Abstract
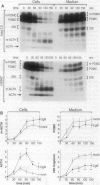
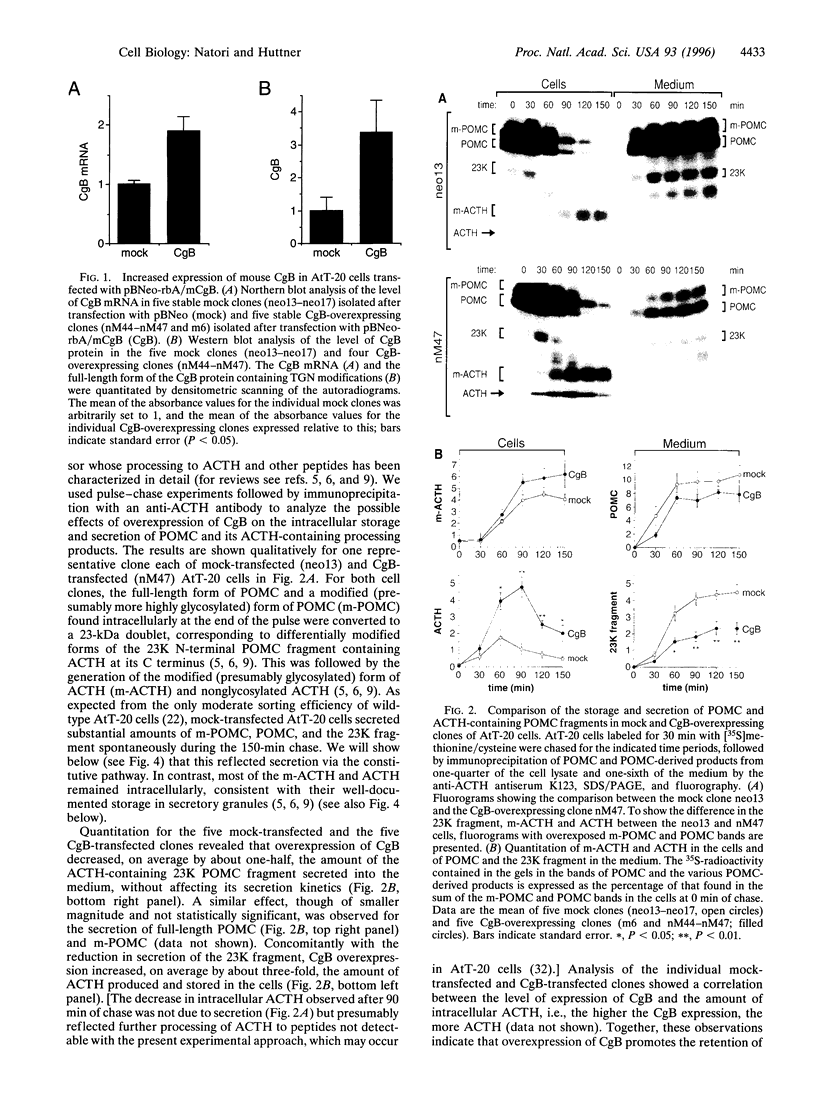
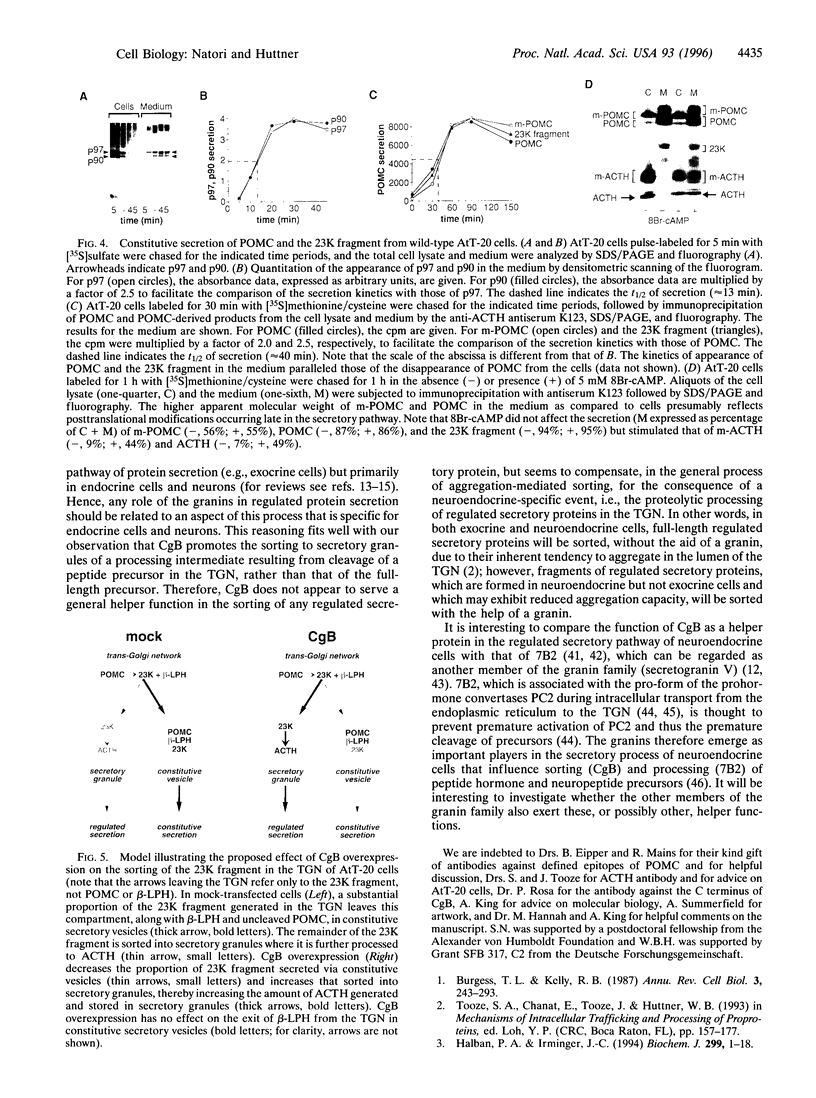
Chromogranin B (CgB, secretogranin I) is a widespread constituent of neuroendocrine secretory granules whose function is unknown. To determine whether CgB affects the sorting of peptide hormone and neuropeptide precursors to secretory granules, we overexpressed CgB in AtT-20 cells, which exhibit an only moderate capacity to sort proopiomelanocortin and proteolytic fragments derived therefrom. In mock-transfected AtT-20 cells, a substantial proportion of newly synthesized proopiomelanocortin and its two primary proteolytic products generated in the trans-Golgi network, the N-terminal 23-kDa fragment containing adrenocorticotropin and the C-terminal beta-lipotropin fragment, was secreted via the constitutive pathway. Two- to three-fold overexpression of CgB markedly reduced the constitutive secretion of the 23-kDa fragment, but not beta-lipotropin and tripled the amount of adrenocorticotropin generated and stored in secretory granules. Our results indicate the existence of neuroendocrine-specific helper proteins which promote the sorting from the trans-Golgi network to secretory granules of certain processing intermediates derived from peptide hormone and neuropeptide precursors and demonstrate that CgB functions as such.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R., Régnier-Vigouroux A., Flatmark T., Huttner W. B. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993 Jul;11(1):105–121. doi: 10.1016/0896-6273(93)90275-v. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Savaria D., Chrétien M., Seidah N. G. 7B2 is a specific intracellular binding protein of the prohormone convertase PC2. J Neurochem. 1995 May;64(5):2303–2311. doi: 10.1046/j.1471-4159.1995.64052303.x. [DOI] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Friederich E., Fritz H. J., Huttner W. B. Inhibition of tyrosine sulfation in the trans-Golgi retards the transport of a constitutively secreted protein to the cell surface. J Cell Biol. 1988 Nov;107(5):1655–1667. doi: 10.1083/jcb.107.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Halban P. A., Irminger J. C. Sorting and processing of secretory proteins. Biochem J. 1994 Apr 1;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi K. L., Seidah N. G., De Serres G., Chrétien M. Isolation and NH2-terminal sequence of a novel porcine anterior pituitary polypeptide. Homology to proinsulin, secretin and Rous sarcoma virus transforming protein TVFV60. FEBS Lett. 1982 Oct 18;147(2):261–266. doi: 10.1016/0014-5793(82)81055-7. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Gerdes H. H., Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991 Jan;16(1):27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Natori S. Regulated secretion. Helper proteins for neuroendocrine secretion. Curr Biol. 1995 Mar 1;5(3):242–245. doi: 10.1016/s0960-9822(95)00049-2. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Ohashi M., Kehlenbach R. H., Barr F. A., Bauerfeind R., Bräunling O., Corbeil D., Hannah M., Pasolli H. A., Schmidt A. Biogenesis of neurosecretory vesicles. Cold Spring Harb Symp Quant Biol. 1995;60:315–327. doi: 10.1101/sqb.1995.060.01.036. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986 May;5(5):931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. The tissue-specific processing of Pro-ACTH/Endorphin recent advances and unsolved problems. Trends Endocrinol Metab. 1990 Nov-Dec;1(8):388–394. doi: 10.1016/1043-2760(90)90097-m. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M., Fischer-Colbrie R., Falgueyret J. P., Benjannet S., Seidah N. G., Lazure C., Winkler H., Chrétien M. Two-dimensional immunoblotting analysis and immunocytochemical localization of the secretory polypeptide 7B2 in adrenal medulla. Neurosci Lett. 1988 Dec 19;95(1-3):81–87. doi: 10.1016/0304-3940(88)90636-2. [DOI] [PubMed] [Google Scholar]
- Milgram S. L., Mains R. E. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J Cell Sci. 1994 Mar;107(Pt 3):737–745. doi: 10.1242/jcs.107.3.737. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Kelly R. B. Secretory protein targeting in a pituitary cell line: differential transport of foreign secretory proteins to distinct secretory pathways. J Cell Biol. 1985 Nov;101(5 Pt 1):1773–1781. doi: 10.1083/jcb.101.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori S., Huttner W. B. Peptides derived from the granins (chromogranins/secretogranins). Biochimie. 1994;76(3-4):277–282. doi: 10.1016/0300-9084(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Pohl T. M., Phillips E., Song K. Y., Gerdes H. H., Huttner W. B., Rüther U. The organisation of the mouse chromogranin B (secretogranin I) gene. FEBS Lett. 1990 Mar 26;262(2):219–224. doi: 10.1016/0014-5793(90)80194-n. [DOI] [PubMed] [Google Scholar]
- Rosa P., Bassetti M., Weiss U., Huttner W. B. Widespread occurrence of chromogranins/secretogranins in the matrix of secretory granules of endocrinologically silent pituitary adenomas. J Histochem Cytochem. 1992 Apr;40(4):523–533. doi: 10.1177/40.4.1552186. [DOI] [PubMed] [Google Scholar]
- Rosa P., Fumagalli G., Zanini A., Huttner W. B. The major tyrosine-sulfated protein of the bovine anterior pituitary is a secretory protein present in gonadotrophs, thyrotrophs, mammotrophs, and corticotrophs. J Cell Biol. 1985 Mar;100(3):928–937. doi: 10.1083/jcb.100.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Gerdes H. H. The granin protein family: markers for neuroendocrine cells and tools for the diagnosis of neuroendocrine tumors. J Endocrinol Invest. 1994 Mar;17(3):207–225. doi: 10.1007/BF03347721. [DOI] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Mantovani S., Rosboch R., Huttner W. B. Monensin and brefeldin A differentially affect the phosphorylation and sulfation of secretory proteins. J Biol Chem. 1992 Jun 15;267(17):12227–12232. [PubMed] [Google Scholar]
- Rosa P., Weiss U., Pepperkok R., Ansorge W., Niehrs C., Stelzer E. H., Huttner W. B. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989 Jul;109(1):17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier-Vigouroux A., Tooze S. A., Huttner W. B. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991 Dec;10(12):3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Mains R. E., Farquhar M. G. Proteolytic processing of pro-ACTH/endorphin begins in the Golgi complex of pituitary corticotropes and AtT-20 cells. Mol Endocrinol. 1989 Aug;3(8):1223–1235. doi: 10.1210/mend-3-8-1223. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M. Proprotein and prohormone convertases of the subtilisin family Recent developments and future perspectives. Trends Endocrinol Metab. 1992 May-Jun;3(4):133–140. doi: 10.1016/1043-2760(92)90102-7. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Hendy G. N., Hamelin J., Paquin J., Lazure C., Metters K. M., Rossier J., Chrétien M. Chromogranin A can act as a reversible processing enzyme inhibitor. Evidence from the inhibition of the IRCM-serine protease 1 cleavage of pro-enkephalin and ACTH at pairs of basic amino acids. FEBS Lett. 1987 Jan 26;211(2):144–150. doi: 10.1016/0014-5793(87)81425-4. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Hsi K. L., De Serres G., Rochemont J., Hamelin J., Antakly T., Cantin M., Chrétien M. Isolation and NH2-terminal sequence of a highly conserved human and porcine pituitary protein belonging to a new superfamily. Immunocytochemical localization in pars distalis and pars nervosa of the pituitary and in the supraoptic nucleus of the hypothalamus. Arch Biochem Biophys. 1983 Sep;225(2):525–534. doi: 10.1016/0003-9861(83)90063-2. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Frank R., Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT20 cells before its cleavage. J Cell Biol. 1987 Jul;105(1):155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Flatmark T., Tooze J., Huttner W. B. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991 Dec;115(6):1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992 Aug;49(3):497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Bloomquist B. T., Mains R. E. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993 Jan 25;268(3):1763–1769. [PubMed] [Google Scholar]
- Zhou A., Mains R. E. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994 Jul 1;269(26):17440–17447. [PubMed] [Google Scholar]