Abstract
Liposome surface functionalization facilitates enormous potential applications of liposomes, such as enhanced stability, bioactive liposome conjugates, and targeted drug, gene and image agent delivery. Anchoring lipids are needed for grafting ligands of interest and play important roles in ligands grafting density, liposome stability, and liposome chemical and physical characteristics as well. In this report, glyco-functionalized liposome systems based on two kinds of anchoring lipid, phosphatidylethonalamine (PE) and cholesterol (Chol) were prepared by post chemically selective functionalization via Staudinger ligation. The size and stability of the liposomes were confirmed by dynamic light scattering (DLS). Particularly, the impact of anchor lipids on the stability of glyco-functionalized liposomes was investigated by comparing two different anchor lipids, namely Chol-PEG2000-TP and DSPE-PEG2000-TP. In addition, the encapsulation and releasing capacity of the glycosylated liposome based on the two anchoring lipids were investigated by entrapping 5, 6-carboxyfluorescein (CF) dye and monitoring the fluorescence leakage, respectively. Furthermore, the density and accessibility of grafted carbohydrate residues on the liposome surface were evaluated for the two anchoring lipids-derived liposomes with lectin binding, respectively.
Keywords: Liposome, surface functionlization, anchor lipids, cholesterol, Staudinger ligation
Introduction
Liposomes as self-assembled lipid bi-layers have been widely investigated as biomimetic models of cell membranes, biomimetic conjugates, and drug, gene and image agent delivery vehicles.1,2 Liposome surface functionalization facilitates enormous potential applications of liposomes, such as enhanced stability, bioactive liposome conjugates, and targeted delivery.3,4 Various biomolecules have been conjugated onto liposome surface for variety of biomedical applications. Among them, carbohydrate molecules, particularly, which play important roles in various biological recognition processes such as fertilization, metastasis, inflammations and host–pathogen adhesion,5 serve as attractive molecules for liposome surface modification. For example, glyco-funcationalizaed liposomes has been investigated for targeted drug delivery6 and as bioactive membrane mimetic conjugates.7
Many methods have been developed for liposome surface functionalization, of which the most commonly used method involves the initial synthesis of an anchor lipid-ligand conjugate, followed by formulation of the liposome with all lipid components or post-insertion into the preformed liposome. Alternatively, chemical modifications of the surface of preformed vesicles that carry functionalized anchoring lipids with biomolecules have been well explored.8,9 So far, variable conjugation methods such as using amide10 or thiol-maleimide coupling11 as well as by imine12 or hydrazone linkage,13 have been developed. However, most methods lack specificity and often result in uncontrolled formation of covalent bonds between liposome and ligands of interest. In addition, the reactive anchoring lipid might react with other membrane components or the encapsulated drugs as well in this conventional conjugation chemistry. Recently, bio-orthogonal chemistry such as click chemistry14 and Staudinger ligation15 have been investigated as novel generic chemical tools for the facile in situ surface modification of liposomes.
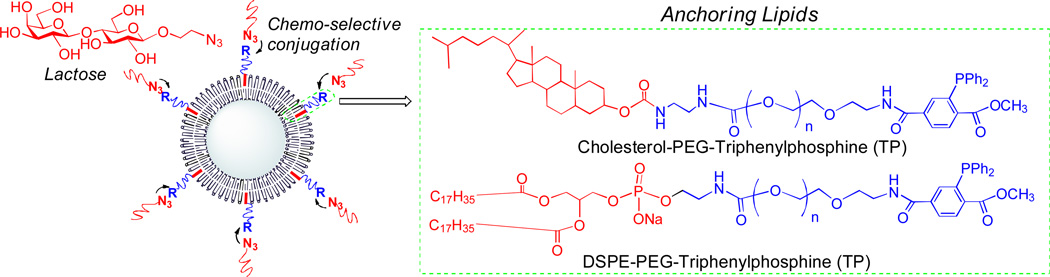
In our previous study, 15 we demonstrated that the Staudinger ligation reaction occur in high yield at room temperature in aqueous solvents and without any catalyst, and is compatible with the unprotected functional groups of biomolecules to be introduced. In the present study, we want to investigate the anchoring lipid effect on liposome surface functionalization, such as ligand grafting density, liposome stability, and liposome chemical and physical characteristics. Two anchor lipids, phosphatidylethonalamine (PE) and cholesterol (Chol) are investigated for liposome surface glyco-functionalization via Staudinger ligation, with the consideration of size, stability and grafting carbohydrate density as well as activity of glyco-liposome conjugates (Figure 1). Further, the impact of anchoring lipids on encapsulation and releasing capacity of the glycosylated liposomes were investigated by entrapping 5, 6-carboxyfluorescein dye and monitoring the fluorescence dye leakage, respectively.
Fig. 1.
Liposome surface glyco-functionalization based on two kinds of anchoring lipids via Staudinger ligation.
Results and discussion
The aim of this paper was to study the anchoring lipid effects on liposome stability, ligand grafting density and liposome chemical and physical characteristics upon liposome surface glyco-functionalization and their lectin binding activity. In this study, two anchoring lipids namely Chol-PEG2000-TP and DSPE-PEG2000-TP were proposed for liposome surface glyco-functionalization with an azide derivative of lactose as a model carbohydrate via Staudinger ligation. The major difference between these two anchoring lipids is their respective hydrophobic molecules inserted in the lipid bilayer of liposomes, a sterol in the case of Chol-PEG, while a phospholipid with long saturated fatty acid chains in the case of DSPE-PEG. In addition, sterol is a neutral molecule which stabilizes liposomes and prevents liposome aggregation, while phospholipid imparts negative charge to the liposome surface, which may lead to additional binding interactions with plasma proteins or the drugs encapsulated and released.18 Therefore, it is expected that these anchoring lipids will have impact on both the chemistry upon liposome surface modification and their chemical and physical characteristics, and in vitro and in vivo behavior as well.
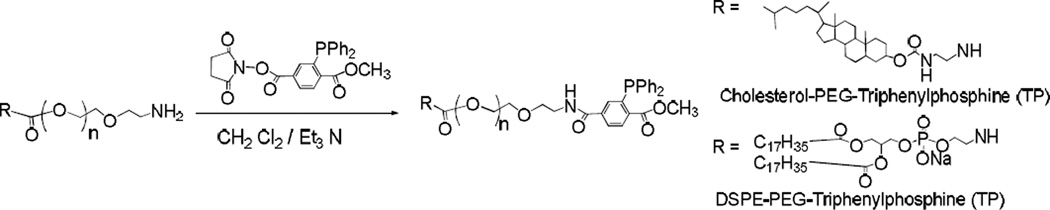
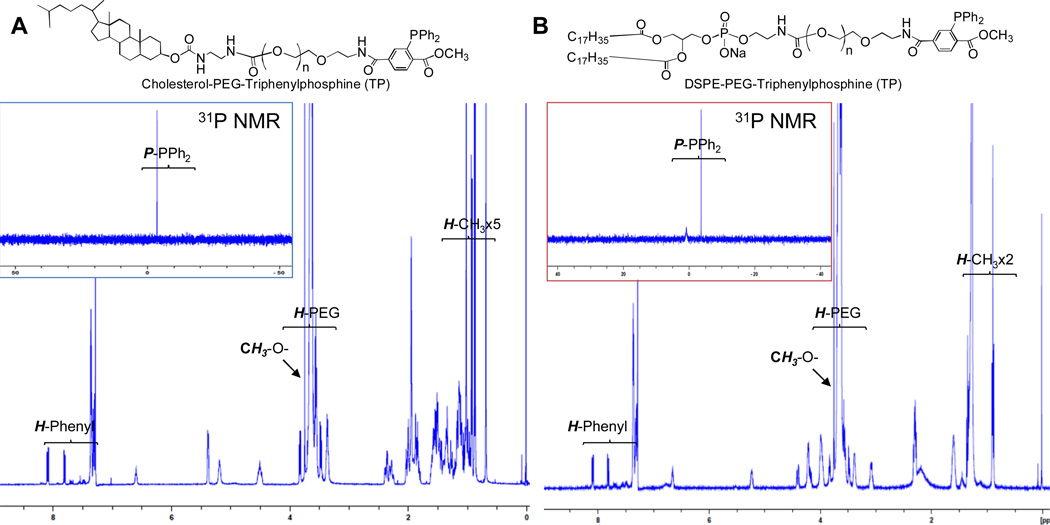
First, the terminal triphenylphosphine carrying anchoring lipids were synthesized by amidation of synthetic Chol-PEG2000-NH216 and commercially available DSPE-PEG2000-NH2 (Avanti Polar Lipid) with 3-diphenylphosphino-4-methoxycarbonylbenzoic acid NHS active ester17 in good yield, respectively (Scheme 1). The resultant anchoring lipids were characterized by 1H, 13C and 31P NMR spectra (Figure 2) (Detail Spectra see Supporting Information). As previously reported, the triphenylphosphine is air sensitive, which is a drawback of Staudinger ligation.15 However, there is no oxidized product formed for both Chol-PEG2000-TP and DSPE-PEG2000-TP after purification in the present study, as shown in 31P NMR spectra, in which the phosphine in both compounds gave a chemical shift at −3.74 ppm (Figure 2A and 2B).
Scheme 1.
Syntheses of anchoring lipids Chol-PEG2000-TP and DSPE-PEG2000-TP
Fig. 2.
NMR spectra of Chol-PEG2000-TP (A) and DSPE-PEG2000-TP (B) in CDCl3.
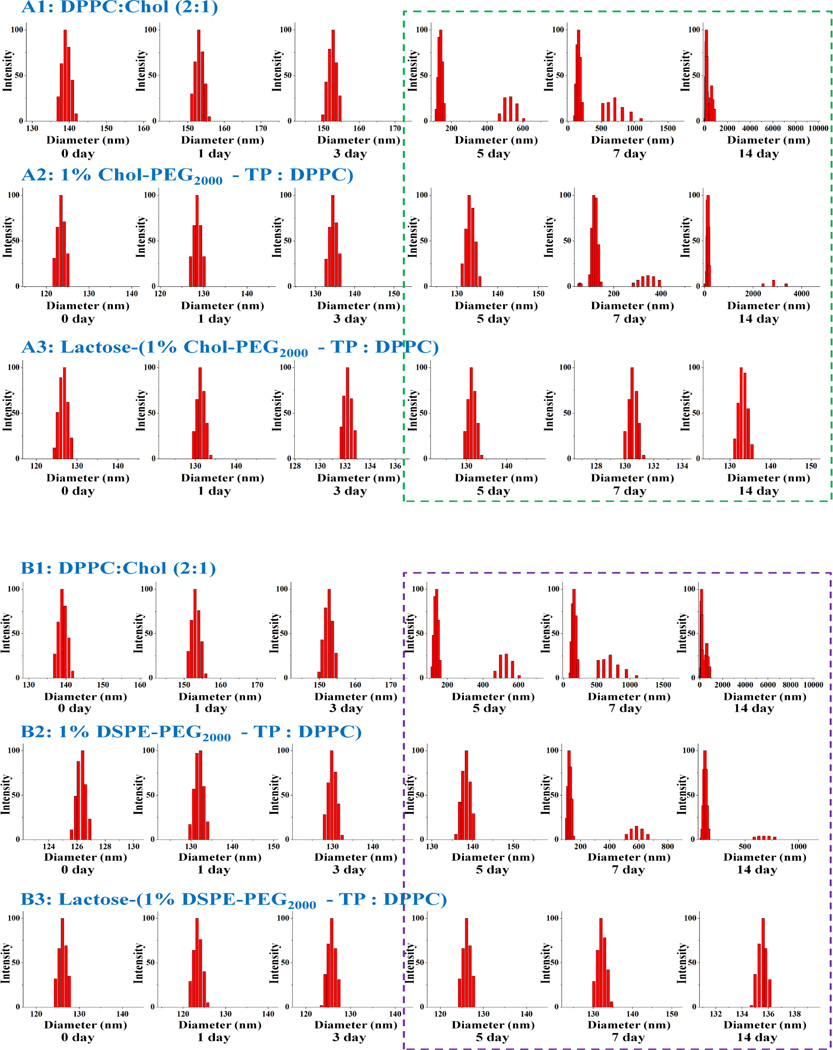
Next, the azido-reactive liposomes composed of saturated phospholipid DPPC and the anchoring lipid in different lipid ratios (see Table 1) were prepared by thin-film hydration and extrusion through polycarbonate membranes with pore size of 800 nm, 600 nm, 400 nm, 200 nm, and 100 nm sequentially at 65 °C. This produced predominantly small unilamellar vesicles, which displayed different average mean diameters of the liposomes of different lipids used, which were confirmed by DLS. Liposome with Chol-PEG2000-TP anchoring lipid is relatively larger than liposome with DSPE-PEG2000-TP anchoring lipid in the same percentage in the liposomes (Table 1). Glyco-surface modification of the preformed liposomes with lactosyl azide19 as a model ligand was performed in PBS buffer (pH 7.4) at room temperature under an argon atmosphere for 6 hours (Fig. 1). DLS was used to verify the integrity of the vesicles during and after the coupling reaction. As a result, there was 10 to 20 nm size increase in the average mean diameter after glyco-modification for all liposomes of different lipids and anchoring lipids (Table1). There was no liposome aggregates formation observed during and after conjugation reaction as monitored by DLS (Figure 3, liposomes with 1% anchoring lipid). Therefore, the reaction conditions described above do not alter the integrity of the liposomes. Continued stability of the glycosylated liposomes were investigated by DLS as well. As shown in Fig. 3 (liposomes with 1% anchoring lipid), the glycosylated liposomes with either Chol-PEG or DSPE-PEG anchoring lipids showed good stability during the 14 days period. However, the liposome with and without anchoring lipids began to collapse and aggregate since 7th and 5th day, respectively (Fig.3). There is no apparent difference between the two kinds of anchoring lipids at different concentrations (data no shown). These results demonstrated that the presence of lactose on the liposome surface provides a steric barrier that prevents liposome aggregation. Also, the PEG linkers also stabilize the liposomes as well since PEG polymers are often used to stabilize liposomes.20 In addition, critical micelle concentration (CMC) was measured for these two anchor lipids by using the pyrene assay.21 As a result, Chol-PEG2000-TP had 3.4 times higher critical micelle concentration (CMC) value than that of DSPE-PEG2000-TP and conjugation with lactose resulted in only 1.1 and 1.4 times increments in CMC for Chol-PEG2000-TP and DSPE-PEG2000-TP, respectively as CMC is strongly dependent on the alkyl chain length (hydrophobicity) (Table 2).
Table 1.
Liposome with different anchoring lipids and their sizes determined by DLS
| Anchoring lipid and in liposome with DPPC | Mean Diameter (nm) | ||
|---|---|---|---|
| Before Conjugation | After Conjugation | ||
| DSPE-PEG2000-TP | 0.5% | 96 | 102 |
| 1.0% | 122 | 129 | |
| 1.5% | 145 | 156 | |
| 2.0% | 168 | 186 | |
| CHL-PEG2000-TP | 0.5% | 100 | 110 |
| 1.0% | 120 | 123 | |
| 1.5% | 149 | 161 | |
| 2.0% | 174 | 195 | |
Fig. 3.
DLS monitoring of liposomes before and after glyco-functionalization: Chol-PEG2000-TP anchored liposome (A) and DSPE-PEG2000-TP anchored liposome (B) and their stabilities monitored with DLS.
Table 2.
Critical micelle concentration (CMC) of anchor lipids
| Anchor lipid | CMC (µM) | |
|---|---|---|
| Before Conjugation | After Conjugation | |
| DSPE-PEG2000-TP | ~8 µM | ~11 µM |
| CHL-PEG2000-TP | ~27 µM | ~31 µM |
The drug encapsulation capacity and releasing property of liposomes over time are important factors for their biomedical applications, especially in drug/gene delivery applications. Therefore, in our study, both Chol and DSPE-based anchoring lipids were investigated for the consideration of drug encapsulation capacity and releasing property of glycosylated liposomes. The same liposomes with 1% anchoring lipid and having encapsulated self-quenching concentrations of 5,6-carboxyfluorescein (5,6-CF, 85 mM) were used as a model compound to determine encapsulation capacity and to monitor the releasing kinetics over two weeks period. Briefly, 5,6-CF-encapsulated liposomes containing TP were prepared in the same conditions as above along with 85 mM 5,6-CF, followed by glycosylation with lactosyl azide and separation from un-reacted lactosyl azide and the non-encapsulated 5,6-CF dye. As a result, encapsulation efficiency of conventional liposome (DPPC : Chol (2 : 1)) was found to be 2.25% ± 0.05 while that of Chol-PEG and DSPE-PEG containing liposomes were found to be 1.77% ± 0.05 and 1.98% ± 0.02, respectively. These decreases in encapsulation efficiency by incorporation of Chol-PEG and DSPE-PEG are probably due to the reduction in the internal vesicular volume by bulky PEG chains covering both the inner and outer surfaces of liposomes. The results are in agreement with previous reports by Schneider et al. who observed concentration dependent reduction in encapsulation efficiencies of water soluble contrast agents in liposomes containing different concentrations of cholesteryl hemisuccinate.22
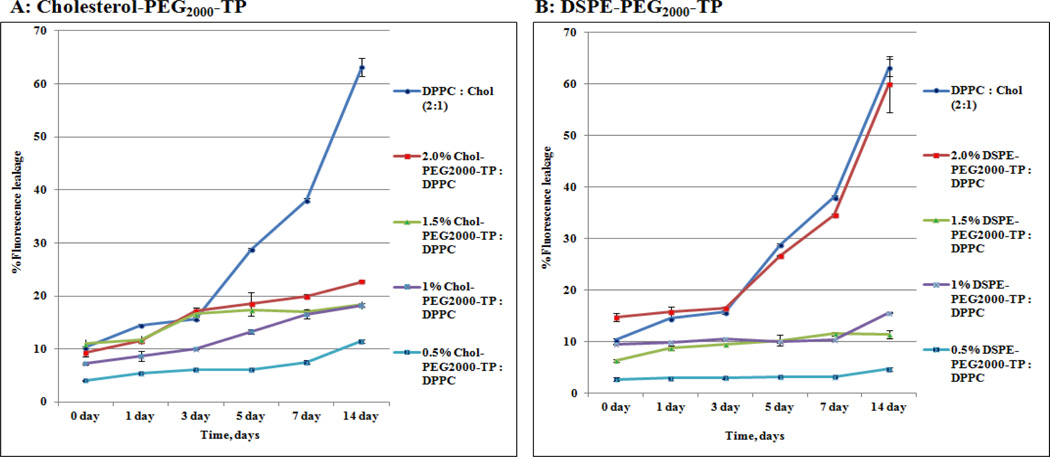
Next, the releasing behaviors of glycosylated liposomes with Chol-PEG and DSPE-PEG anchoring lipid and plain liposome of DPPC and Cholesterol (2:1) were investigated. As shown in Fig. 4, the cumulative release of 5,6-CF in the plain liposome was 5% daily and sped up after 5th day due to its instability, while, 5,6-CF releasing was consistent in a reduced rate (1% daily) for two weeks in the liposomes with the presence of Chol–PEG anchoring lipid in 0.5, 1.0, 1.5, and 2%. Also, liposomes with the DSPE-PEG anchoring lipid in 0.5, 1.0 and 1.5% released 5,6-CF in consistent reduced rate (less 1% daily) for two weeks. However, liposome with the DSPE-PEG anchoring lipid in 2.0% has the same 5, 6-CF releasing pattern of plain liposome (5% daily and sped up after 5th day). Overall, Chol–PEG anchored liposome showed relatively faster releasing (Figure 4A) than DSPE-PEG anchored liposomes (Figure 4B). The difference could be explained by the fact that Chol increases rigidity of the lipid membrane and thus lead to fast leakage of liposomes.23
Fig. 4.
5,6-CF releasing kinetics of lactosylated liposomes with anchoring lipid in different ratios: Chol-PEG2000-TP (A) and DSPE-PEG2000-TP (B).
Furthermore, the grafted lactose on the surface of liposome was quantified by using the Lactose Assay kit.24 Briefly, a reaction mix (85 µL Assay buffer + 1 µL Lactase enzyme + 1 µL dye reagent) was added to samples containing lactose and were incubated for 30 min at room temperature. The optical density readings were obtained at 570 nm using UV-vis Spectroscopy. As a result, 90% glyco-functionalization yield was determined for Chol-PEG2000-TP anchored liposome, while 35% yield was obtained for DSPE-PEG2000-TP anchored liposome with 1% anchoring lipids in both liposome preparations. The reason for this lower coupling efficiency is unknown; however, it might be due to the incorporation of less phospholipid anchoring lipid in the outer leaf surface of the reactive liposome.
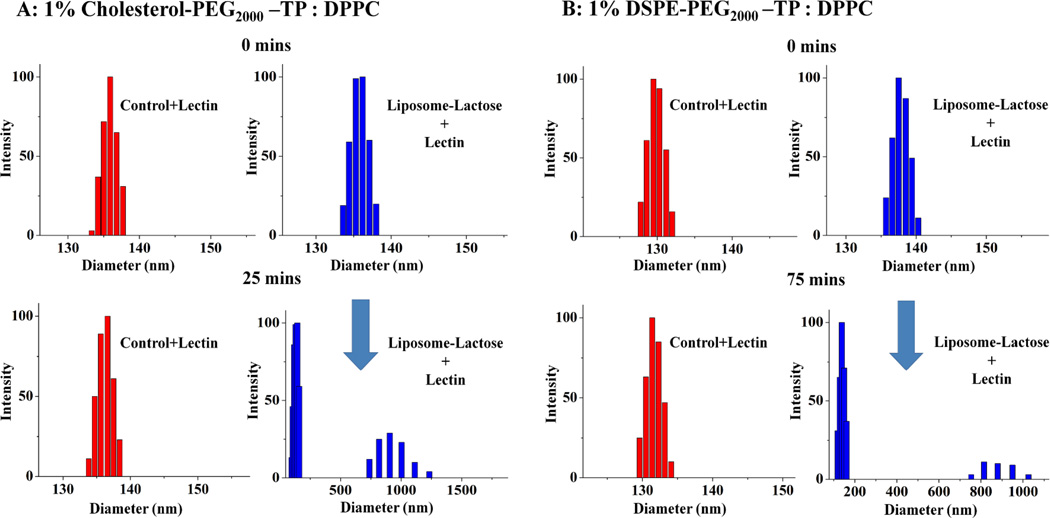
To determine whether the grafted lactose residues are easily accessible at the surface of liposomes, lectin binding assay was conducted by incubating lactosylated liposome in the presence of β-galactose binding lectin (Arachis hypogae, 120 kDa, SIGMA) in PBS (pH 7.4). As a result, apparent visible aggregation was observed and was monitored in DLS experiment for glycosylated liposome with Chol-PEG anchoring lipid after 25 minutes incubation (Figure 5A), while, it took 75 minutes to observe the similar apparent visible aggregation for glycosylated liposome with DSPE-PEG anchoring lipid, that was also monitored in DLS experiment (Fig. 5B). In contrast, neither aggregation nor size change was observed with control liposomes without lactose. Furthermore, the presence of free lactose (5.0 mM) prevented aggregates formation for both glycosylated lipsomes, confirming that the agglutination was due to a specific recognition of the lactose residues on the surface of the liposomes by lectin. The lectin binding difference might be due to the lactose density on the liposome surface, in which multivalent interactions contribute to the lectin induced aggregation of the glycosylated liposomes.
Fig. 5.
DLS monitoring of agglutination due to multivalent lectin binding between lectin and lactosylated liposomes with anchor lipid of Chol-PEG2000-TP (A) and DSPE-PEG2000-TP (B).
Conclusions
In this study, we have investigated the anchor lipid effects on liposome surface functionalization via Staudinger ligation. Two different anchor lipids, namely Chol-PEG2000-TP and DSPE-PEG2000-TP were synthesized and evaluated for liposome surface glyco-functionalization with latosyl azide as a model ligand via Staudinger ligation. The reaction could be performed under mild conditions in aqueous buffers without catalyst and in high yields under reasonable reaction time. The reaction conditions developed in the present work did not alter the integrity of the bilayers, in terms of liposome sizes and leakiness, and provided perfectly functional vesicles. The anchoring lipids showed their effects on the liposome size, stability, encapsulation and releasing capacity, glycosylation efficiency and their lectin binding property. The reported anchor lipids and efficient and chemoselective conjugation approach, which is particularly suitable for the ligation of water soluble molecules and can accommodate many chemical functions, are anticipated to be useful in the coupling of many other ligands onto liposomes for a wide range of applications.
Experimental
Materials
All solvents and reagents were purchased from commercial sources and were used as received, unless otherwise noted. Deionized water was used as a solvent in all procedures. Monocholesteryl-PEG2000-amine was synthesized as literature method.16 3-Diphenylphosphino-4-methoxy-carbonylbenzoic acid NHS active ester (TP-NHS) was synthesized as literature method.17 Lactosyl azide was synthesized in our lab as previously reported.19
Method
1H NMR spectra were recorded with Bruker 400 MHz spectrometer. In all cases, the sample concentration was 10 mg/mL, and the appropriate deuterated solvent was used with TMS as an internal standard. Dynamic Light Scattering (DLS) was recorded with 90 Plus particle size analyzer (BIC). Fluorescent spectrum was measured with FluoroMax-2 (ISA). UV-Vis Spectroscopy was recorded with Cary 50 UV-Vis spectrophotometer (VARIAN).
Synthesis of Cholesterol – PEG2000-TP (Chol-PEG2000-TP)
Et3N (14 µL, 0.1 mmol) was added to Monocholesteryl-PEG2000-amine (50 mg, 20.1 µmol) in anhydrous CH2Cl2 (4 mL) and was stirred for 30 min. at room temperature under Argon gas atmosphere, then TP-NHS (14 mg, 30.1 µmol, 1.5 equiv.) dissolved in anhydrous CH2Cl2 (1 mL) was added and the reaction solution was stirred overnight under darkness. TLC analysis was used to check the formation of Chol-PEG2000-TP (visualized under UV lamp and Ninhydrin staining). The reaction mixture was concentrated by rotary evaporator and then the reaction mixture residual was separated by passing through Sephadex LH-20 column with MeOH as eluent. The fractions containing Cholesterol-PEG2000-TP were collected and evaporated to dryness under vacuum (32 mg, 50.1% yield). 1H NMR (CDCl3, 400 MHz): 8.09 (dd, J = 8.0, 3.6 Hz, 1H), 7.81 (dd, J = 8.0, 1.4 Hz, 1H), 7.37-7.29 (m, 8H), 6.60 (m, 1H), 5.38 (m, 3H), 5.19 (m, 2H), 4.50 (m, 3H), 3.85 (m, 2H), 3.75 (s, 3H), 3.80-3.50 (br. m, 100H, O-CH2-CH2-O), 3.57-3.55 (m, 8H), 3.49-3.34 (m, 2H), 3.37-3.36 (m, 5H), 2.38 (dd, J = 7.8, 1.9 Hz, 2H), 2.23 (m, 2H), 2.04-1.80 (m, 7H), 1.89 (s, 3H), 1.62-0.95 (m, 25H), 1.02 (s, 6H), 0.93 (d, J = 6.6 Hz, 3H), 0.87 (d, J = 6.7 Hz, 6H) ppm, 13C NMR (CDCl3, 100 MHz) (δ): 166.70, 166.68, 166.38, 156.22, 139.88, 137.39, 137.28, 137.17, 134.00, 133.79, 132.79, 130.79, 128.96, 128.66, 128.58, 126.72, 122.42, 70.57, 56.69, 56.14, 52.17, 50.03, 42.31, 40.70, 39.86, 39.74, 39.5038.59, 37.00, 36.56, 36.18, 35.77, 31.88, 28.21, 28.19, 27.99, 24.27, 23.81, 21.03, 19.32, 18.71, 11.85 ppm, and 31P NMR (CDCl3, 170 MHz) (δ): −3.67 ppm.
Synthesis of DSPE-PEG2000-TP
0.2 mL of Et3N was added to DSPE-PEG2000-NH2 (25 mg, 8.9 µmol) dissolved in 10 mL anhydrous CH2Cl2 and was stirred for 30 min at room temperature under Argon gas atmosphere. Then a solution of succinimidyl-3-diphenylphosphino-4-methoxycarbonylbenzoate (TP-NHS) (8 mg, 17.9 µmol) dissolved in 5 mL of anhydrous CH2Cl2 was added to the above solution under Argon gas. The reaction mixture was further allowed to stir for 24 hr at room temperature under Argon gas and then the solution was concentrated under vacuum to give a residue. TLC analysis (chloroform: methanol 1:10, v/v, visualized under UV lamp and Ninhydrin staining) was used to check the reaction. The reaction mixture was concentrated by rotary evaporator and then the concentrated reaction mixture was separated by passing through Sephadex LH-20 column with MeOH as eluent. The fractions containing DSPE-PEG2000-TP were collected and evaporated to dryness under vacuum (10 mg, 30.1 % yield). 1H NMR (CDCl3, 400 MHz) (δ): 8.09 (dd, J = 8.0, 3.6 Hz, 1H), 7.81 (dd, J = 8.0, 1.4 Hz, 1H), 7.36-7.28 (m, 8H), 6.65 (m, 1H), 5.22 (m, 1H), 4.40 (m, 1H), 4.20-4.15 (m, 3H), 4.00-3.95 (m, 3H), 3.75 (s, 3H), 3.80-3.50 (br. m, 100H, O-CH2-CH2-O), 3.58-3.54 (m, 1H), 3.39-3.38 (m, 1H), 3.09-3.06 (m, 1H), 2.29 (m, 4H), 2.28 (br.s, 1H), 1.59 (br.s, 2H), 1.34 (t, J = 6.9 Hz, 2H), 1.66 (br. s, 32H), 0.89 (t, J = 6.9 Hz, 6H) ppm, 13C NMR (CDCl3, 100 MHz) (δ): 173.38, 172.99, 166.70, 166.39, 156.54, 141.28, 137.39, 137.28, 137.17, 136.54, 134.79, 133.80, 132.81, 130.79, 128.96, 128.66, 128.59, 126.73, 71.57, 70.41, 70.25, 70.08, 69.61, 63.38, 62.63, 52.19,45.62, 39.86, 34.28, 34.10, 31.91, 29.70, 29.65, 29.52, 29.34, 29.17, 29.15, 24.92, 24.88, 22.67, 14.10 8.55 ppm, and 31P NMR (CDCl3, 170 MHz) (δ): −3.68 ppm.
Liposome preparation
DPPC (30 mg, 40.9 µmol) and different ratios of DSPE-PEG2000-TP or Cholesterol-PEG2000-TP (0.5%–2.0%) were dissolved in 3.0 mL anhydrous chloroform. The solvent was slowly removed on a roto-evaporator under reduced pressure in order to form a thin lipid film on the flask wall that was further dried in vacuum chamber overnight. Then, the lipid film was swelled in the dark with 3 mL PBS buffer (pH 7.4) to form multilamellar vesicle suspension. It was further subjected to 10 freeze-thaw cycles involving quenching in liquid N2 and then immersed in a 65 °C water-bath. The crude lipid suspension thus formed was passed through an extruder with different polycarbonate membranes (pore sizes 800 nm, 600 nm, 400 nm, 200 nm and finally 100 nm) to afford the liposomes.
Conjugation of lactose onto liposomes surface
lactosyl azide (0.5 mg) in 0.5 mL PBS (pH 7.4) buffer (Argon bubbled before use) was added into 1 mL of liposomes obtained as above. The Staudinger ligation reaction was conducted at room temperature for 6 h under Argon atmosphere, after which the unreacted lactosyl azide was removed by gel filtration (1.5 × 20 cm column of Sephadex G-75). The size of liposomes during the Staudinger ligation was monitored over time by using 90 Plus particle analyzer. Similarly, control experiments were conducted in the absence of lactosyl azide.
Determination of concentration of lactose on the liposome surface
A standard curve was generated as described by Lactose Assay kit (Bioassay systems) with free lactose solution. To 20 µL of lactose-grafted liposome obtained above, 80 µL of reaction mix (85 µL Assay buffer + 1 µL Lactase enzyme + 1 µL dye reagent) was added and mixed. The mixture was kept on a shaker, and was allowed to stand for 30 min at room temperature. The optical readings were taken at 570 nm. The amount of lactose conjugated onto the liposome surface was thus calculated from the standard calibration curve.
Determination of critical micelle concentration (CMC) of the anchor lipids
A series of anchor lipids (DSPE-PEG2000-TP and Chol-PEG2000-TP) solutions ranging from 1µM – 100 µM, containing 1µM pyrene were prepared. Briefly, 100 µL of pyrene solution was added to different vials and the solvent was evaporated, to which 500 µL of different concentrations of the anchor lipids were added. The solutions were allowed to stir at room temperature for overnight. Finally, the emission spectra at two wavelengths (I385 and I374) were recorded using a Hitachi FluoroMax-2 (ISA) spectrophotometer. The graphs were plotted by taking the intensity ratios of I385 and I374, which correspond to the third and first peaks of pyrene molecule. The CMC values of the two anchor lipids before and after glyco-functionalization were determined by taking the intersection of the straight lines of the graphs.
5, 6-Carboxyfluorescein (5, 6-CF) Encapsulation Efficiency
5, 6-CF-encapsulated liposomes containing TP were prepared in the same conditions as above with PBS buffer (pH 7.4) containing 85 mM 5,6-CF. Next, glycosylation of the liposomes with lactosyl azide were conducted at room temperature for 6 h under Argon atmosphere, after which the un-reacted lactosyl azide and the non-encapsulated 5,6-CF dye were removed by gel filtration (1.5 × 20 cm column of Sephadex G-75). The 5,6-CF encapsulation efficiency (EE %) was determined by measuring the amount of encapsulated dye relative to the total initial amount, using FluoroMax-2 (ISA). The free dye was removed from the dye encapsulated liposomes by passing through a 1.5 × 20 cm column of Sephadex G-75. The amount of encapsulated dye was determined after disrupting the liposome using 5 µL Triton-X 1%. The readings were obtained by adding 20 µL of reaction solution + 1980 µL PBS buffer (pH 7.4). The EE% is calculated by using the formula:
Where V and C are volume and concentration and i represents initial values taken from the original preparation and f represents values taken from the encapsulated dye liposomes.
5,6-CF dye leakage assay
5,6-CF-encapsulated liposomes were prepared in the same conditions as above along with 85 mM 5,6-CF. The fluorescent leakage was measured using FluoroMax-2 (ISA) to monitor the stability of liposomes. Briefly, 20 µL reaction solution and 1980 µL PBS buffer (pH 7.4) were mixed together and then the fluorescent intensity was measured. 5 µL solution of Triton-X 1% was added to obtain the total amount of encapsulated dye present in the liposome, from which the % leakage of 5, 6-CF dye was determined. Similarly, the control experiments were conducted with the plain liposome without lactose modification.
Characterization of specific binding between lactose on the liposome surface and lectin
To study the accessibility of lactose grafted on to the liposome surface, 100 µL lectin (β-galactose binding lectin, Arachis hypogae, 120 kDa, Sigma) in PBS buffer solution (1 mg/mL, pH 7.4) was added into 100 µL solution of lactose grafted liposomes. The stability and size of the liposomes were monitored with DLS over time. Control was conducted with liposome without lactose in the same manner above.
Supplementary Material
Acknowledgements
This work was partly supported by grants from the NIH (1R01HL102604-04, X.-L. Sun), National Science Foundation MRI Grant (CHE-1126384, X.-L. Sun) and Cleveland State University Faculty Research Development Grant (X.-L. Sun).
References
- 1.Philippot J, Schuber F, editors. Liposomes as Tools in Basic Research and Industry. Boca Raton: CRC Press; 1995. [Google Scholar]
- 2.Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Nat. Rev. Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 4.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Biochim. Biophys. Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 5.Lis H, Sharon N. Chem. Rev. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Ma Y, Sun X-L. Med. Res. Rev. 2010;30:270–289. doi: 10.1002/med.20171. [DOI] [PubMed] [Google Scholar]
- 7.Sun -L, Kanie Y, Guo C-T, Kanie O, Suzuki Y, Wong C-H. Eur. J. Org. Chem. 2000;14:2643–2653. [Google Scholar]
- 8.Sapra P, Allen TM. Lipid Res. 2003;42:439–462. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 9.Nobs L, Buchegger F, Gurny R, Allemann E. J. Pharm. Sci. 2004;93:1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- 10.Kung VT, Redemann CT. Biochim. Biophys. Acta. 1986;862:435–439. doi: 10.1016/0005-2736(86)90247-6. [DOI] [PubMed] [Google Scholar]
- 11.Schelte P, Boeckler C, Frisch B, Schuber F. Bioconjugate Chem. 2000;11:118–123. doi: 10.1021/bc990122k. [DOI] [PubMed] [Google Scholar]
- 12.Nakano Y, Mori M, Nishinohara S, Takita Y, Naito S, Kato H, Taneichi M, Komuro K, Uchoda T. Bioconjugate Chem. 2001;12:391–395. doi: 10.1021/bc0001185. [DOI] [PubMed] [Google Scholar]
- 13.Bourel-Bonnet L, Pecheur EI, Grandjean C, Blanpain A, Baust T, Melnyk O, Hoflack B, Gras-Masse H. Bioconjugate Chem. 2005;16:450–457. doi: 10.1021/bc049908v. [DOI] [PubMed] [Google Scholar]
- 14.Said Hassane F, Frisch B, Schuber F. Bioconjugate Chem. 2006;17:849–854. doi: 10.1021/bc050308l. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Ma Y, Sun X-L. Chem. Commun. 2009;21:3032–3034. doi: 10.1039/b822420j. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Xu G, Yang W, Barth RF, Tjarks W, Lee RJ. Bioconjug Chem. 2007;18:101–108. doi: 10.1021/bc060174r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CC-Y, Seo TS, Li Z, Ruparel H, Ju J. Bioconjugate Chem. 2003;14:697–701. doi: 10.1021/bc0256392. [DOI] [PubMed] [Google Scholar]
- 18.Presti FT, Pace RJ, Chen SI. Biochemistry. 1982;21:3831–3835. doi: 10.1021/bi00259a017. [DOI] [PubMed] [Google Scholar]
- 19.Sun X-L, Grande D, Baskaran S, Chaikof EL. Biomacromolecules. 2002;3:1065–1070. doi: 10.1021/bm025561s. [DOI] [PubMed] [Google Scholar]
- 20.Immordino ML, Dosio F, Cattel L. Int. J. Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider T, Sachse A, Leike J, Robling G, Schmidtgen M, Drechsler M, Brandl M. Int. J. Pharm. 1996;132:9–21. [Google Scholar]
- 22.Semple SC, Chonn A, Cullis PR. Biochemistry. 1996;35:2521–2525. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 23.Priem B, Gilbert M, Wakarchuk WW, Heyraud A, Samain E. Glycobiology. 2002;12:235–240. doi: 10.1093/glycob/12.4.235. [DOI] [PubMed] [Google Scholar]
- 24.Song S, Cheong L-Z, Falkeborg M, Liu L, Dong M, Jensen HM, Bertelsen K, Thorsen M, Tan T, Xu X, Guo Z. PLOS One. 2013;8:e73891. doi: 10.1371/journal.pone.0073891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.