Abstract
Osteoporosis and obesity are worldwide health problems. Interestingly, both are associated with significant morbidity and mortality. Both the diseases have common linkage as bone marrow mesenchymal stromal cells are the common precursors for both osteoblasts and adipocytes. Aging may shift composition of bone marrow by increasing adipocytes, osteoclast activity, and decreasing osteoblast activity, resulting into osteoporosis. Adipocytes secret leptin, adiponectin, adipsin, as well as proinflammatory cytokines, that contributes in pathogenesis of osteoporosis. This new concept supports the hypothesis, that the positive correlation of weight and body mass index (BMI) with bone mineral density (BMD) is not confirmed by large population-based studies. Thus, the previous concept, that obesity is protective for osteoporosis may not stand same as bone marrow fat deposition (adipogenesis) seen in obesity, is detrimental for bone health.
Keywords: Adipocytes, adiponectin, aging, leptin, obesity of bone, osteoporosis
INTRODUCTION
Osteoporosis and obesity are worldwide health problems associated with significant morbidity and mortality as well as both has been suggested to result from dysregulation of a common precursor cell, that is, bone marrow mesenchymal stromal cells.[1]
The ongoing debate regarding the previous concept, that obesity is protective for osteoporosis may not stand same in view of the new concept of obesity of bone (adipogenesis), which is considered detrimental for bone health. This new concept has emerged as a result of the fact that weight and body mass index (BMI) have positive correlation with bone mineral density (BMD),[2,3] but large population-based studies did not verify and confirm such positive correlation between bone mass and BMI.[4,5]
The major factors considered in past for obesity as protective factor for osteoporosis include increased load on the cortical skeleton, direct stimulation of bone formation by leptin, greater aromatase activity, increased estradiol leading decrease bone resorption, and stimulation of bone formation.[6]
However, recent data suggest childhood obesity is associated with increased risk of lower extremity fractures in spite of increase BMD.[7] Another study[8] suggested that obesity is associated with higher BMD, but its protective effect on fracture risk is controversial. Higher absolute values of BMD, cortical and trabecular architecture, and strength indices have not been in proportion to the excess of BMI in obese postmenopausal women. Obesity was associated with reduced risk of clinical spine, hip, pelvis, and wrist/forearm fractures, but increased risk of multiple rib fracture when compared to normal or underweight population.[9] The research findings that many if not most osteoporotic fracture occur in overweight or obese women and men, further refute that obesity is protective.[10]
HOW OBESITY OF BONE EXPLAIN THESE CONTRADICTIONS
Bone marrow mesenchymal stromal cells are the common precursors for both osteoblasts and adipocytes. Aging may shift composition of bone marrow by increasing adipocytes, osteoclast activity, and decreasing osteoblast activity, resulting into osteoporosis.[1] The factors secreted by adipocytes known to affect bone remodeling are leptin, adiponectin, and adipsin, as well as proinflammatory cytokines, such as tumor necrosis factor and interleukin-6.[6] Therefore, bone marrow adipogenesis, appear to exert lypotoxic effect on osteoblast. The role of leptin, adiponectin, and adipsin in exerting lypotoxic effect on bone is explained below [Figure 1].
Figure 1.
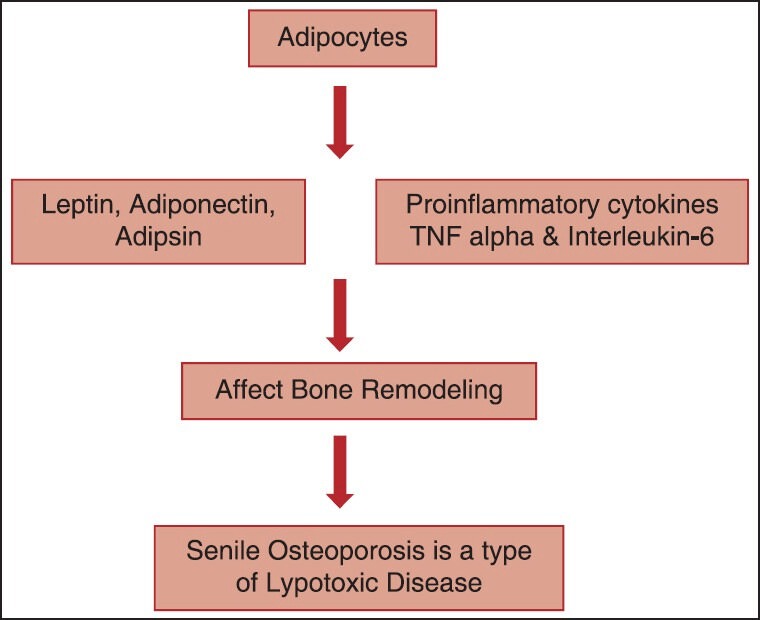
Showing the role of Leptin, Adiponectin, and Adipsin in Osteoporosis
Leptin and insulin growth factor-1 have been shown to possess positive peripheral roles in preserving bone mass during postmenopause.[10] More than that serum leptin has been shown a useful indicator of risk for osteoporosis associated with diet-induced obesity.[11] However, the recent data suggested that leptin controls bone formation through a hypothalamic relay, thereby suggesting central mechanism also to be involved in its action on bone. Adrenergic receptors deficiency (i.e., beta-less) has been shown to have increased body weight and fat mass, and exhibit greater total body bone mass, trabecular bone volume, and femoral cross-sectional size.[12,13] Thereby, indicating that the integrity of sympathetic signaling is necessary for the increase in bone resorption. Which will mean that catecholamine-deficient state might have a high bone mass and sympathomimetics administered may decrease bone formation and bone mass. Conversely, beta-blockers are likely to increase bone formation and bone mass and blunt the bone loss induced by estrogen deficient state. Thus, leptin acts similar to the estrogen.
Adiponectin has been shown to increase osteoclast formation and negatively affects osteoporosis. This action is mediated by stimulating the production of receptor activator of receptor activator of nuclear factor kappa-B ligand (RANKL) that stimulates osteoclast differentiation. Furthermore, adiponectin can inhibit the production of osteoprotegerin, a known inhibitor of osteoclastogenesis, in osteoblasts. However, its level in lean mass person is seen higher and it is on lower side in fat person. Thereby, suggesting that low levels of adiponectin in obesity may confer osteoprotection.[14]
However, contradictory evidences emerged in favor of adiponectin denying osteoprotective effect among obese persons, from recent study, wherein a unique adipokine released from adipocytes has been shown antiapoptotic, anti-inflammatory, and anti-oxidative. In one of the study, human osteoblasts have been reported to express adiponectin and its receptors and shown to increase bone mass by suppressing osteoclastogenesis and by activating osteoblastogenesis.[15]
Adipsin similarly has been shown to negatively affect osteoporosis particularly diabetes mellitus (DM) induces osteoporosis. Increased expression is seen among DM and obese patients and it has been shown to decrease bone formation.[16]
Other: Beside these factors some other important factors have been proposed in genesis of osteoporosis among obese, that is, obesity and vitamin D insufficiency decreased bioavailability of vitamin D3 because cutaneous body fat has been reported in some studies may influence on long-term negatively with osteoporosis. This factor becomes very important as there is already a high prevalence of Vitamin D deficiency.[17]
CLINICAL AND DIAGNOSTIC IMPLICATION OF THE CONCEPT- OBESITY OF BONE
Errors in BMD determinations commonly seen in markedly obese individuals are because of fat deposition in bone marrow. Dual-energy X-ray absorptiometry measurements may be falsely elevated by increased body fat; whereas, measurements of trabecular BMD by quantitative computed tomography may be decreased by greater marrow fat.[6]
Growth failure, pubertal arrest leading to reduced final adult height, and peak bone mass is usual explanation for poor bone health in Cushing's syndrome.[18] Whereas, insulin resistance and hyperglycemia are considered the reasons for deleterious effects on osteoblast function in patients of DM.[19] Similarly, glucocorticoids are known to alter the cellular composition of bone by regulating the supply and lifespan of osteoclasts and osteoblasts.[20] Immobility contributing to osteoporosis as in Parkinsonism is considered to be a result of decreased muscle strength.[21]
However, all the secondary causes of osteoporosis, like Cushing's syndrome, DM, glucocorticoids, and immobility, are associated with obesity. It was difficult in past with pervious hypothesis that obesity is protective for osteoporosis to explain the cause of osteoporosis in such cases. However, this can now be explained on the basis of associated increase in bone marrow adiposity seen in such cases.[6] Similarly, in extremes of life, puberty, and old age, fat infiltration in the bone marrow might be detrimental for skeletal strength and may negatively affect the optimal function of the bone remodeling unit.[6]
TREATMENT IMPLICATION OF THIS CONCEPT
Treatment implications are going to be immense. The present treatment options for osteoporosis primarily known are either antiosteoclastogenesis or pro-osteoblastogenesis in nature. However, recently vitamin D has been shown to act by inhibiting bone marrow adipogenesis as an additional mechanism beside its known actions on bone.[22] However, further studies in this direction demonstrated dose-dependent effects of 1,25(OH)2 D3 on the transdifferentiation of muscle cells into adipose cells. Low physiological concentrations (possibly mimicking a deficient state) induced adipogenesis; whereas, higher (physiological and supraphysiological) concentrations attenuated this effect.[23] Furthermore, there is evidence that vitamin D affects body fat mass by inhibiting adipogenic transcription factors and lipid accumulation during adipocyte differentiation. Some recent studies demonstrate that vitamin D metabolites also influence adipokine production and the inflammatory response in adipose tissue.[24]
Similarly alendronate, a widely used bisphosphonate recently has been reported to stimulate osteoblastic differentiation while inhibiting adipogenesis in vitro; thereby, suggesting anabolic effect on bone through the differentiation of mesenchymal stem cells.[25]
Parathyroid hormone (PTH) also has been shown in past to induce osteoblast differentiation, inhibits adipogenesis and suppresses osteoblast apoptosis.[26] Similarly, Parathyroid hormone related protein (1-36) (PTHrP(1-36)) has been shown to induce a mild osteogenic effect and inhibit adipocytic effect in human mesenchymal stem cell and thereby helping in osteoporosis beside its known action to modulate bone formation through promoting osteoblast differentiation.[27]
Strontium ranelate, has both antiresorptive and anabolic effects on bone. However, it has been recently shown that adipogenesis is negatively affected in the presence of strontium ranelate with a concomitant dose-dependent decrease in the expression of adipogenic markers and changes in adipokine profile, thereby generating a favorable osteogenic effect within the bone marrow milieu.[28]
Similarly in another current study, dietary relevant mixtures of isoflavones and their metabolites, lignans and their metabolites, coumestrol, and a mixture containing all of them have been shown to concentration-dependently inhibit adipocyte differentiation as their additional mechanism of action in preventing osteoporosis.[29]
CONCLUSION
The previous concept that obesity is protective for osteoporosis may not stand same and new concept of bone marrow fat deposition seen in obesity has emerged supporting the detrimental effect of obesity for bone health. Thus, obesity may not be considered as protective for osteoporosis. Furthermore, exploring the connection between bone and fat at a molecular and cellular level is likely to lead to a better understanding of several risk factors and pathogenesis basis and to the development of drugs with dual mechanism for both osteoporosis and obesity in future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Horowitz MC, Lorenzo JA. The origin of osteoclasts. Curr Opin Rheumatol. 2004;16:464–8. doi: 10.1097/01.bor.0000127825.05580.eb. [DOI] [PubMed] [Google Scholar]
- 2.Greendale GA, Judd HL. The menopause: Health implications and clinical management. J Am Geriatr Soc. 1993;41:426–36. doi: 10.1111/j.1532-5415.1993.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 3.Carranza-Lira S, Rosas M, Murillo A, Martínez N, Santos J. Osteoporosis in postmenopausal women (Mexico City): 2.Validation of a predictive clinical index. Int J Fertil Womens Med. 2002;47:26–31. [PubMed] [Google Scholar]
- 4.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–6. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–7. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 6.Rosen CJ, Bouxsein ML. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 7.Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of lower extremity fractures. Clin Orthop Relat Res. 2013;471:1199–207. doi: 10.1007/s11999-012-2621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sornay-Rendu E, Boutroy S, Vilayphiou N, Claustrat B, Chapurlat RD. In Obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: The Os des Femmes de Lyon (OFELY) Study. J Bone Miner Res. 2013;28:1679–87. doi: 10.1002/jbmr.1880. [DOI] [PubMed] [Google Scholar]
- 9.Premaor MO, Compston JE, Fina Avilés F, Pagès-Castellà A, Nogués X, Díez-Pérez A, et al. The association between fracture site and obesity in men: A population-based cohort study. J Bone Miner Res. 2013;28:1771–7. doi: 10.1002/jbmr.1878. [DOI] [PubMed] [Google Scholar]
- 10.Morcov C, Vulpoi C, Brãniºteanu D. Correlation between adiponectin, leptin, insulin growth factor-1 and bone mineral density in pre and postmenopausal women. Rev Med Chir Soc Med Nat Iasi. 2012;116:785–9. [PubMed] [Google Scholar]
- 11.Fujita Y, Watanabe K, Maki K. Serum leptin levels negatively correlate with trabecular bone mineral density in high-fat diet-induced obesity mice. J Musculoskelet Neuronal Interact. 2012;12:84–94. [PubMed] [Google Scholar]
- 12.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 13.Petzel M. Action of leptin on bone and its relationship to menopause. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:195–9. doi: 10.5507/bp.2007.034. [DOI] [PubMed] [Google Scholar]
- 14.Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, et al. EY Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648–56. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 15.Takeda Y, Nakanishi K, Tachibana I, Kumanogoh A. Adiponectin: A novel link between adipocytes and COPD. Vitam Horm. 2012;90:419–35. doi: 10.1016/B978-0-12-398313-8.00016-6. [DOI] [PubMed] [Google Scholar]
- 16.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–31. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 18.Mancini T, Doga M, Mazziotti G, Giustina A. Cushing's syndrome and bone. Pituitary. 2004;7:249–52. doi: 10.1007/s11102-005-1051-2. [DOI] [PubMed] [Google Scholar]
- 19.Lechleitner M, Pils K, Roller-Wirnsberger R, Beubler E, Gasser R, Mrak P, et al. Diabetes and osteoporosis: Pathophysiological interactions and clinical importance for geriatric patients. Z Gerontol Geriatr. 2013;46:390–7. doi: 10.1007/s00391-013-0518-4. [DOI] [PubMed] [Google Scholar]
- 20.Manolagas SC. Steroids and osteoporosis: The quest for mechanisms. J Clin Invest. 2013;123:1919–21. doi: 10.1172/JCI68062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Bos F, Speelman AD, Samson M, Munneke M, Bloem BR, Verhaar HJ. Parkinson's disease and osteoporosis. Age Ageing. 2013;42:156–62. doi: 10.1093/ageing/afs161. [DOI] [PubMed] [Google Scholar]
- 22.Duque G, Macoritto M, Kremer R. Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity. Biogerontology. 2004;5:421–9. doi: 10.1007/s10522-004-3192-5. [DOI] [PubMed] [Google Scholar]
- 23.Ryan KJ, Daniel ZC, Craggs LJ, Parr T, Brameld JM. Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. J Endocrinol. 2013;217:45–58. doi: 10.1530/JOE-12-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915–23. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 25.Duque G, Rivas D. Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. J Bone Miner Res. 2007;22:1603–11. doi: 10.1359/jbmr.070701. [DOI] [PubMed] [Google Scholar]
- 26.Chan GK, Miao D, Deckelbaum R, Bolivar I, Karaplis A, Goltzman D. Parathyroid hormone-related peptide interacts with bone morphogenetic protein 2 to increase osteoblastogenesis and decrease adipogenesis in pluripotent C3H10T 1/2 mesenchymal cells. Endocrinology. 2003;144:5511–20. doi: 10.1210/en.2003-0273. [DOI] [PubMed] [Google Scholar]
- 27.Casado-Diaz A, Santiago-Mora R, Quesada JM. The N- and C-terminal domains of parathyroid hormone-related protein affect differently the osteogenic and adipogenic potential of human mesenchymal stem cells. Exp Mol Med. 2010;42:87–98. doi: 10.3858/emm.2010.42.2.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal C, Gunaratnam K, Tong J, Duque G. Biochemical changes induced by strontium ranelate in differentiating adipocytes. Biochimie. 2013;95:793–8. doi: 10.1016/j.biochi.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Taxvig C, Specht IO, Boberg J, Vinggaard AM, Nellemann C. Dietary relevant mixtures of phytoestrogens inhibit adipocyte differentiation in vitro. Food Chem Toxicol. 2013;55:265–71. doi: 10.1016/j.fct.2012.12.060. [DOI] [PubMed] [Google Scholar]


