers, the lining of metal food and beverage cans, toys, dental sealants, thermal receipts, cigarette filters, and medical devices (Geens et al. 2011; Sasaki et al. 2005; Vandenberg et al. 2009). The primary route of exposure in the general population is thought to be through ingestion (Biedermann et al. 2010; Christensen et al. 2012; Reuss and Leblanc 2010; Wilson et al. 2007), although other exposure routes (e.g., dermal absorption) are plausible (Biedermann et al. 2010; Reuss and Leblanc 2010). Human exposure is widespread with BPA being detected in urine samples from 93% of the U.S. general population (Calafat et al. 2008), including 96% of pregnant women (Woodruff et al. 2011). BPA has also been detected in amniotic fluid, cord blood, placental tissue, and breast milk (Chou et al. 2011; Vandenberg et al. 2007); and it can also cross the placenta from the pregnant mother to the fetus (Balakrishnan et al. 2010).
In animals, prenatal exposure to low doses of BPA [i.e., doses below the U.S. Environmental Protection Agency’s reference dose of 50 µg/kg·day; (U.S.EPA Integrated Risk Information System (IRIS), http://www.epa.gov/ncea/iris/subst/0356.htm)] has been linked to adverse neurodevelopmental, reproductive, and metabolic effects (Richter et al. 2007; Shelby 2008; Vandenberg et al. 2009; vom Saal and Hughes 2005; Welshons et al. 2006). Results on the association between prenatal BPA exposure and birth weight are inconsistent (Chou et al. 2011; Lee et al. 2008; Miao et al. 2011; Padmanabhan et al. 2008; Philippat et al. 2012; Wolff et al. 2008). Nonetheless, the animal evidence and limited human studies raise concerns that developing fetuses may be susceptible to adverse health effects associated with prenatal BPA exposure.
Targeted studies have shown that drinking from polycarbonate water bottles (Carwile et al. 2009) and eating canned (Carwile et al. 2011; Teeguarden et al. 2011) or processed (Rudel et al. 2012) foods increase BPA exposure in adults. In non-pregnant adults, consuming sodas or meals not prepared at home has been positively associated with urinary BPA concentrations (Lakind and Naiman 2010), while age and household income are negatively associated with urinary BPA concentrations (Calafat et al. 2008). Mexican-Americans have been reported to have lower urinary BPA concentrations compared with other ethnic groups in the U.S. general population (Calafat et al. 2008). Studies in pregnant and non-pregnant adults have also reported high intra-individual variability in urinary BPA concentrations potentially due to factors such as BPA toxicokinetics (e.g., the short half-life of BPA) and changes in xenobiotic metabolism during pregnancy (Braun et al. 2011; Braun et al. 2012; Mahalingaiah et al. 2008).
To date, only a few large population-based studies have evaluated determinants of BPA exposure in pregnant women (Braun et al. 2011; Casas et al. 2013; Hoepner et al. 2013; Meeker et al. 2013). Smoking, lower education level, consuming canned vegetables at least once per day, and working as a cashier were all positively associated with urinary BPA concentrations in a Cincinnati cohort of predominantly non-Hispanic white pregnant women. BPA concentrations were also positively correlated with serum cotinine (marker for environmental tobacco smoke) and urinary phthalate concentrations. Additionally, urinary BPA concentrations were reported to vary according to time of day samples were collected. In Spanish pregnant women, BPA concentrations were positively associated with mothers who were: younger, smoked, less educated, exposed to second-hand tobacco smoke, and consumed high amounts of canned fish (Casas et al. 2013). In Puerto Rican pregnant women, an increasing trend was reported between BPA concentrations and pre-pregnancy BMI (Meeker et al. 2013). Another study conducted in New York City reported that African American women had higher urinary BPA concentrations than Dominican women during pregnancy and reported a positive association between urinary BPA concentrations and urinary phthalate concentrations (Hoepner et al. 2013).
Determinants of BPA exposure may vary across populations (Braun et al. 2011; Calafat et al. 2008; Casas et al. 2013; He et al. 2009; Hoepner et al. 2013; Meeker et al. 2013) and identification of modifiable exposure factors may help to minimize exposures during critical windows of development. Additionally, although BPA concentrations are reported to be lower in Mexican-Americans (Calafat et al. 2008), it is not known what factors contribute to exposures within this population, particularly among pregnant women, and whether BPA exposure changes with acculturation. BPA exposure data on minority populations in the U.S. is also limited.
In the present study, we evaluated variability and identified predictors of urinary BPA concentrations measured at two time points during pregnancy in a sample of predominantly low-income Mexican/Mexican-American women living in California. We also explored the role of residence time in the United States on significant dietary predictors of BPA exposure.
Methods and Materials
Study Participants
Participants were pregnant women participating in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal birth cohort study of environmental exposures and children’s health. Participating families are predominantly lowincome, Mexican-Americans or Mexican immigrants, and live in the Salinas Valley, California, an agricultural region. Pregnant women who were ≥18 years old, <20 weeks gestation, Spanish-or English-speaking, eligible to receive government health insurance, receiving prenatal care from local community clinics, and planning to deliver at the county hospital were recruited in 1999 and 2000 (Eskenazi et al. 2003). In total, 601 pregnant women were enrolled in the study. All protocols were reviewed and approved by the Committee for Protection of Human Subjects at the University of California, Berkeley and the Centers for Disease Control and Prevention (CDC) and written informed consent was obtained from participants prior to data and sample collection. Women were interviewed and provided urine samples during two prenatal visits. The first prenatal visit took place at approximately (Mean ± SD) 14.0 ± 5.1 weeks gestation (Range: 5 to 28 weeks gestation), while the second prenatal visit took place at approximately 26.4 ± 2.4 weeks gestation (Range: 18 to 39 weeks gestation). The present analysis includes all women of Mexican descent who provided at least one prenatal sample of sufficient volume for analysis for BPA and specific gravity. Specific gravity measurements were not available for 79 of the urine samples collected at the first prenatal visit, so these samples were excluded from analysis. The final sample included 491 women (n=866 urine samples). Urinary BPA concentrations were available from 407 women at the first prenatal visit and 459 at the second prenatal visit; 375 women contributed BPA measurements at both prenatal visits. Demographic characteristics were similar between women who provided one urine sample and women who provided two urine samples (data not shown).
Data Collection
Bilingual study staff conducted interviews in Spanish or English at each prenatal visit to collect maternal information on demographic characteristics, general dietary habits, and health. During the second prenatal visit, study staff also administered a modified version of the Block food frequency questionnaire to document participants’ dietary nutritional intake throughout the pregnancy (Harley et al. 2005).
Urine sample collection and laboratory analyses
Spot urine samples were collected in polypropylene urine cups and aliquotted into glass vials. Samples were stored at −80 °C until shipment to the CDC in Atlanta, GA for analysis. Concentration of total (free plus conjugated) species of urinary BPA was quantified using automated online solid-phase extraction-high performance liquid chromatography-isotope-dilution tandem mass spectrometry using previously validated methods (Ye et al. 2005). Analytical runs included quality control (QC) samples (~3 µg/L and ~10 µg/L), which were analyzed with standards, blanks, and study samples. The coefficients of variation of repeated measurements of the QC materials ranged between 3.9 and 5.8%, depending on the concentration. Analysis of field blanks showed no detectable BPA contamination using our collection protocol; analysis of reagent blanks indicated no BPA contamination during the laboratory sample processing. The limit of detection (LOD) was 0.4 µg/L. Concentrations below the LOD for which a signal was detected were reported as measured. Concentrations below the LOD with no signal detected were randomly imputed based on a log-normal probability distribution using maximum likelihood estimation (Lubin et al. 2004).
Although some previous studies of BPA have accounted for urine dilution by adjusting urine concentrations by creatinine (Braun et al. 2011; Calafat et al. 2008), this may not be appropriate particularly in populations undergoing rapid physiologic changes, such as pregnant women, due to high intra-individual variability in creatinine concentrations (Boeniger et al. 1993). Furthermore, as reported by Mahalingaiah et al. (Mahalingaiah et al. 2008), creatinine adjustment may not be appropriate for organic compounds such as BPA which are glucuronidated in the liver and eliminated by active tubular secretion. Other factors may also confound creatinine concentrations (e.g., muscularity, urine flow, age, exercise, diet, and diurnal variation) (Mahalingaiah et al. 2008). Thus, we normalized for dilution using the specific gravity of each sample using previously reported methods (Mahalingaiah et al. 2008). Specific gravity was measured at room temperature with a refractometer (National Instrument Company Inc., Baltimore, MD), which was calibrated with deionized water before each measurement. For comparison with other studies, we also provide general statistics and report on the variability of BPA levels in urine using creatinine-corrected concentrations (µg/g). Creatinine (mg/dL) was measured using a commercially available diagnostic enzyme method (Vitros CREA slides, Ortho Clinical Diagnostics, Raritan, NJ, USA).
Data Analysis
We first summarized demographic characteristics for women who provided at least one urine sample. We then calculated descriptive statistics for BPA concentrations at each prenatal visit. BPA concentrations were log-normally distributed, therefore, we log10-transformed concentrations prior to further analysis.
To evaluate the within- and between-woman variability and reproducibility of BPA concentrations (uncorrected and corrected for specific gravity and creatinine) and specific gravity in urine samples for women who provided both prenatal samples, we calculated the intraclass correlation coefficient (ICC) using mixed effects models (Rabe-Hesketh and Skrondal 2012). The ICC is a measure of reproducibility and commonly used to assess the suitability of biomarkers to properly characterize exposure. An ICC > 0.75 indicates excellent reproducibility, an ICC value between 0.4 and 0.75 indicates fair to good reproducibility, and an ICC of < 0.4 indicates poor reproducibility (Rosner 2006). Thus, low ICC values indicate great within-person variability and that more samples per person are needed to properly characterize exposure.
Previous studies have reported that sample collection time, independent of other factors, may influence urinary BPA concentrations (Calafat et al. 2005; Mahalingaiah et al. 2008). To test this in our study participants, we used generalized estimating equations (GEE) models (Jewell and Hubbard 2009) using log10-transformed urinary BPA concentrations (uncorrected and specific gravity-corrected) as the dependent variable and sample collection time as the independent variable; sample collection time was assessed as a continuous (military time) variable. Because consumption of processed/packaged foods may be a significant source of BPA, we also assessed collection time as a categorical variable in separate GEE models; collection time categories were based on potential meal times and included: 8:00 am to 11:59am (assumed to be after breakfast, but before lunch), 12:00pm to 1:59pm (could be before or after lunch), 2:00pm to 5:59 pm (assumed to be after lunch, but before dinner), 6:00 pm to 8:30pm (assumed before or after dinner). GEE models were conducted since they provide robust standard errors and take into account the non-independence of repeated urine samples collected from the same individual.
GEE models (Jewell and Hubbard 2009) were also used to evaluate predictors of BPA exposure during pregnancy. Separate models were run using uncorrected and specific gravity-corrected urinary BPA concentrations as the dependent variables. We assessed several sociodemographic factors, maternal characteristics, and dietary factors as potential predictors of exposure including those previously reported in the published literature (Braun et al. 2011; Calafat et al. 2008; Cao et al. 2011; Lakind and Naiman 2010; Mahalingaiah et al. 2008). Potential predictors of BPA exposure considered in the models included: maternal age, education, parity, pre-pregnancy body mass index (BMI), income poverty ratio (ratio of family income to the respective poverty threshold based on 2000 U.S. Census data), years spent living in the United States, consumption of: soda, alcohol, canned fruit, bottled water, pizza, fish, and hamburgers during pregnancy; gestational age at the time of urine sample collection, and collection time of each urine sample provided.
Information on demographic characteristics and pre-pregnancy BMI was collected at the first prenatal visit. Pre-pregnancy BMI (kg/m2) was calculated based on self-reported weight and measured height. Information on dietary consumption throughout the pregnancy was extracted from the food frequency questionnaire administered in the second prenatal visit. This food frequency questionnaire was originally designed to document women’s nutrient intake during pregnancy and lists 124 food items but has limited information about food packaging. Thus, of the 124 food items, we only included the limited number of available food items previously associated with BPA or potentially packaged in containers with BPA. Time-varying covariates included in the models were gestational age at the time the urine samples were collected, maternal smoke exposure (personal and second hand exposure), soda consumption, and alcohol consumption. Information on these time-varying covariates was collected at the time of each urine collection (e.g., at the first interview, mothers were asked about soda consumption habits since they became pregnant and at the second interview they were asked about these habits since the first interview). With the exception of gestational age, collection time, and income poverty ratio, covariates were examined as categorical variables in our GEE model; variables were categorized as specified in Table 1. Values for missing covariates (<5%) were randomly imputed based on observed probability distributions. All potential predictors of BPA exposure were included in the GEE models as independent variables; statistical significance of individual predictors was considered as a p-value ≤0.05.
Table 1.
General demographic characteristics for CHAMACOS pregnant womena
| Characteristic | N | (%) | |
|---|---|---|---|
| Maternal Age | |||
| 18–22 | 156 | (31.8) | |
| 23–25 | 116 | (23.6) | |
| 26–29 | 110 | (22.4) | |
| >30 | 109 | (22.2) | |
| Maternal Age (mean + sd) | 25.6 + 5.2 years | ||
| Marital Status | |||
| Married or living as married | 399 | (81.3) | |
| Single | 92 | (18.7) | |
| Maternal Education | |||
| <6th grade | 221 | (45.0) | |
| 7–12th grade | 181 | (36.9) | |
| >high school | 89 | (18.1) | |
| Parity | |||
| No previous children | 169 | (34.4) | |
| One child | 150 | (30.6) | |
| 2 or more children | 172 | (35.0) | |
| Years in the United States | |||
| <5 yrs | 258 | (52.5) | |
| 6–10 yrs | 115 | (23.4) | |
| 11+ yrs | 76 | (15.5) | |
| Entire Life | 42 | (8.6) | |
| Years in the United States (mean + sd) | 7.2 + 7.2 years | ||
| Poverty Levelb | |||
| <100% poverty line | 307 | (62.5) | |
| 100–200% poverty line | 170 | (34.6) | |
| >200% of poverty line | 14 | (2.9) | |
| Employed at any point during pregnancyc | |||
| Yes | 304 | (62.9) | |
| No | 179 | (37.1) | |
| Mother smoked during pregnancy | |||
| Yes | 20 | (4.1) | |
| No | 471 | (95.9) | |
| Second hand smoke exposure during pregnancy | |||
| Yes | 20 | (4.1) | |
| No | 471 | (95.9) | |
| Mother drank any alcohol during pregnancyc | |||
| Yes | 106 | (22.4) | |
| No | 367 | (77.6) |
Information is provided for Latina pregnant women who provided at least one urine sample and had specific gravity information.
Poverty level was used to calculate income poverty ratio calculated as the ratio of family income to the respective poverty threshold based on 2000 U.S. Census data. Income poverty ratio was used in the final GEE model.
Information was missing for some women (n=8 for employment status and n=18 for a n=18 for alcohol consumption during pregnancy).
All statistical analyses were conducted using Stata 10 for Windows (StataCorp, College Station, TX).
Results
Participant Characteristics
Mothers were primarily young (Mean ± SD: 25.6 ±5.2 years), married or living as married (81%), and relatively few had education beyond high school (18%). Thirty four percent of the mothers were expecting their first child, while the other 66% of the women had at least one child. The mean residence time in the United States of participating women at the time of the pregnancy was 7.2 years (SD: 7.2 years). Over 60% of women lived below the federal poverty threshold and most of them (63%) worked at some point during their pregnancy. Few of them smoked (<5%), were exposed to second hand smoke (33%), or drank any alcohol during pregnancy (<23%) (Table 1).
BPA concentrations at each prenatal visit
Table 2 presents summary statistics for BPA concentrations corrected and uncorrected for urinary dilution at each collection. BPA was detected in ≥79% of the samples provided at each prenatal visit. Median and geometric mean BPA urinary concentrations were similar at both prenatal visits regardless of whether concentrations were uncorrected or corrected for dilution using creatinine or specific gravity. For urine samples collected at the first prenatal visit, urinary BPA concentrations ranged from <LOD to 63.2 µg/L (<LOD to 27 µg/gCre) and from <LOD to 32.8 µg/L (<LOD to 47.6 µg/gCre) at the second prenatal visit. Specific gravity-corrected concentrations ranged from <LOD to 50.6 µg/g and from <LOD to 31.5 µg/g in the first and second prenatal visit, respectively. Maximum concentrations for creatinine-corrected BPA concentrations were also observed to be higher in the first visit (versus the second visit), in contrast to the uncorrected and specific gravity-corrected concentrations.
Table 2.
Urinary Bisphenol A concentrations in CHAMACOS Pregnant Women.a
| Collectionb | n | %>LOD | p25 | p50 | p75 | Max | GM(GSD) | ||
|---|---|---|---|---|---|---|---|---|---|
| Uncorrected, µg/L | |||||||||
| 1st prenatal visit | 407 | 79.1 | 0.5 | 1.0 | 1.7 | 63.2 | 0.9 | (2.8) | |
| 2nd prenatal visit | 459 | 82.1 | 0.5 | 1.0 | 1.8 | 32.8 | 1.0 | (2.6) | |
| Creatinine-Corrected, µg/gCre | |||||||||
| 1st prenatal visit | 407 | 79.1 | 0.6 | 1.1 | 1.8 | 27.0 | 1.1 | (2.4) | |
| 2nd prenatal visit | 459 | 82.1 | 0.7 | 1.1 | 1.8 | 47.6 | 1.2 | (2.2) | |
| Specific Gravity-Corrected, µg/L | |||||||||
| 1st prenatal visit | 407 | 79.1 | 0.7 | 1.1 | 1.9 | 50.6 | 1.2 | (2.4) | |
| 2nd prenatal visit | 459 | 82.1 | 0.7 | 1.2 | 1.9 | 31.5 | 1.2 | (2.2) |
Values reported are for Latina women who provided at least one urine sample and had data on specific gravity.
Samples were collected around (mean + sd) 14.0 + 5.1 weeks and 26.4 + 2.4 weeks gestation for the 1st and 2nd prenatal visits, respectively.
Abbreviations: LOD=Limit of detection (0.4 µg/L); GM=Geometric mean; GSD= Geometric standard deviation.
Variability of urinary BPA concentrations
We observed greater within- than between-woman variability in urinary BPA concentrations for the 375 women who provided urine samples at both prenatal visits. Intraclass correlation coefficient (ICC) values were 0.22, 0.14, and 0.16 for uncorrected, creatinine-corrected and specific gravity- corrected urinary BPA concentrations, respectively, indicating that 78 to 86% of the variability in urinary BPA concentrations was due to intra-individual variability. Additionally, specific gravity values were found to vary more within- than between-women (ICC=0.26).
Sample collection time
Independent of other factors, BPA urinary concentrations were slightly higher when the sample was collected later in the day. For every one-hour increase in sample collection time, we observed a 3.13% (p=0.03) and 3.3% (p=0.007) increase in uncorrected and specific gravity-corrected BPA concentrations, respectively. When we evaluated time as a categorical variable based on potential meal times, we observed a 16.8% (p=0.04) and 19.6% (p=0.006) increase in uncorrected and specific gravity-corrected urinary BPA concentrations, respectively, in samples collected between 2:00 and 5:59 pm relative to samples collected before 12:00 pm. We also observed an increase (~8–18% increase), albeit non-significant (p≥0.14), in uncorrected and specific-gravity corrected urinary BPA concentrations in samples collected at or after 12 noon compared to concentrations in samples collected earlier.
Predictors of BPA exposure during pregnancy
Our analysis from GEE multiple linear regression models revealed that years spent living in the United States, servings of soda and hamburgers, and sample collection time were significant predictors of BPA exposure during pregnancy in our study participants after controlling for other factors (Table 3). Pregnant women who reported living in the United States for their entire lives had 38% (95% CI: −0.1, 89.3; p=0.05) and 35% (95% CI: 2.6, 78.0; p=0.03) higher uncorrected and specific gravity-corrected urinary BPA concentrations, respectively, compared with women who reported living in the United States for 5 years or less. Additionally, women who reported drinking at least three sodas per day had approximately 58% (95% CI: 18.0, 112.1; p=0.002) and 41% (95%CI: 9.9, 80.9; p=0.01) higher uncorrected and specific gravity-corrected urinary BPA concentrations, respectively, compared with women who did not consume soda. Compared with women who reported not consuming any hamburgers, women who reported eating hamburgers three times per week or more had 20% (95%CI: −0.2, 45.2; p=0.05) and 17.3% (95%CI: 0.5, 36.9; p=0.04) higher uncorrected and specific gravity-corrected urinary BPA concentrations, respectively. Lastly, we observed that for every one-hour increase in sample collection time, there was a 3% (95% CI: 0.3, 6.0; p=0.03 and 95%CI: 0.8, 5.8; p=0.01 for uncorrected and specific gravity-corrected concentrations, respectively) increase in urinary BPA concentrations. Results were similar when we restricted our analysis to women with no missing covariate data (i.e., no imputed covariates) and when we included collection time as a categorical variable based on potential meal times (i.e., higher BPA concentrations were observed as samples were collected later in the day and associations with other predictor variables were largely unchanged).
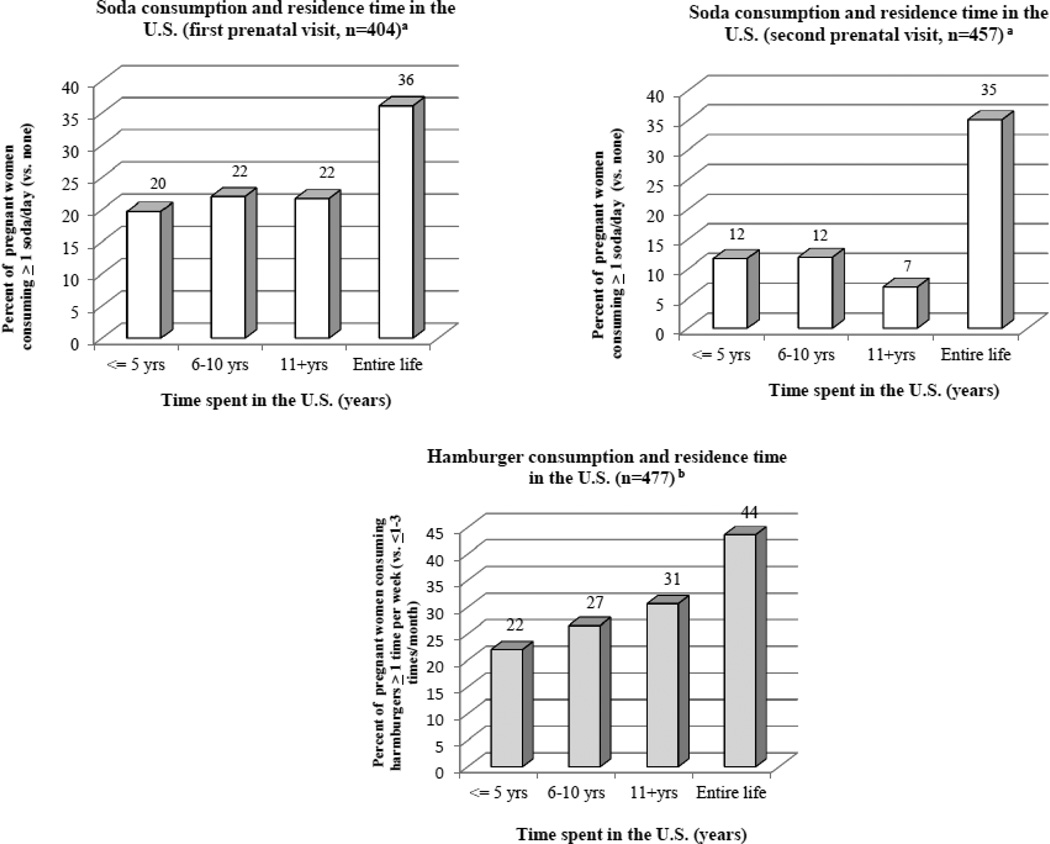
When we evaluated the relationship between time spent living in the United States and significant dietary predictors, we observed that there was a higher percentage of women who reported consuming sodas (≥ 1 soda/day vs. no sodas) and hamburgers (≥1 time per week vs. ≤ 1–3 times per month) in women who reported living in the United States their entire lives compared with women who had lived less time in the country (Figure 1).
Figure 1. Residence time in the U.S. and consumption of soda and hamburgers during pregnancy.
a. Data is for women with BPA and specific gravity measurements at each prenatal visit and with information on soda consumption. Data on soda consumption was missing for three and two women at the first and second prenatal visit, respectively. b. Data is for women who contributed BPA and specific gravity measurements at one or both prenatal visits. Data on hamburger consumption was collected only at the second prenatal visit.
Discussion
We observed significantly higher BPA concentrations with longer residence in the United States among pregnant women of Mexican descent. Pregnant women who consumed more servings of soda and hamburgers also had higher BPA concentrations. Urinary BPA concentrations from samples collected twice during pregnancy varied greatly, with high within-versus between-woman variability, and seemed to be marginally higher in samples collected in the afternoon/evening hours.
The higher BPA concentrations in pregnant women in our study who lived in the United States their entire lives compared with recent immigrants may reflect differences in diet that accompany U.S. acculturation. We previously reported that longer residence in the United States was associated with poorer diet and nutrient intake in the CHAMACOS pregnant women (Harley and Eskenazi 2006) and other studies have also shown that more acculturated individuals consume more packaged and processed foods (Ayala et al. 2008; Buzby JC et al. 2008; Cuellar S 2006). We found that a higher percentage of Mexican-American women who lived in the United States their entire lives also reported consuming more sodas and hamburgers, significant dietary predictors of BPA exposure in study participants, compared with immigrant women. Fast food intake was not explicitly measured in our study, but soda and hamburger consumption may be a marker for processed or fast food consumption. However, differences in BPA concentrations by time in the United States persisted after controlling for these factors, suggesting that hamburger and soda consumption alone do not explain all the differences in BPA exposure between US-born women and Mexican immigrants.
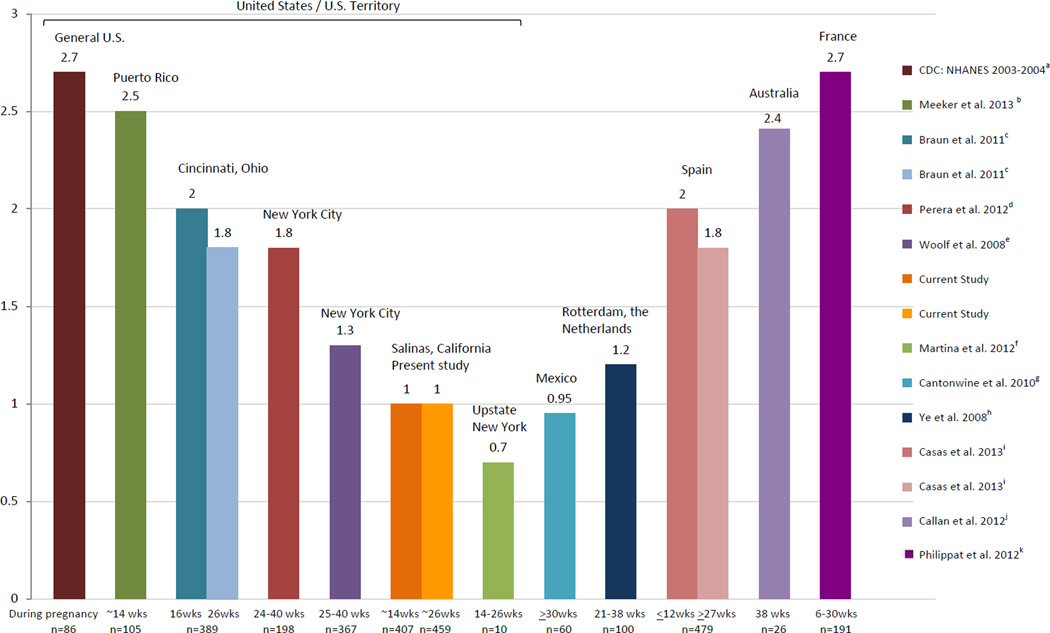
Geometric mean (GM) urinary BPA concentrations in the CHAMACOS pregnant women were about one third lower than those reported in pregnant women in the U.S. general population (GM: 1.0 vs. 2.8 µg/L) (CDC 2003–2004). With the exception of Old Order Mennonite pregnant women living in a community near Rochester, New York (Martina et al., 2012), uncorrected median urinary BPA concentrations (including creatinine- and/or specific gravity-corrected if available in other studies for comparison) in CHAMACOS pregnant women were lower than those reported previously for pregnant women in Puerto Rico (Meeker et al. 2013) and other U.S. studies (Braun et al. 2011; Casas et al. 2011; Perera et al. 2012; Philippat et al. 2012; Wolff et al. 2008). Median uncorrected concentrations in CHAMACOS pregnant women were also lower than those reported in pregnant women from Europe (Ye et al. 2008; Callan et al. 2012) (Figure 2). However, median BPA concentrations in Mexican-origin pregnant women in our study were comparable to those observed in pregnant women from Mexico City (Cantonwine et al. 2010), further suggesting that varying BPA concentrations among populations of pregnant women may be due to cultural differences in diet and behavior. For example, the comparatively low concentrations in our Mexican/Mexican-American participants and in the Mexican pregnant women studied by Cantonwine et al. (Cantonwine et al. 2010) may be related to the traditional Mexican diet that tends to favor fresh foods over packaged or processed foods (Buzby JC et al. 2008; Cuellar S 2006).
Figure 2. Median Uncorrected Urinary BPA Concentrations in Pregnant Women (µg/L).
Participants were pregnant mothers from: a the National Health and Nutrition Examination Survey (NHANES): representative sample form the general U.S. population; b the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) Study;c the Health Outcomes and Measures of the Environment (HOME) Study; d the Columbia Center for Children’s Environmental Health NYC Cohort; e the Children’s Environmental Health Study (75% of the samples were collected between 31–40 weeks gestation); f the Old Order Mennonites Study; gthe Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) study; h the Generation R Study; i the Infancia y Medio Ambiente (INMA) project; j the Australian Maternal Exposure to Toxic Substances (AMETS); and k the Etude des Déterminants pré et post natals du développement et de la santé de l’Enfant (EDEN) and PELAGIE mother-child cohorts.
Our findings of higher urinary BPA concentrations with increased soda and hamburger consumption is supported by other studies. A positive association between urinary BPA concentrations and soda consumption was also reported in a representative sample of the U.S. general population (Lakind and Naiman 2010). Additionally, a recent study conducted in Canada found that hamburgers had relatively high levels of BPA compared with other fast food items and noted that this may have been due to the wrapping paper and/or ingredients used to make the hamburgers (Cao et al. 2011).
Although canned goods are a major source of dietary exposure to BPA, we did not observe an association between BPA and canned fruit consumption. The lack of association between BPA urinary concentrations and canned fruit consumption in our study participants is consistent with findings in a Cincinnati, Ohio pregnancy cohort (Braun et al. 2011). A small survey of canned foods also reported high levels of BPA in some soups and vegetables, but no detectable levels in canned fruit (Schecter et al. 2010).
We observed high within-subject variability in urinary BPA concentrations in samples collected during two prenatal visits. This variability is likely due to the short half-life and episodic nature of BPA exposure. Less within-subject variability of BPA concentrations has been reported in non-pregnant women of child-bearing age compared with pregnant women in our study (ICC=0.43 vs. 0.14, respectively, using creatinine-corrected concentrations) (Nepomnaschy et al. 2009). It is possible that women’s changes in dietary habits during pregnancy could, in part, explain the higher variability we observed (Mirel LB et al. 2009). Our finding is very similar to that of the Cincinnati cohort, where Braun et al. (Braun et al. 2011) reported ICCs of 0.28 and 0.11 for uncorrected and creatinine-corrected BPA concentrations, respectively, for samples collected at approximately 16 and 26 weeks gestation (vs. ICCs of 0.22 and 0.14 for uncorrected and creatinine-corrected concentrations, respectively, in CHAMACOS pregnant women). We also observed great within-woman variability (ICC=0.16) in specific gravity-corrected urinary BPA concentrations as also reported in pregnant women in Boston (ICC=0.12) (Braun et al. 2012) and pregnant women from Puerto Rico (ICC=0.24) (Meeker et al. 2013). Interestingly, the CHAMACOS and Cincinnati studies (Braun et al. 2011) found that ICC values decreased when concentrations were corrected by creatinine concentrations (vs. when BPA concentrations were not corrected for dilution); decreased ICCs were also observed in our study participants when using specific gravity- corrected urinary BPA concentrations. Additionally, specific gravity values in urine samples were found to vary greatly within women (ICC=0.26) as reported in pregnant women in Boston (ICC=0.37) (Braun et al. 2012). Maximum concentrations for creatinine-corrected BPA concentrations were also observed to be higher in the first visit (vs. the second visit), in contrast to the uncorrected and specific gravitycorrected concentrations which may be due to lower creatinine excretion later in pregnancy as reported previously (Becker et al. 1992; Bradman et al. 2005; Davison et al. 1980; Davison and Noble 1981). Regardless of dilution correction method, results suggest that multiple urine samples are necessary to properly characterize BPA exposure during pregnancy given the high intra-individual variability in urinary concentrations. The suitability of other biomarkers of BPA exposure such as blood has been explored; however, BPA concentrations in blood are considerably lower than those observed in urine and decrease rapidly after exposure. Hence, a large proportion of BPA in blood will be non-detectable with the current analytical methods. Additionally, even when concentrations are detectable, BPA concentrations in blood also vary greatly within individuals (Calafat 2010).
As in the present study, several other studies have reported differences in concentrations based on sample collection time (Calafat et al. 2005; Mahalingaiah et al. 2008). Mahalingaiah et al. (Mahalingaiah et al. 2008) reported that urinary BPA concentrations in men and women were highest in samples collected between 1200 and 1600 hours compared with concentrations in morning or late afternoon/evening samples. Teeguarden et al. showed a dramatic increase in urinary BPA concentrations following lunch and dinner, but not breakfast, of meals containing canned foods (Carwile et al. 2011; Teeguarden et al. 2011). Given the short half-life of BPA in humans (<6 hours (Volkel et al. 2002)), differences in exposure levels according to sample collection time may reflect sleep and dietary intake patterns (e.g., concentrations may be lower in the morning after a long period of no intake during sleep, and levels increase during the day after consuming meals contaminated with BPA or that BPA content in foods consumed later in the day is higher than that in foods consumed earlier in the day) (Calafat et al. 2008).
Limitations of this study include imperfect data on predictor variables. For example, our questionnaire did not distinguish between canned or bottled soda consumption, with the latter not likely to be a significant source of BPA (Lakind and Naiman 2010). Moreover, we did not collect information on fasting time or time of last urination when we collected urine samples, both of which may impact BPA urinary concentrations (Stahlhut et al. 2009). Because we did not obtain information on time of day meals were consumed, we were not able to confirm whether higher BPA urinary concentrations observed in the afternoon/evening hours resulted from ingestion of BPA-contaminated food during the day. We also did not collect information on other potential sources of BPA exposure (e.g., dental treatment, medical devices, or exposure to thermal receipts). Furthermore, the study instruments administered were originally designed to assess exposures to pesticides rather than BPA. The food frequency questionnaire was also designed to document women’s nutrient intake during pregnancy and only limited information was gathered about food packaging. Although one question asked about consumption of canned fruit, there were no questions specifically about canned vegetables, soups, or tuna fish. The question about fish consumption included both fresh and canned fish, so fish was included as one of the food items of interest. Thus, information on potentially important BPA exposure sources such as consumption of packaged or processed foods other than canned fruits was not available. Although we gathered detailed dietary information during the second prenatal visit using a food frequency questionnaire, a 24-hour recall survey at both visits might have also been more appropriate given that the short half-life of BPA (Volkel et al. 2002). Additionally, although working as a cashier has been reported to be associated with higher BPA exposure in pregnant women (Braun et al. 2011), we were not able to assess this in our population due to the low number of women reporting this occupation (n=5). Even so, median uncorrected urinary BPA concentrations in these five women were not that different than those observed in women who were unemployed or reported another profession at the time of urine sample collection (1.1 µg/L vs. 1.0 µg/L in the first prenatal visit and 1.0 µg/L vs. 1.1 µg/L in the second prenatal visit).
Despite study limitations, findings from our study have several implications. First, consistent with other studies (Braun et al. 2011; Nepomnaschy et al. 2009), urinary BPA concentrations varied greatly within women suggesting the need for collection of multiple urine samples to better characterize BPA exposure over time and avoid exposure misclassification. The episodic nature of the exposures and the relatively short half-life of BPA (<6 hours (Volkel et al. 2002)) result in the observed high within-woman variability, and concentrations reflect recent exposures. Also, variations in urinary BPA concentrations throughout the day highlight the need to consider sample collection time and the time of the last urination to correctly categorize exposure in future epidemiological investigations (Stahlhut et al. 2009; Ye et al. 2011). Findings also suggest that, for women participating in this study, residence time in the United States is associated with different dietary habits that influence BPA exposure.
Conclusions
In summary, our findings suggest that there are some factors that could be modified to minimize exposures during pregnancy in Mexican-origin women (e.g., reducing soda and hamburger intake) and that sociodemographic factors may influence BPA exposure. This study supports other findings of relatively lower BPA urinary concentrations in Mexican-American populations compared with other populations, but is the first to show that factors associated with acculturation might increase BPA concentrations. Additional studies are needed to confirm our findings and evaluate determinants of BPA exposure in other populations.
RESEARCH HIGHLIGHTS.
-
-
Longer residence time in the U.S. was associated with higher BPA exposure.
-
-
High soda or hamburger consumption was associated with higher BPA exposure.
-
-
Women who lived their entire lives in the U.S. consumed more sodas and hamburgers.
First study to show that acculturation-related factors may increase BPA exposure.
Acknowledgements
This publication was supported by grant numbers: RD 83171001 from the U.S. EPA, and RC ES018792 and P01 ES009605 from NIEHS. This work is solely the responsibility of the authors and does not necessarily represent the official views of the funders or CDC. We gratefully acknowledge the study participants, community partners, and our field and Center staff for their contributions to this work. We would also like to thank Xiaoliu Zhou, Tao Jia, and Ryan Hennings for measuring the urinary BPA concentrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of Abbreviations: BPA: Bisphenol A; CHAMACOS: Center for the Health Assessment of Mothers and Children of Salinas; ICC: intraclass correlation coefficient; GEE: generalized estimating equations; GM: geometric mean; GSD: geometric standard deviation; CI: confidence interval; CDC: Centers for Disease Control and Prevention; SD: standard deviation; LOD: Limit of detection
Competing Interests: The authors declare that they have no competing interests.
Contributor Information
Lesliam Quirós-Alcalá, Email: lquiros@berkeley.edu.
Brenda Eskenazi, Email: eskenazi@berkeley.edu.
Asa Bradman, Email: abradman@berkeley.edu.
Xiaoyun Ye, Email: xay5@cdc.gov.
Antonia M. Calafat, Email: aic7@cdc.gov.
Kim Harley, Email: kharley@berkeley.edu.
References
- Ayala GX, Baquero B, Klinger S. A systematic review of the relationship between acculturation and diet among Latinos in the United States: implications for future research. J Am Diet Assoc. 2008;108:1330–1344. doi: 10.1016/j.jada.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JG, Whitworth JA, Kincaid-Smith P. Clinical Nephrology in Medical Practice. Boston: Blackwell Scientific Publications; 1992. [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr D, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly M, McKone T. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119:131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby JC, Lin B, Wells HF, Lucier G, A P. Canned Fruit and Vegetable Consumption in the United States: A Report to the United States Congress. USDA Economic Research Service. 2008 Available at: http://www.ers.usda.gov/publications/ap/ap032/ap032.pdf.
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan AC, Hinwood AL, Heffernan A, Eaglesham G, Mueller J, Odland JO. Urinary bisphenol A concentrations in pregnant women. Int J Hyg Environ Health. 2012 doi: 10.1016/j.ijheh.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, Fortenberry GZ, Calafat AM, Tellez-Rojo MM. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environmental health : a global access science source. 2010;9:62. doi: 10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XL, Perez-Locas C, Dufresne G, Clement G, Popovic S, Beraldin F, Dabeka RW, Feeley M. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:791–798. doi: 10.1080/19440049.2010.513015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Use of Polycarbonate Bottles and Urinary Bisphenol A Concentrations. Environ Health Perspect. 2009 doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. JAMA. 2011;306:2218–2220. doi: 10.1001/jama.2011.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodriguez LS, Riano I, Tardon A, Vrijheid M, Calafat AM, Sunyer J. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37:858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, Koch HM, Mendez MA, Sunyer J, Rubio S, Vrijheid M. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int. 2013;56C:10–18. doi: 10.1016/j.envint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- CDC 2003–20004. National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed September 2012]. National Center for Health Statistics (NCHS) [ http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/lab03_04.htm]. [Google Scholar]
- Chou WC, Chen JL, Lin CF, Chen YC, Shih FC, Chuang CY. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environmental health : a global access science source. 2011;10:94. doi: 10.1186/1476-069X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar S. The Hispanic Market in the U.S.–Opportunities and Challenges for the Food Industry. Dept. of Applied Economics and Management, Cornell University; 2006. [Google Scholar]
- Davison JM, Dunlop W, Ezimokhai M. 24-hour creatinine clearance during the third trimester of normal pregnancy. Br J Obstet Gynaecol. 1980;87:106–109. doi: 10.1111/j.1471-0528.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88:10–17. doi: 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Gladstone E, Jaramillo S, Birch K, Holland N. CHAMACOS, A Longitudinal Birth Cohort Study: Lessons from the Fields. Journal of Children's Health. 2003;1:3–27. [Google Scholar]
- Harley K, Eskenazi B. Time in the United States, social support and health behaviors during pregnancy among women of Mexican descent. Soc Sci Med. 2006;62:3048–3061. doi: 10.1016/j.socscimed.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley K, Eskenazi B, Block G. The association of time in the US and diet during pregnancy in low-income women of Mexican descent. Paediatr Perinat Epidemiol. 2005;19:125–134. doi: 10.1111/j.1365-3016.2005.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Miao M, Herrinton LJ, Wu C, Yuan W, Zhou Z, Li DK. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ Res. 2009;109:629–633. doi: 10.1016/j.envres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Hoepner LA, Whyatt RM, Just AC, Calafat AM, Perera FP, Rundle AG. Urinary concentrations of bisphenol A in an urban minority birth cohort in New York City, prenatal through age 7 years. Environ Res. 2013;Volume 122:38–44. doi: 10.1016/j.envres.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell NP, Hubbard A. Epidermology. Taylor & Francis Group; 2009. Analysis Of Longitudinal Studies. [Google Scholar]
- Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005–2006. National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2010;21:272–279. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Ryu HY, Kim HK, Min CS, Lee JH, Kim E, Nam BH, Park JH, Jung JY, Jang DD, Park EY, Lee KH, Ma JY, Won HS, Im MW, Leem JH, Hong YC, Yoon HS. Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol. 2008;25:413–419. doi: 10.1016/j.reprotox.2008.05.058. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116:173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina CA, Weiss B, Swan SH. Lifestyle behaviors associated with exposures to endocrine disruptors. Neurotoxicology. 2012;33:1427–1433. doi: 10.1016/j.neuro.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernandez N, Jimenez-Velez B, Alshawabkeh AN, Cordero JF. Distribution, Variability, and Predictors of Urinary Concentrations of Phenols and Parabens among Pregnant Women in Puerto Rico. Environ Sci Technol. 2013;47:3439–3447. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M, Yuan W, Zhu G, He X, Li DK. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod Toxicol. 2011;32:64–68. doi: 10.1016/j.reprotox.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Mirel LB, Curtin LR, Gahche J, V B. JSM Proceedings. Alexandria, VA: American Statistical Association; 2009. Characteristics of pregnant women from the 2001–06 National Health and Nutrition Examination Survey; pp. 2592–2601. Available at: https://www.amstat.org/membersonly/proce edings/2009/papers/304082.pdf. [Google Scholar]
- Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long-term frozen storage. Environ Res. 2009;109:734–737. doi: 10.1016/j.envres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman J, Calafat A, Xiong W, Rauh V, Wang S. Prenatal Bisphenol A Exposure and Child Behavior in an Inner City Cohort. Environ Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA, Cordier S, Slama R. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120:464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata Volume I: Continuous Responses. Third Edition. College Station, TX: 2012. [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 6th ed. Pacific Grove, CA: Duxbury Press; 2006. [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2012;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Malik N, Haffner D, Smith S, Harris TR, Paepke O, Birnbaum L. Bisphenol A (BPA) in U.S. food. Environ Sci Technol. 2010;44:9425–9430. doi: 10.1021/es102785d. [DOI] [PubMed] [Google Scholar]
- Shelby MD. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol, A. NTP CERHR MON:v. 2008:vii–ix. 1–64. passim. [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. Bisphenol A Data in NHANES Suggest Longer Than Expected Half-Life, Substantial Non-Food Exposure, or Both. Environmental Health Perspectives. 2009;117 doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK. Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol Sci. 2011;123:48–57. doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- U.S.EPA Integrated Risk Information System (IRIS) [Accessed on: January 2012];Bisphenol A (CASRN 80-05-7) (Last updated 1993). Available at: http://www.epa.gov/ncea/iris/subst/0356.htm Accessed on: January 2012 (Last updated 1993). Available at: http://www.epa.gov/ncea/iris/subst/0356.htm.
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP, Steegers EA, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]