Abstract
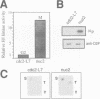
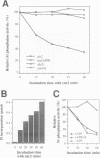
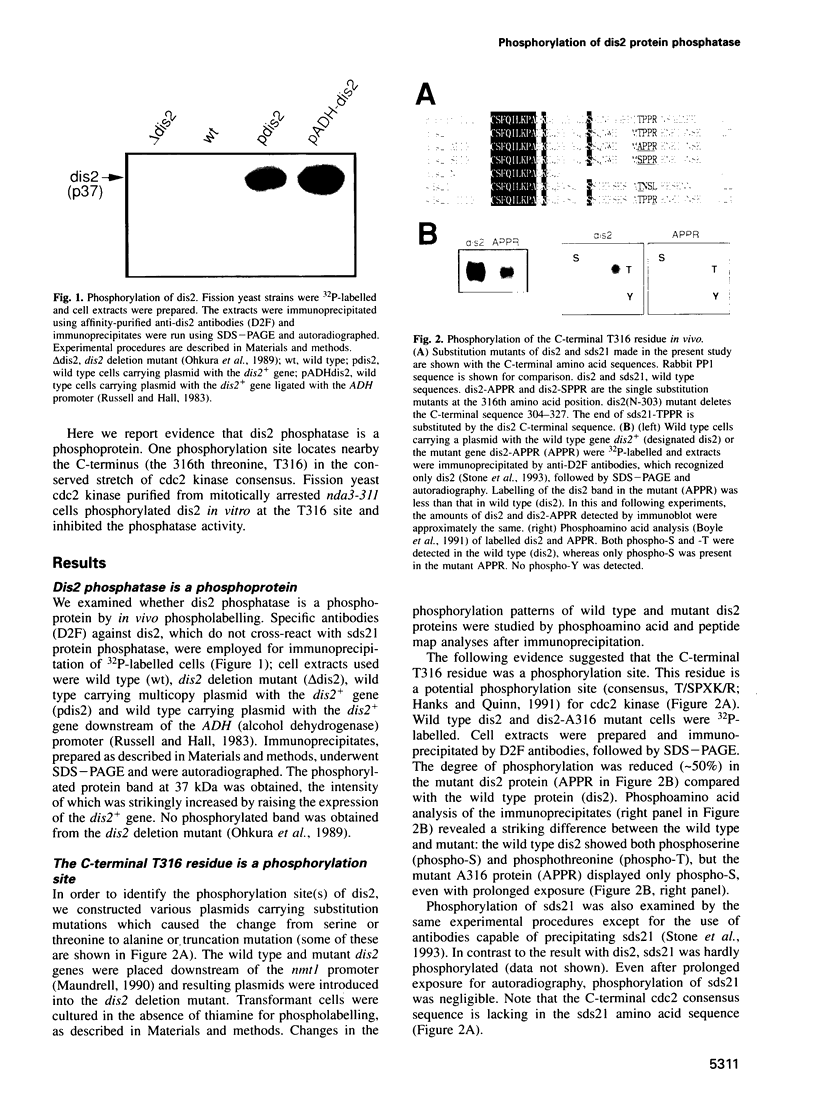
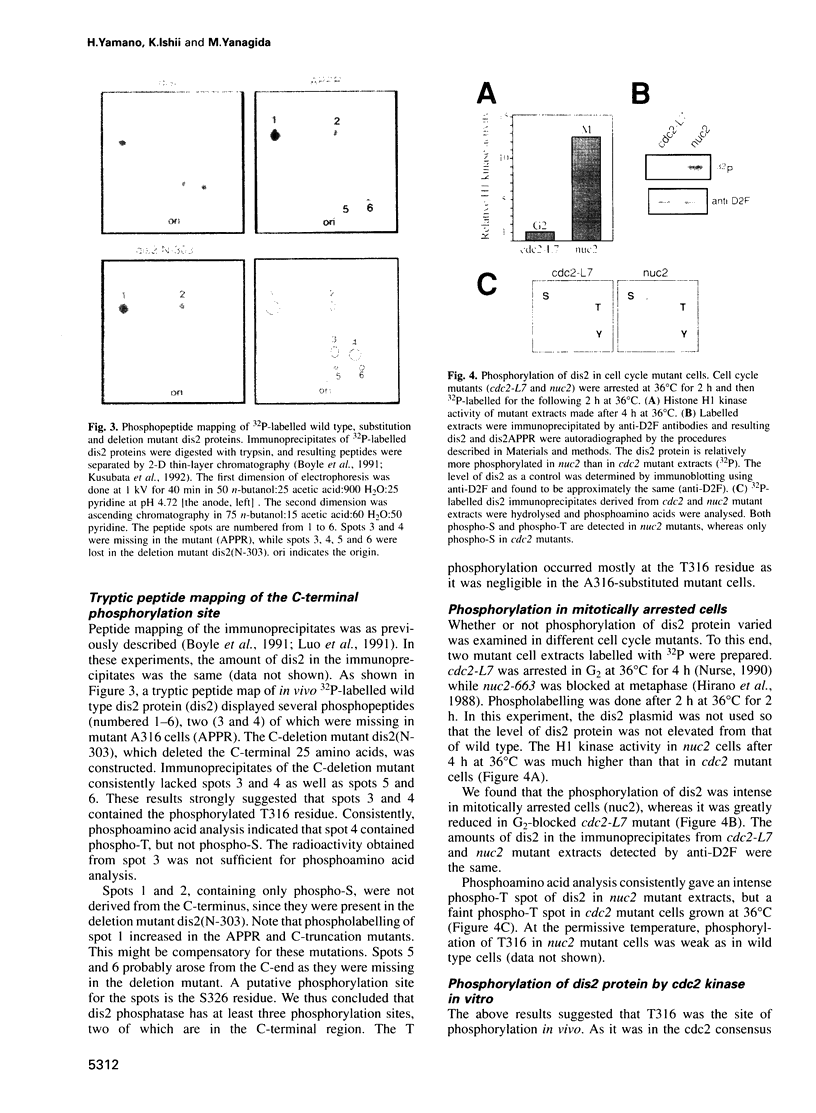
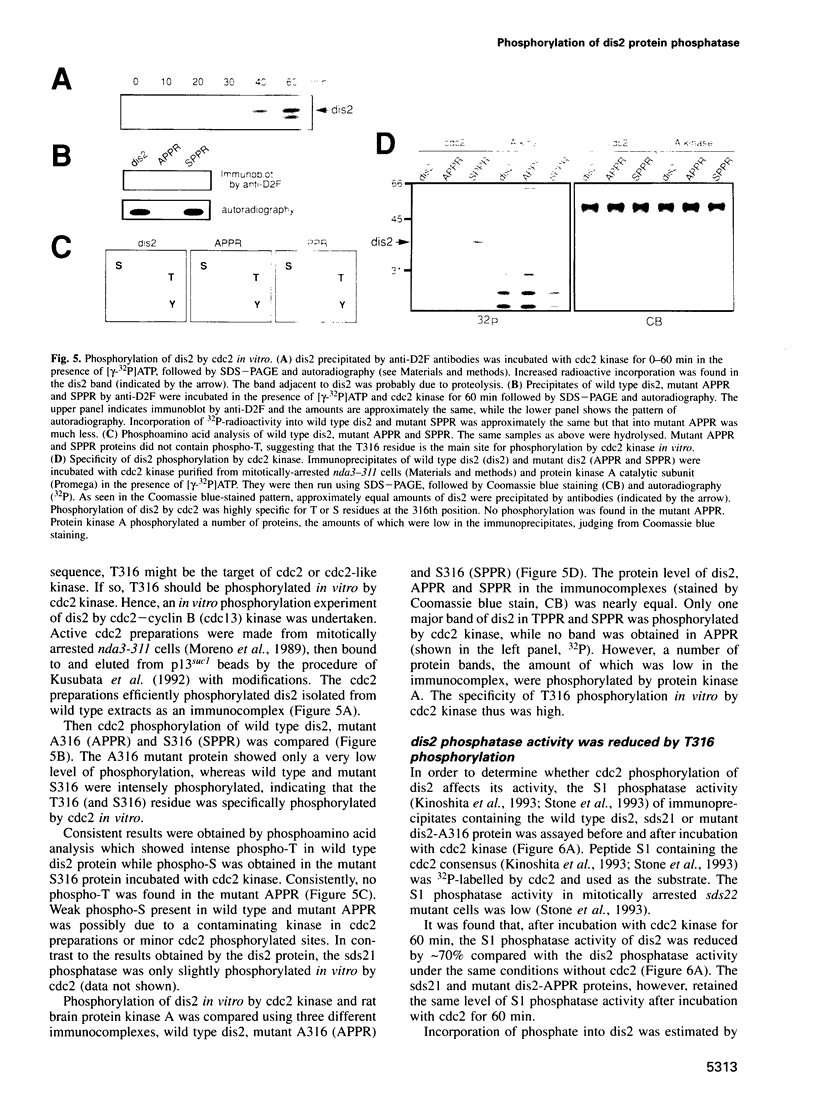
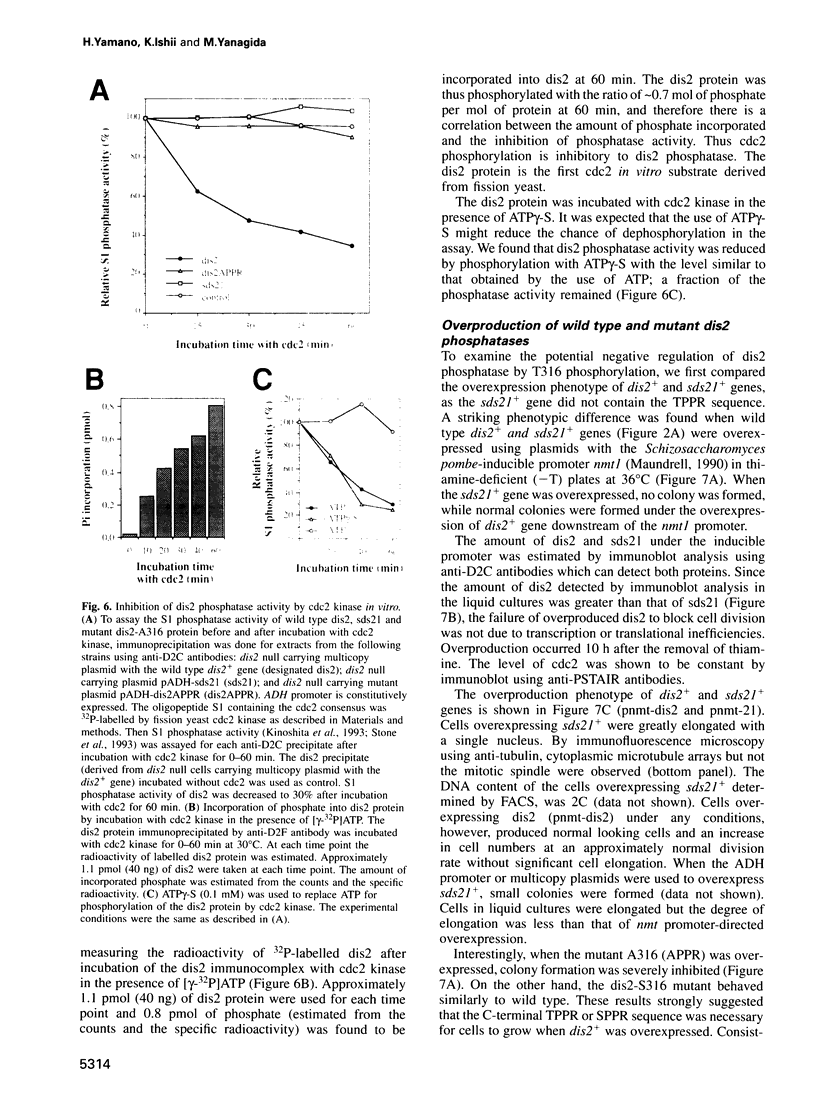
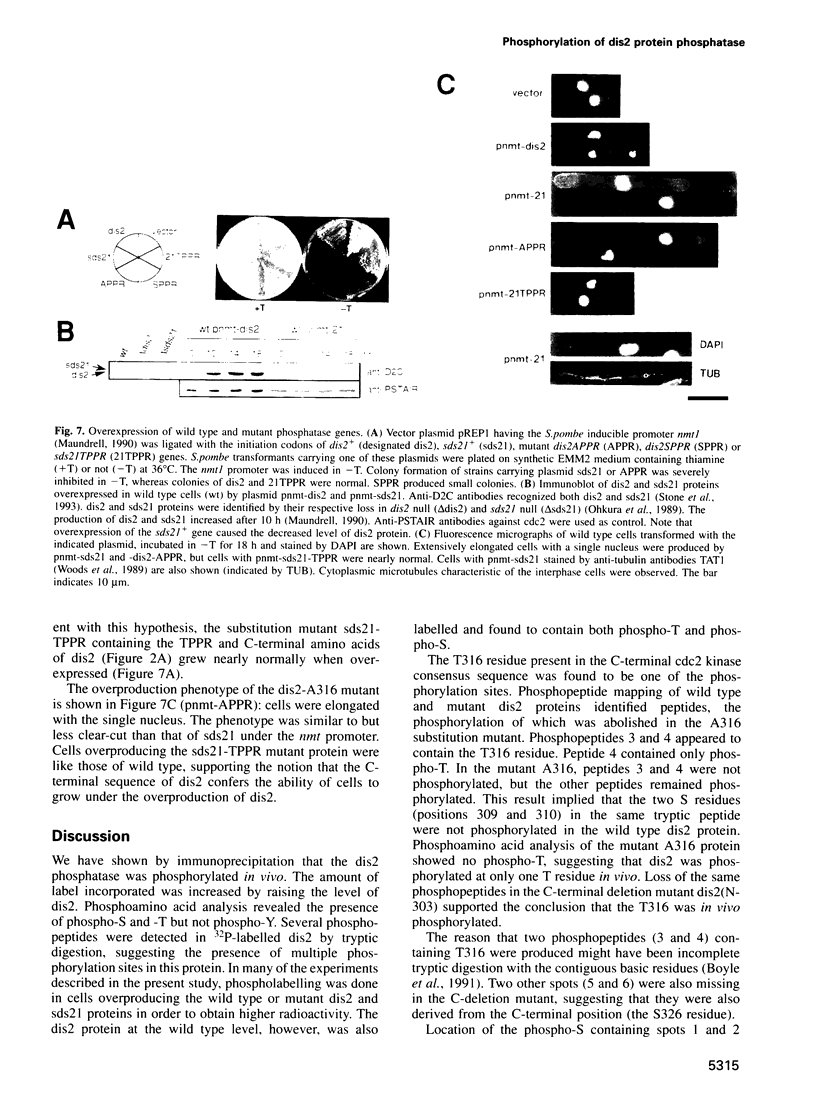
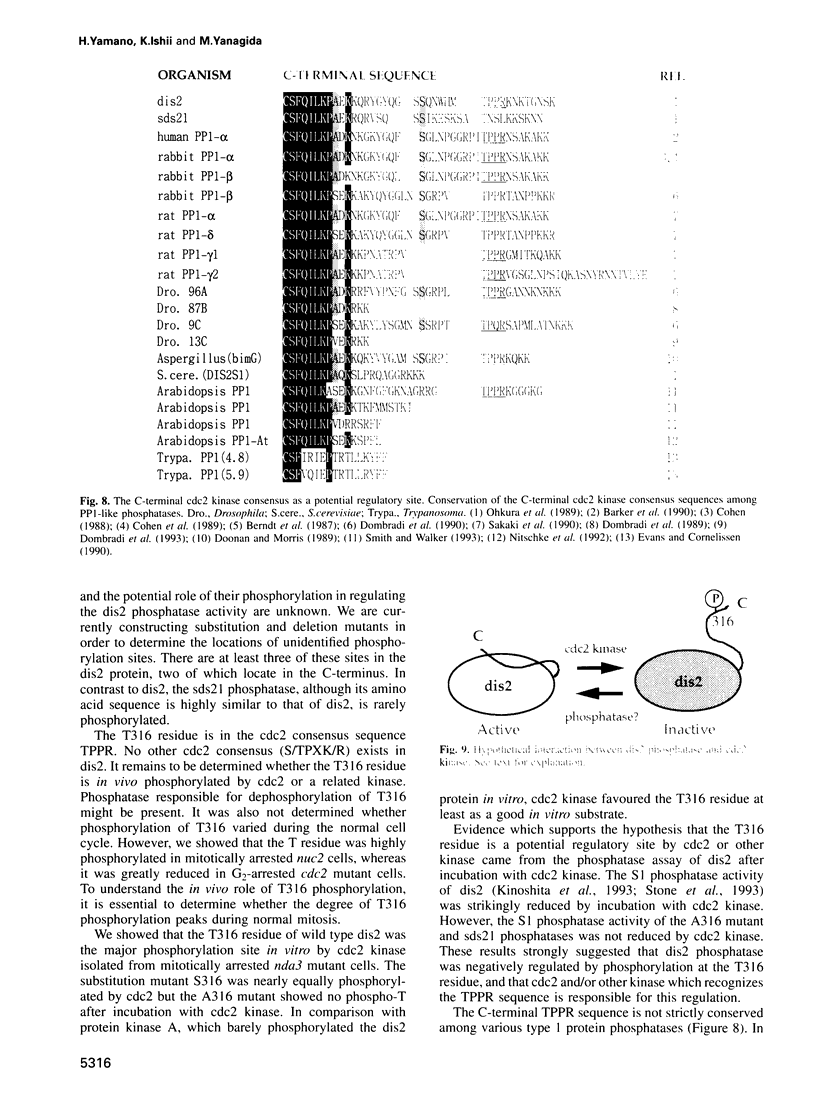
We show that the fission yeast dis2 protein phosphatase, which is highly similar to mammalian type 1 phosphatase, is a phosphoprotein containing phosphoserine (phospho-S) and threonine (phospho-T). It has several phosphorylation sites, two of which locate in the C-terminus. Phospho-T was abolished in the alanine substitution mutant at the C-terminal T316, which is conserved as a residue in the cdc2 consensus, TPPR, in a number of type 1-like phosphatases. In G2-arrested cdc2-L7 cells, the degree of T316 phosphorylation was reduced, whereas it was enhanced in metaphase-arrested nuc2-663 mutant cells. Phospho-T was produced in dis2 by fission yeast cdc2 kinase, but not in the substitution mutant A316, indicating that the T316 residue was the site for cdc2 kinase in vitro. Phosphatase activity of wild type dis2 was reduced by incubation with cdc2 kinase, but that of mutant dis2-A316 was not. Phosphorylation of T316 hence has a potential significance in cell cycle control in conjunction with cdc2 kinase activation and inactivation. Overexpression phenotypes of wild type dis2+, sds21+ and mutant dis2-A316, sds21-TPPR genes were consistent with negative regulation of dis2 by phosphorylation. This type of regulation would explain why cells harboring the dis2-11 mutation enter mitosis but fail to exit from it.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Yanagida M. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. J Cell Biol. 1989 Apr;108(4):1195–1207. doi: 10.1083/jcb.108.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axton J. M., Dombrádi V., Cohen P. T., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990 Oct 5;63(1):33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Barker H. M., Jones T. A., da Cruz e Silva E. F., Spurr N. K., Sheer D., Cohen P. T. Localization of the gene encoding a type I protein phosphatase catalytic subunit to human chromosome band 11q13. Genomics. 1990 Jun;7(2):159–166. doi: 10.1016/0888-7543(90)90536-4. [DOI] [PubMed] [Google Scholar]
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Berndt N., Campbell D. G., Caudwell F. B., Cohen P., da Cruz e Silva E. F., da Cruz e Silva O. B., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding a type-1 protein phosphatase catalytic subunit: homology with protein phosphatase 2A. FEBS Lett. 1987 Nov 2;223(2):340–346. doi: 10.1016/0014-5793(87)80316-2. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Schelling D. L., da Cruz e Silva O. B., Barker H. M., Cohen P. The major type-1 protein phosphatase catalytic subunits are the same gene products in rabbit skeletal muscle and rabbit liver. Biochim Biophys Acta. 1989 Jun 1;1008(1):125–128. doi: 10.1016/0167-4781(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Cohen P. T. Two isoforms of protein phosphatase 1 may be produced from the same gene. FEBS Lett. 1988 May 9;232(1):17–23. doi: 10.1016/0014-5793(88)80378-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Dohadwala M., da Cruz e Silva E. F., Hall F. L., Williams R. T., Carbonaro-Hall D. A., Nairn A. C., Greengard P., Berndt N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrádi V., Axton J. M., Brewis N. D., da Cruz e Silva E. F., Alphey L., Cohen P. T. Drosophila contains three genes that encode distinct isoforms of protein phosphatase 1. Eur J Biochem. 1990 Dec 27;194(3):739–745. doi: 10.1111/j.1432-1033.1990.tb19464.x. [DOI] [PubMed] [Google Scholar]
- Dombrádi V., Axton J. M., Glover D. M., Cohen P. T. Cloning and chromosomal localization of Drosophila cDNA encoding the catalytic subunit of protein phosphatase 1 alpha. High conservation between mammalian and insect sequences. Eur J Biochem. 1989 Aug 15;183(3):603–610. doi: 10.1111/j.1432-1033.1989.tb21089.x. [DOI] [PubMed] [Google Scholar]
- Dombrádi V., Mann D. J., Saunders R. D., Cohen P. T. Cloning of the fourth functional gene for protein phosphatase 1 in Drosophila melanogaster from its chromosomal location. Eur J Biochem. 1993 Feb 15;212(1):177–183. doi: 10.1111/j.1432-1033.1993.tb17648.x. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Evers R., Cornelissen A. W. The Trypanosoma brucei protein phosphatase gene: polycistronic transcription with the RNA polymerase II largest subunit gene. Nucleic Acids Res. 1990 Sep 11;18(17):5089–5095. doi: 10.1093/nar/18.17.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I. M., Hyams J. S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988 Mar;89(Pt 3):343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hirano T., Hiraoka Y., Yanagida M. A temperature-sensitive mutation of the Schizosaccharomyces pombe gene nuc2+ that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1988 Apr;106(4):1171–1183. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Toda T., Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984 Dec;39(2 Pt 1):349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Honda R., Ohba Y., Yasuda H. The cell cycle regulator, human p50weel, is a tyrosine kinase and not a serine/tyrosine kinase. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1333–1338. doi: 10.1016/s0006-291x(05)81552-9. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Yamano H., Le Bouffant-Sladeczek F., Kurooka H., Ohkura H., Stone E. M., Takeuchi M., Toda T., Yoshida T., Yanagida M. Sister-chromatid separation and protein dephosphorylation in mitosis. Cold Spring Harb Symp Quant Biol. 1991;56:621–628. doi: 10.1101/sqb.1991.056.01.071. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Yamano H., Niwa H., Yoshida T., Yanagida M. Negative regulation of mitosis by the fission yeast protein phosphatase ppa2. Genes Dev. 1993 Jun;7(6):1059–1071. doi: 10.1101/gad.7.6.1059. [DOI] [PubMed] [Google Scholar]
- Kusubata M., Tokui T., Matsuoka Y., Okumura E., Tachibana K., Hisanaga S., Kishimoto T., Yasuda H., Kamijo M., Ohba Y. p13suc1 suppresses the catalytic function of p34cdc2 kinase for intermediate filament proteins, in vitro. J Biol Chem. 1992 Oct 15;267(29):20937–20942. [PubMed] [Google Scholar]
- Luo K. X., Hurley T. R., Sefton B. M. Cyanogen bromide cleavage and proteolytic peptide mapping of proteins immobilized to membranes. Methods Enzymol. 1991;201:149–152. doi: 10.1016/0076-6879(91)01014-s. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Ohkura H., Gomes R., Sunkel C. E., Baumgartner S., Hemmings B. A., Glover D. M. The 55 kd regulatory subunit of Drosophila protein phosphatase 2A is required for anaphase. Cell. 1993 Feb 26;72(4):621–633. doi: 10.1016/0092-8674(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Moreno S., Hayles J., Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989 Jul 28;58(2):361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Nitschke K., Fleig U., Schell J., Palme K. Complementation of the cs dis2-11 cell cycle mutant of Schizosaccharomyces pombe by a protein phosphatase from Arabidopsis thaliana. EMBO J. 1992 Apr;11(4):1327–1333. doi: 10.1002/j.1460-2075.1992.tb05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Yanagida M. S. pombe gene sds22+ essential for a midmitotic transition encodes a leucine-rich repeat protein that positively modulates protein phosphatase-1. Cell. 1991 Jan 11;64(1):149–157. doi: 10.1016/0092-8674(91)90216-l. [DOI] [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Sasaki K., Shima H., Kitagawa Y., Irino S., Sugimura T., Nagao M. Identification of members of the protein phosphatase 1 gene family in the rat and enhanced expression of protein phosphatase 1 alpha gene in rat hepatocellular carcinomas. Jpn J Cancer Res. 1990 Dec;81(12):1272–1280. doi: 10.1111/j.1349-7006.1990.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K., Yanagida M. Functional dissection of the phosphorylated termini of fission yeast DNA topoisomerase II. J Cell Biol. 1992 Dec;119(5):1023–1036. doi: 10.1083/jcb.119.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Walker J. C. Expression of multiple type 1 phosphoprotein phosphatases in Arabidopsis thaliana. Plant Mol Biol. 1993 Jan;21(2):307–316. doi: 10.1007/BF00019946. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Yamano H., Kinoshita N., Yanagida M. Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Curr Biol. 1993 Jan;3(1):13–26. doi: 10.1016/0960-9822(93)90140-j. [DOI] [PubMed] [Google Scholar]
- Woods A., Sherwin T., Sasse R., MacRae T. H., Baines A. J., Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989 Jul;93(Pt 3):491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]