Abstract
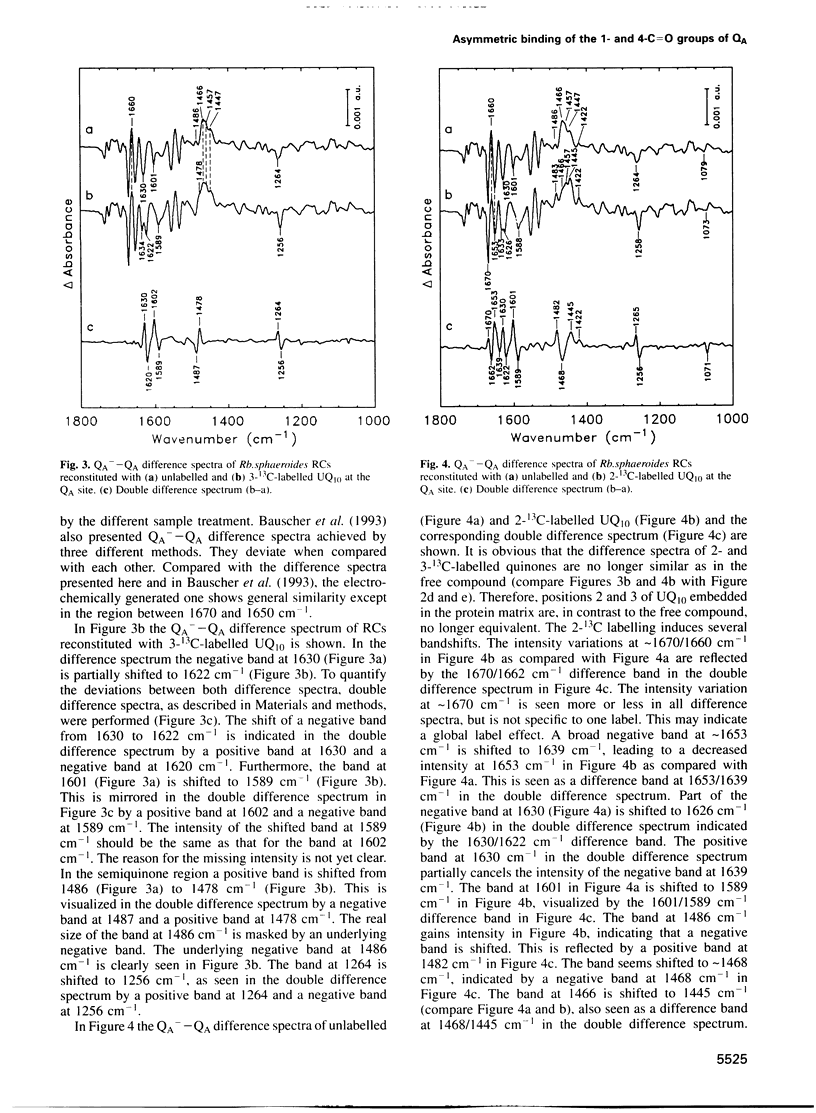
Using 1-, 2-, 3- and 4-13C site-specifically labelled ubiquinone-10, reconstituted at the QA site of Rhodobacter sphaeroides R26 reaction centres, the infra-red bands dominated by the 1- and 4-C = O vibration of QA are assigned in the QA(-)-QA difference spectra. The mode dominated by the 4-C = O vibration is drastically downshifted in the reaction centres as compared with its absorption frequency in free ubiquinone-10. In contrast, the mode dominated by the 1-C = O vibration absorbs at similar frequencies in the free and the bound forms. The frequency shift of the 4-C = O vibration is due to a large decrease in bond order and indicates a strong interaction with the protein microenvironment in the ground state. In the charge-separated state the mode dominated by the semiquinone 4-C = O vibration is characteristic of strong hydrogen bonding to the microenvironment, whereas the mode dominated by the 1-C = O vibration indicates a weaker interaction. The asymmetric binding of the 1- and 4-C = O groups to the protein might contribute to the factors governing different redox reactions of ubiquinone-10 at the QA site as compared with its reactions at the QB site.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: protein-cofactor (quinones and Fe2+) interactions. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8487–8491. doi: 10.1073/pnas.85.22.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt T., Schmidt S., Kaiser W., Lauterwasser C., Meyer M., Scheer H., Zinth W. The accessory bacteriochlorophyll: a real electron carrier in primary photosynthesis. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11757–11761. doi: 10.1073/pnas.90.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilibin A. F., Gracheva N. M. Lekarstvennaia bolezn' v klinike infektsionnykh zabolevanii. Sov Med. 1974 Jul;0(7):3–10. [PubMed] [Google Scholar]
- Boxer S. G. Mechanisms of long-distance electron transfer in proteins: lessons from photosynthetic reaction centers. Annu Rev Biophys Biophys Chem. 1990;19:267–299. doi: 10.1146/annurev.bb.19.060190.001411. [DOI] [PubMed] [Google Scholar]
- Breton J., Berthomieu C., Thibodeau D. L., Nabedryk E. Probing the secondary quinone (QB) environment in photosynthetic bacterial reaction centers by light-induced FTIR difference spectroscopy. FEBS Lett. 1991 Aug 19;288(1-2):109–113. doi: 10.1016/0014-5793(91)81014-y. [DOI] [PubMed] [Google Scholar]
- Breton J., Burie J. R., Berthomieu C., Berger G., Nabedryk E. The binding sites of quinones in photosynthetic bacterial reaction centers investigated by light-induced FTIR difference spectroscopy: assignment of the QA vibrations in Rhodobacter sphaeroides using 18O- or 13C-labeled ubiquinone and vitamin K1. Biochemistry. 1994 Apr 26;33(16):4953–4965. doi: 10.1021/bi00182a026. [DOI] [PubMed] [Google Scholar]
- Breton J., Thibodeau D. L., Berthomieu C., Mäntele W., Verméglio A., Nabedryk E. Probing the primary quinone environment in photosynthetic bacterial reaction centers by light-induced FTIR difference spectroscopy. FEBS Lett. 1991 Jan 28;278(2):257–260. doi: 10.1016/0014-5793(91)80129-q. [DOI] [PubMed] [Google Scholar]
- Buchanan S., Michel H., Gerwert K. Light-induced charge separation in Rhodopseudomonas viridis reaction centers monitored by Fourier-transform infrared difference spectroscopy: the quinone vibrations. Biochemistry. 1992 Feb 11;31(5):1314–1322. doi: 10.1021/bi00120a006. [DOI] [PubMed] [Google Scholar]
- Chang C. H., el-Kabbani O., Tiede D., Norris J., Schiffer M. Structure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides. Biochemistry. 1991 Jun 4;30(22):5352–5360. doi: 10.1021/bi00236a005. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwert K., Siebert F. Evidence for light-induced 13-cis, 14-s-cis isomerization in bacteriorhodopsin obtained by FTIR difference spectroscopy using isotopically labelled retinals. EMBO J. 1986 Apr;5(4):805–811. doi: 10.1002/j.1460-2075.1986.tb04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessling B., Souvignier G., Gerwert K. A model-independent approach to assigning bacteriorhodopsin's intramolecular reactions to photocycle intermediates. Biophys J. 1993 Nov;65(5):1929–1941. doi: 10.1016/S0006-3495(93)81264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hienerwadel R., Thibodeau D., Lenz F., Nabedryk E., Breton J., Kreutz W., Mäntele W. Time-resolved infrared spectroscopy of electron transfer in bacterial photosynthetic reaction centers: dynamics of binding and interaction upon QA and QB reduction. Biochemistry. 1992 Jun 30;31(25):5799–5808. doi: 10.1021/bi00140a016. [DOI] [PubMed] [Google Scholar]
- Leonhard M., Mäntele W. Fourier transform infrared spectroscopy and electrochemistry of the primary electron donor in Rhodobacter sphaeroides and Rhodopseudomonas viridis reaction centers: vibrational modes of the pigments in situ and evidence for protein and water modes affected by P+ formation. Biochemistry. 1993 May 4;32(17):4532–4538. doi: 10.1021/bi00068a007. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Bagley K. A., Thibodeau D. L., Bauscher M., Mäntele W., Breton J. A protein conformational change associated with the photoreduction of the primary and secondary quinones in the bacterial reaction center. FEBS Lett. 1990 Jun 18;266(1-2):59–62. doi: 10.1016/0014-5793(90)81506-j. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke K., Dutton P. L. Experimental resolution of the free energies of aqueous solvation contributions to ligand-protein binding: quinone-QA site interactions in the photosynthetic reaction center protein. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2920–2924. doi: 10.1073/pnas.90.7.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke K., Dutton P. L. Influence of QA site redox cofactor structure on equilibrium binding, in situ electrochemistry, and electron-transfer performance in the photosynthetic reaction center protein. Biochemistry. 1993 May 11;32(18):4769–4779. doi: 10.1021/bi00069a011. [DOI] [PubMed] [Google Scholar]


