Abstract
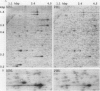
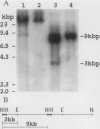
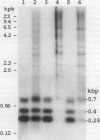
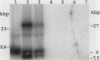
We have implemented an approach for the detection of DNA alterations in cancer by means of computerized analysis of end-labeled genomic fragments, separated in two dimensions. Analysis of two-dimensional patterns of neuroblastoma tumors, prepared by first digesting DNA with the methylation-sensitive restriction enzyme Not I, yielded a multicopy fragment which was detected in some tumor patterns but not in normal controls. Cloning and sequencing of the fragment, isolated from two-dimensional gels, yielded a sequence with a strong homology to a subtelomeric sequence in chimpanzees and which was previously reported to be undetectable in humans. Fluorescence in situ hybridization indicated the occurrence of this sequence in normal tissue, for the most part in the satellite regions of acrocentric chromosomes. A product containing this sequence was obtained by telomere-anchored PCR using as a primer an oligonucleotide sequence from the cloned fragment. Our data suggest demethylation of cytosines at the cloned Not I site and in neighboring DNA in some tumors, compared with normal tissue, and suggest a greater similarity between human and chimpanzee subtelomeric sequences than was previously reported.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa J., Kuick R., Neel J. V., Kodaira M., Satoh C., Hanash S. M. Genetic variation detected by quantitative analysis of end-labeled genomic DNA fragments. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9052–9056. doi: 10.1073/pnas.91.19.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B., Fearon E. R., Vogelstein B., de Bustros A., Sharkis S. J., Burke P. J., Staal S. P., Nelkin B. D. Hypermethylation of the 5' region of the calcitonin gene is a property of human lymphoid and acute myeloid malignancies. Blood. 1987 Aug;70(2):412–417. [PubMed] [Google Scholar]
- Baylin S. B., Höppener J. W., de Bustros A., Steenbergh P. H., Lips C. J., Nelkin B. D. DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res. 1986 Jun;46(6):2917–2922. [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Brown W. R. Molecular cloning of human telomeres in yeast. Nature. 1989 Apr 27;338(6218):774–776. doi: 10.1038/338774a0. [DOI] [PubMed] [Google Scholar]
- Cheng J. F., Smith C. L., Cantor C. R. Isolation and characterization of a human telomere. Nucleic Acids Res. 1989 Aug 11;17(15):6109–6127. doi: 10.1093/nar/17.15.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. H., Allshire R. C., McKay S. J., McGill N. I., Cooke H. J. Cloning of human telomeres by complementation in yeast. Nature. 1989 Apr 27;338(6218):771–774. doi: 10.1038/338771a0. [DOI] [PubMed] [Google Scholar]
- Cross S., Lindsey J., Fantes J., McKay S., McGill N., Cooke H. The structure of a subterminal repeated sequence present on many human chromosomes. Nucleic Acids Res. 1990 Nov 25;18(22):6649–6657. doi: 10.1093/nar/18.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Girvitz S. C., Bacchetti S., Rainbow A. J., Graham F. L. A rapid and efficient procedure for the purification of DNA from agarose gels. Anal Biochem. 1980 Aug;106(2):492–496. doi: 10.1016/0003-2697(80)90553-9. [DOI] [PubMed] [Google Scholar]
- Goodman M., Tagle D. A., Fitch D. H., Bailey W., Czelusniak J., Koop B. F., Benson P., Slightom J. L. Primate evolution at the DNA level and a classification of hominoids. J Mol Evol. 1990 Mar;30(3):260–266. doi: 10.1007/BF02099995. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Buckley J. D. The role of DNA methylation in cancer. Adv Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- Kuick R., Asakawa J., Neel J. V., Satoh C., Hanash S. M. High yield of restriction fragment length polymorphisms in two-dimensional separations of human genomic DNA. Genomics. 1995 Jan 20;25(2):345–353. doi: 10.1016/0888-7543(95)80032-h. [DOI] [PubMed] [Google Scholar]
- Lemieux N., Dutrillaux B., Viegas-Péquignot E. A simple method for simultaneous R- or G-banding and fluorescence in situ hybridization of small single-copy genes. Cytogenet Cell Genet. 1992;59(4):311–312. doi: 10.1159/000133277. [DOI] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Miwa W., Yashima K., Sekine T., Sekiya T. Demethylation of a repetitive DNA sequence in human cancers. Electrophoresis. 1995 Feb;16(2):227–232. doi: 10.1002/elps.1150160138. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Moyzis R. K., Meyne J., Burke D. T., Olson M. V. Cloning human telomeric DNA fragments into Saccharomyces cerevisiae using a yeast-artificial-chromosome vector. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6240–6244. doi: 10.1073/pnas.86.16.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N. J., Baird D. M., Jeffreys A. J. A subterminal satellite located adjacent to telomeres in chimpanzees is absent from the human genome. Nat Genet. 1994 Jan;6(1):52–56. doi: 10.1038/ng0194-52. [DOI] [PubMed] [Google Scholar]
- Wilkie A. O., Higgs D. R., Rack K. A., Buckle V. J., Spurr N. K., Fischel-Ghodsian N., Ceccherini I., Brown W. R., Harris P. C. Stable length polymorphism of up to 260 kb at the tip of the short arm of human chromosome 16. Cell. 1991 Feb 8;64(3):595–606. doi: 10.1016/0092-8674(91)90243-r. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Prakash O. The origin of man: a chromosomal pictorial legacy. Science. 1982 Mar 19;215(4539):1525–1530. doi: 10.1126/science.7063861. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]