Abstract
Background and Purpose
Lacunar infarction is due to a perforating arteriolar abnormality. Possible causes include embolism, atheromatosis or intrinsic disease. We examined whether the size, shape or location of the lacunar infarct varied with embolic sources, systemic atheroma or vascular risk factors.
Methods
We examined data from three prospective studies of patients with clinical and diffusion-weighted imaging (DWI) positive symptomatic lacunar infarction who underwent full clinical assessment and investigation for stroke risk factors. Lacunar infarct size (maximum diameter; shape, oval/tubular; location, basal ganglia/centrum semiovale/brainstem) were coded blind to clinical details.
Results
Amongst 195 patients, 48 infarcts were tubular, 50 were 15-20mm diameter, 97 were in the basal ganglia and 74 in the centrum semiovale. There was no association between infarct size or shape and any risk factors. Centrum semiovale infarcts were less likely to have a potential relevant embolic source (4% v 11%, OR 0.16 95% confidence interval (CI) 0.03-0.83) and caused a lower National Institute of Health Stroke Scale (NIHSS) (2 v 3, OR 0.78 95% CI 0.62-0.98) than basal ganglia infarcts. There were no other differences by infarct location.
Conclusions
Lacunar infarcts in the basal ganglia caused marginally more severe strokes and were three times as likely to have a potential embolic source than those in the centrum semiovale but the overall rate of carotid or known cardiac embolic sources (11%) was low. We found no evidence that other risk factors differed with location, size or shape suggesting that most lacunar infarcts share a common intrinsic arteriolar pathology.
Keywords: Lacunar Stroke, Stroke/pathology, stroke/aetiology
Introduction
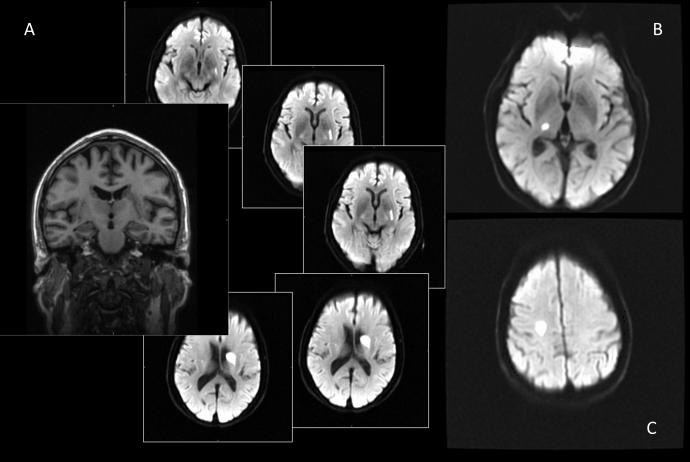
In 1982 Fisher1 described two possible arteriolar pathologies that led to recent small subcortical infarction: lipohyalinosis, associated with smaller infarcts, and arteriolosclerosis associated with larger ones. Atheroma of the parent artery, e.g. middle cerebral artery, could also affect the perforating arteriole and might cause larger basal ganglia lacunar infarcts, e.g. if several perforating arterioles were affected simultaneously.2 However, these pathological examinations were mostly performed late after the stroke, making it difficult to determine the cause of the acute event. The recent wider availability of sagittal and coronal views on diagnostic imaging has increased the recognition that some recent lacunar infarcts may be long or ‘tubular’, leading to the suggestion that such infarcts are a distinct sub-group of lacunar stroke recognizable by their tubular shape3 and location in the basal ganglia, which may have a different aetiology (Figure 1). This sub-group of lacunar stroke has also been associated with progressive sub-acute neurological deterioration after initial presentation4, 5
Figure 1.
Examples of lacunar infarcts of varying size and shape: A) a tubular lacunar infarction on a coronal T1 weighted imaging (left) and axial DWI images (right), B) a small infarction in the right basal ganglia, C) a larger infarction in the right centrum semiovale.
In general, patients with lacunar ischaemic stroke have a different risk factor profile to other non-lacunar stroke sub-types,6 with fewer ipsilateral embolic sources (e.g. cardioembolic or carotid stenosis) and less evidence of large artery atheroma elsewhere (e.g. ischaemic heart disease).
An association between the larger, tubular lacunar infarcts in the basal ganglia and a risk factor profile similar to other atheromatous conditions would imply that such infarcts were atheromatous in nature. However, studies that examined whether lacunar infarcts of varying size, shape and locations had different risk factors or potential stroke causes have produced inconsistent or incomplete results (Table 1).3,7-11 Hence, we investigated patients with a clinical and magnetic resonance (MR) diffusion-weighted imaging (DWI) confirmed diagnosis of lacunar ischaemic stroke to determine if clinical features and risk factors varied with the size, shape or location of the lacunar infarct.
Table 1.
Other studies examining size, shape and location of lacunar infarctions
| Study | Factor examined, (Size, shape or location) |
Number of subjects |
Inclusion / exclusion | Risk factor- free sub- typing? |
Results |
|---|---|---|---|---|---|
| Horowitz 19927 | Size | 108 | Consecutive patients in a stroke data-bank with a final diagnosis of lacunar infarction in the lenticulostriate distribution |
Yes : patients had a clinical lacunar. |
No difference in hypertension between patients with large and small infarcts |
| Ohara 20059 | Size | 130 | Consecutive patients with a first-ever lacunar infarction. |
Not clear (only able to access abstract) |
Large infarcts associated with gender, large artery disease progressing stroke and higher thrombin-anti thrombin complex. |
| Yonemura 200211 |
Location | 106 | 106 patients selected from 582 consecutive patients with stroke/TIA. All infarcts < 15mm. Centrum semiovale: in territory of the white matter medullary artery arising from the cortical branches of the MCA. Excluded infarcts in: thalamus, brain stem, and subcortical white matter of the anterior and posterior cerebral artery territories, corona radiata. |
Yes: All with DWI- MRI |
Infarct in centrum semiovale associated with large artery disease and an embolic source. |
| Yamamoto 201010 |
Location | 392 | Compared infarcts in lenticulostriate territory to those in anterior pontine territory |
Yes: lacunar syndrome and DWI-MRI |
Diabetes and large artery disease significantly more common in the APA group. |
| Ryu 20123 | Shape | 105 | Consecutive patients with infarcts up to 20mm in size (15mm if intra-tentorial), whatever the TOAST subtype. Compared ‘conglomerated beads’ to ‘oval’ shape. |
Yes | No difference in risk factors, or in TOAST subtype ‘Bead shaped’ infarcts were larger and associated with early neurologic deterioration. |
| Lee 20058 | Size and shape. |
103 | From a series of consecutive patients with TIA or Stroke. Included all infarcts in the territory of the white matter medullary artery. No upper size limit. Stroke sub-type was then classified according to ‘TOAST’. |
Yes: MRI-DWI features only. |
Sausage or chain shaped infarcts associated with large artery disease / cardio-embolic source. |
Methods
Patient recruitment
We examined data from three existing prospective stroke studies and identified all patients with a symptomatic MR DWI-confirmed lacunar infarction, who had both an electrocardiogram (ECG) and a carotid Doppler ultrasound. We included two prospective observational studies from a regional Stroke Service in Edinburgh (one published,12 one now completed recruiting) and consecutive patients with lacunar stroke admitted to the Stroke Unit of Careggi University Hospital, Florence. All studies were approved by the relevant research ethics committee. Patients were recruited in Edinburgh from 2005-2007 and 2010-2012, and in Florence from 2007-2011.
Patient assessment
All patients were assessed at presentation with a structured full clinical assessment by a stroke specialist and MR imaging at 1.5T including DWI, T1-weighted, T2-weighted, fluid-attenuated inversion recovery and T2*-weighted images. The clinical assessment included the National Institutes of Health Stroke Scale (NIHSS) score; if symptoms were improving by the time of presentation we estimated the worst NIHSS from the history. We recorded past medical history including: hypertension (a previous diagnosis of hypertension, or blood pressure ≥140/90 mmHg); diabetes mellitus; hypercholesterolemia (a previous diagnosis or a fasting total cholesterol level >5mmol/L); and smoking (current or within the previous 12 months). All patients had a 12 lead ECG, and carotid Doppler ultrasound performed blind to stroke subtype and brain imaging. In addition, we performed echocardiography in younger patients, and patients with any suspected cardiac abnormality including patent foramen ovale, and 24 hour ECG tape if there was any suspicion of arrhythmia. We defined clinically significant carotid stenosis as 50% or greater using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria.13 We defined a lacunar infarct on DWI based on focal hyper-intense signal in the deep grey or white matter of the cerebral hemispheres or brainstem, not extending to the cerebral cortex and of no more than 20mm in maximum axial diameter. We recognise that the 20mm cut-off is arbitrary, but it is a widely used definition and we considered that infarcts larger than 20mm maximum axial diameter were likely to be striatocapsular infarcts (due to transient middle cerebral artery (MCA) embolic occlusion or persistent MCA occlusion with good peripheral collateral arteries, as described by Donnan et al14). Of the 518 patients recruited into the two studies in Edinburgh, 154 had a lacunar lesion on MRI but the remainder had either a cortical infarct (205), no infarct on imaging (n=142), or were recruited in an early pilot phase and lacked complete risk factor details (n=17). Out of 879 consecutive patients with acute ischemic stroke admitted to the Stroke Unit in Florence, 79 had a lacunar stroke syndrome; of these 41 had a lesion on DWI-MRI. There was no statistically significant difference in demographics or risk factors between the included and non included lacunar stroke patients. All patients gave written informed consent and the studies were approved by the local Research Ethics Committee.
Image analysis
A trained neurologist (ADB) assessed all scans whilst blinded to the patients’ details; classifications were checked with a neuroradiologist (JMW). We classified infarcts by size, shape and location. We measured axial infarct diameter, and then classed infarcts 15-20mm in axial diameter as ‘large’ and those 0-14mm as ‘small’. We classed infarct shape as tubular (long axis at least twice that of the short axis) or oval. We classed infarct location as basal ganglia (if more than half of the infarct was in the lentiform nucleus, thalamus or internal capsule) or centrum semiovale (infarct not involving deep grey matter or internal capsule), or other (brainstem). Examples are given in Figure 1.
Statistical analysis
We performed statistical analysis using R Statistical Software. We performed univariate analysis using Fisher’s Exact Test for dichotomous variables and the Mann Whitney test for continuous, non-parametric variables (age, NIHSS and lesion size). We first assessed the variables individually then assessed two combined variables: ‘extra-cranial atherosclerosis’; one or more of carotid stenosis, peripheral vascular disease (PVD), and/or ischaemic heart disease (IHD); and ‘any potential embolic source’ which consisted of atrial fibrillation (AF) and/or ipsilateral carotid stenosis. We used binary logistic regression for multi-variable analysis using pre-selected parameters and those that were significant on univariate analysis. We then analysed the relationship between size, shape and location of the infarct. To visually examine whether lesion size was normally distributed we plotted a Kernel density plot. We examined the distribution of lesion size by the location and shape of lesions, the presence of a potential embolic source, and the presence of large artery atheromatous disease.
Results
Patient characteristics
We identified 195 suitable patients (Table 2). The infarct was less than 15 mm in axial diameter in 145/195 (74%) patients and 15-20 mm in 50 (26%); tubular in 48/195 (25%), oval in 147/195 (75%); located in the basal ganglia in 97/195 (50%), in the centrum semiovale in 74/195 (38%) and in other locations in 24/195 (12%). Seventy per cent (137/195) of patients were men, with a median age of 68 years (inter quartile range (IQR) 59-75). Most patients (73%) were hypertensive, 38% had hypercholesterolaemia, and 45% were smokers.
Table 2.
Clinical and demographic factors of patients with different types of lesions.
| Demographic and clinical variables |
All subjects (n=195) |
SIZE |
SHAPE |
LOCATION |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <15mm (n=145) |
>15mm (n=50) |
p | Tubular (n=48) |
Oval (n=147) |
p | Basal Ganglia (n=97) |
Centrum Semiovale (n=74) |
p | ||
| Univariable Analysis | ||||||||||
|
| ||||||||||
| Median Age (IQR) | 68(58-75) | 68(59-75) | 67.5(57.5-75) | 0.56 | 64(56-72) | 68(60-76) | 0.08 | 67 (56-74) | 70(62-76) | 0.138 |
| Female (%) | 58(30) | 46(32) | 12(24) | 0.37 | 10(21) | 48(33) | 0.15 | 27 (28) | 28(37) | 0.188 |
| Smoker (%) | 88(45) | 67(46) | 21(42) | 0.74 | 24 (50) | 64(44) | 0.50 | 44 (45) | 32 (43) | 0.76 |
| Prior TIA (%) | 28(14) | 22 (15) | 6 (12) | 0.64 | 7 (15) | 21(14) | 1 | 15 (15) | 9 (12) | 0.658 |
| Prior Stroke (%) | 19(10) | 15(10) | 4(8) | 0.79 | 4 (8) | 15(10) | 1 | 6(6) | 10(14) | 0.118 |
| Prior IHD(%) | 20 (10) | 17 (12) | 3 (6) | 0.29 | 3 (6) | 17 (12) | 0.41 | 12 (12) | 6 (8) | 0.455 |
| Prior PVD(%) | 13(7) | 11 (8) | 2 (4) | 0.52 | 1(2) | 12(8) | 0.19 | 5 (5) | 7 (9) | 0.368 |
| Diabetes (%) | 23(12) | 17(12) | 6(12) | 1 | 3 (6) | 20(14) | 0.21 | 10(10) | 8 (11) | 1.0 |
| Ipsilateral CS(%) | 7(4) | 5(3) | 2 (4) | 1 | 2 (7) | 5 (3) | 0.68 | 6 (6) | 1(1) | 0.141 |
| Controlateral CS(%) | 9(5) | 8 (6) | 1 (2) | 0.45 | 3 (6) | 6 (4) | 0.69 | 3 (3) | 3 (4) | 1.0 |
| Any Large Vessel disease (%) | 39(20) | 32(22) | 7 (14) | 0.31 | 7 (15) | 32 (22) | 0.41 | 20(21) | 14 (19) | 0.848 |
| AF(%) | 11(6) | 7 (5) | 4 (8) | 0.48 | 3 (6) | 8 (5) | 0.41 | 5(5) | 2 (3) | 0.702 |
| Hypertension (%) | 142(73) | 103(71) | 39(78) | 0.36 | 35(73) | 107(73) | 1 | 66(68) | 56(76) | 0.309 |
| High Cholesterol (%) | 74 (38) | 54 (37) | 20 (40) | 0.74 | 18(38) | 56(38) | 1 | 37 (38) | 27 (36) | 0.874 |
| Any Embolic Source (%) | 18(9) | 12 (8) | 6 (12) | 0.41 | 5 (10) | 13 (9) | 0.78 | 11 (11) | 3 (4) | 0.099 |
| Median NIHSS (IQR) | 2(2-4) | 2 (2-4) | 3 (2-4) | 0.48 | 3 (2-4) | 2 (2-3.5) | 0.12 | 3 (2-4) | 2 (2-3) | 0.0473 |
|
| ||||||||||
| Multivariable Analysis | ||||||||||
|
| ||||||||||
| Any Large Vessel Disease | 1.80(0.73-4.91) | 0.68 (0.25-1.67) | 0.94(0.39-2.27) | |||||||
| Embolic Source (AF or ipsilateral carotid stenosis) |
0.52(0.18-1.64) | 1.61(0.47-4.86) | 0.16 (0.03-0.83) | |||||||
| Age | 1.01(0.98-1.04) | 0.97(0.94-1.01) | 1.03 (1.0-1.070) | |||||||
| Worse NIHSS | _ | _-- | 0.78 (0.62-0.98) | |||||||
| Corrected for large vessel disease, embolic source, and age. |
Corrected for large vessel disease, embolic source, and age. |
Corrected for large vessel disease, embolic source, age and NIHSS |
||||||||
IQR=Interquantile Range; TIA=transient ischemic attack; IHD=ischemic heart disease; PVD=peripheral vascular disease; CS=carotid stenosis; LV=large vessel; AF=atrial fibrillation
Univariate analysis of size, shape, location, risk factors and clinical features
On univariate analysis (Table 2) there was no association between infarct size, or shape and risk factor profiles. Lacunar strokes located in the basal ganglia caused more severe strokes than those in the centrum semiovale: median initial NIHSS score was 3 in the basal ganglia and 2 in the centrum semiovale (p=0.04). The association between basal ganglia location and the presence of a relevant embolic source (11 v 4%) did not reach statistical significance (p=0.099). There were no other differences by infarct location. We examined the location of lesions in the basal ganglia and found that 6/41 (14%) thalamic infarcts had an embolic source compared with 5/56 (9%) lesions elsewhere in the basal ganglia (internal capsule, medial lentiform), p=0.0519. Conversely, 6/11 basal ganglia infarcts with an embolic source were in the lateral thalamus.
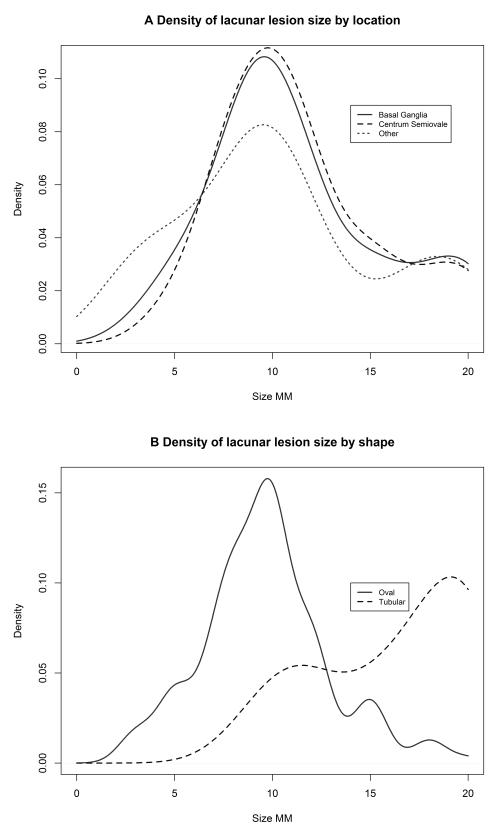
There was no significant difference in the median size of lesion in the basal ganglia, centrum semiovale or posterior circulation locations (all 10mm, p=0.767), Figure 2a. Tubular lesions were larger (median 17.5mm) than oval lesions (median 10mm, p<0.001), Figure 2b.
Figure 2.
Kernel Density plot demonstrating the distribution of lacunar infarction size by shape and location of lesion (area under the curve=1 irrespective of sample size).
There was no statistically significant relationship between infarct shape and location: 23/97 (24%) of basal ganglia infarcts were tubular against 16/74 (22%) of centrum semiovale infarcts. Therefore, there was a subset of larger, tubular infarcts but they did not occur consistently in any particular part of the brain.
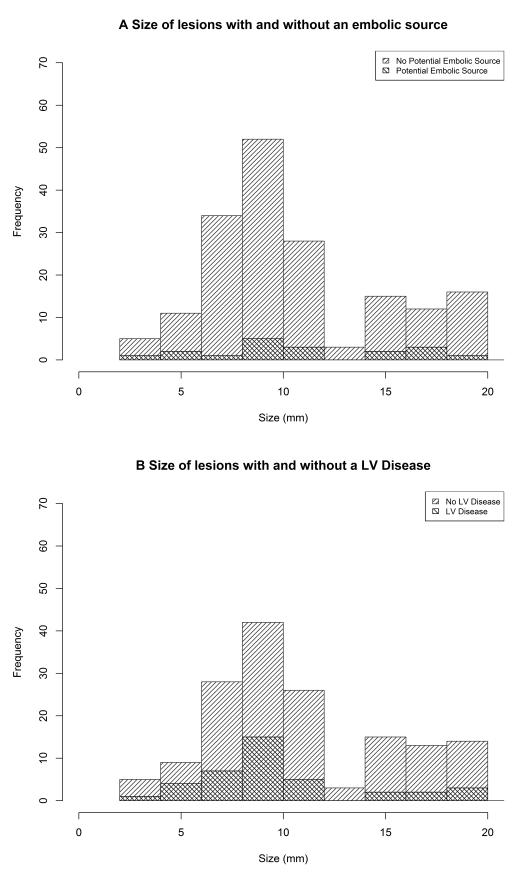
To explore lesion topography further, we plotted the distribution of lesion size (mm) for both tubular and oval lesions shape (Figure 2). Whilst oval lesions were normally distributed, tubular lesions were non-normally distributed (Figure 2b), implying that these may be the tail of a larger normally distributed group of lesions. There was no difference in the distributions of the size of lesions with and without an embolic source, or with or without extra cranial large vessel disease (Figure 3).
Figure 3.
Histograms of lacunar lesion size, by the present of a potential embolic source and by large vessel disease elsewhere.
Multivariable analysis of size, shape, location, risk factors and clinical features
Multivariable analysis demonstrated that patients with a centrum semiovale infarct were less likely to have a potential embolic source (AF or ipsilateral carotid stenosis) than those with a basal ganglia infarct (odds ratio (OR) 0.16, 0.03-0.83) (Table 2). However, patients with an embolic source, or extracranial large vessel disease were not more likely to have a larger lesion that those without embolic sources or extracranial large artery disease (Table 3) in this series.
Table 3.
Odds ratio of an embolic source, and extra cranial large vessel disease, adjusted for age and gender.
| Odds Ratio of an Embolic Source (AF, or ipsilateral carotid stenosis) | |||
|
| |||
| Model 1 Unadjusted | |||
| OR | Lower CI | Upper CI | |
| Size (perMM) | 0.91 | 0.29 | 1.15 |
| Model 2, Adjusted for Age and Gender | |||
| Size | 1.04 | 0.92 | 1.18 |
| Age (per Year) | 1.07 | 1.02 | 1.13 |
| Gender (Male) | 1.02 | 0.34 | 3.50 |
|
| |||
| Odds Ratio of extra cranial large vessel disease | |||
|
| |||
| Model 3 Unadjusted | |||
| OR | Lower CI | Upper CI | |
| Size (perMM) | 0.87 | 1.20 | 1.04 |
| Model 4, Adjusted for Age and Gender | |||
| OR | Lower CI | Upper CI | |
| Size (perMM) | 1.04 | 0.92 | 1.18 |
| Age (per Year) | 1.07 | 1.02 | 1.13 |
| Gender (Male) | 1.02 | 0.34 | 3.50 |
Discussion
Our study showed little association between clinical risk factors and the size, shape or location of a lacunar stroke except for an association between basal ganglia infarcts and a potential relevant embolic source, e.g. ipsilateral carotid stenosis or atrial fibrillation. However, most patients did not have a potential carotid or cardio- embolic source, as detected on carotid ultrasound or ECG in all, or on echocardiography (performed where indicated), whether the lesion was in the basal ganglia (89%) or the centrum semiovale (96%). There was also no significant difference in overall infarct sizes between the centrum semiovale and basal ganglia.
Six other studies (Table 1) have examined associations between risk factors and size, shape or location of lacunar infarcts, but the present study is the only one to examine the relationship with and between all three factors, and to include patients with recent lacunar infarction in all perforating arteriolar territories. The present study is also nearly twice as large as previous studies except for one,9 but this latter compared risk factors in patients with basal ganglia and pontine infarcts only, not the centrum semiovale, nor infarct size or shape. Some studies only examined the centrum semiovale and included deep border zone as well as lacunar infarcts if in white matter.8 Others found that patients with a small centrum semiovale infarct were more likely to have an embolic source than patients with a similar lesion in the basal ganglia,11 although they included intracranial stenosis, which is rare in our population,15 and excluded the thalamus and all other territories supplied by the basilar and posterior cerebral arteries. A pathology study in Edinburgh16 found a potential embolic source in 10 out of 12 subjects with centrum semiovale lacunar infarcts at autopsy who presented with and died of, various conditions. However only half had a history of symptomatic stroke, of uncertain relationship to the infarct seen at post-mortem, at some point prior to death. We found no association between the size of infarcts and risk factors, though Ohara9 found an association between larger infarcts and female sex, as well as intra-cranial stenosis and thrombin-anti-thrombin complex. Ashdaghi et al (published in abstract)17 examined the shape of lacunar lesions in 2264 patients with DWI proven lesions; classifying the lesion as slab, stick, oval or multiple.18 They found that diabetes was more common in patients with oval lesions, however investigation of other risk factors is limited as patients were excluded if they had a potential source of embolism. Our finding that shape was not linked to different risk factors was similar to the findings of Ryu3 who described infarcts shaped like ‘conglomerated beads’ but did not find these to have different risk factors to oval infarcts.
The strengths of our study include a large group of prospectively-recruited stroke patients subtyped using risk factor free methods thus avoiding expectation bias or confounding. The use of acute DWI-MRI allowed accurate diagnosis of lacunar infarction and assessment of infarct characteristics. The risk factors were assessed in a standardised fashion blind to all clinical data, reducing potential for confounding from the belief of the assessing clinician regarding the stroke aetiology. Including both inpatients and outpatients avoided any bias towards more severe stroke as many patients with lacunar stroke are only mildly affected and may not be admitted to hospital.
The cut off of 20mm may have complicated the results, and be influenced by the time of imaging, as acute lesions are larger than those at a later stage. Prior studies used imaging at a later stage after stroke. Future studies should consider the shape of all subcortical lesions regardless of size as this may help to determine the cut off of lacunar versus striatocapsular infarcts in future. A major weakness is that we were not able to examine for intracranial artery stenosis or aortic arch atheroma in the patients, nor for cardioembolic sources with echocardiography or 24 hour ECG in all patients, although these were performed wherever indicated. However, as regards intracranial stenosis, we did perform intracranial arterial imaging in all 120 patients in a previous study (half with recent small subcortical infarction) in a very similar population in Edinburgh and did not find any intracranial stenoses at all although there were cervical carotid stenoses (which we would have detected with carotid ultrasound); many of these 120 patients overlapped with the present population.15 As regards aortic arch atheroma, diagnostic standards for clinically relevant atheroma are not yet established and it would be difficult to examine all patients with transoesophageal echocardiography, or MRA of the aortic arch. We did not gather data on the speed of onset of the infarct, whether sudden or progressive, although previous studies have reported an association between basal ganglia infarcts and progressive symptoms. We had fewer of the larger, tubular, basal ganglia infarcts, which limited the associations that could be tested in multivariable analysis without over fitting. We measured the maximum diameter on acute DWI which may overestimate the true infarct size through ‘blooming’ effects; however this will probably have affected all infarcts equally and therefore not interfered with the analysis of size and other variables. We measured the maximum diameter in the axial plane – this may have underestimated the maximum dimensions of some tubular basal ganglia infarcts. Future studies should describe the lesions’ maximum longitudinal and axial dimensions and evaluate the rapidity of change in visible infarct dimensions on different sequences over time.
We found no evidence of differences in risk factors by lacunar infarct location, size or shape, except for the association between having a potential embolic source and infarcts in the basal ganglia. The low absolute proportion of patients with a relevant carotid or known cardioembolic source (11%) should be compared with the much higher proportion of patients with non-lacunar stroke who have either ipsilateral (22%) or contralateral (8%) carotid stenosis or cardioembolic sources (26%) detected by the same means.6 The size distribution of tubular lesions demonstrated in Figure 2 raises the possibility that these lesions classed as small subcortical are actually the lower tail of a distribution of larger deep infarcts, e.g. striatocapsular,14 and should encourage further measurement of lesions in other datasets and re-examination of the current size limits and risk factor associations.
This work provides further evidence that most lacunar infarcts are due to an intrinsic arteriolar pathology regardless of their morphology and should be tested in other populations using risk factor free clinical subtyping and DWI to be certain of the stroke subtype and location on imaging. Future research should concentrate on defining the aetiology of lacunar ischaemic stroke, avoiding risk factor based subtyping, and developing treatments.
Acknowledgements
We wish to thank K Shuler for her invaluable assistance with the data management of the patients recruited in Edinburgh.
Sources of Funding: Edinburgh Patients: the studies were funded by The Chief Scientist Office of the Scottish Executive (CZB/4/281), the Wellcome Trust (075611 & WT088134/Z/09/A the Row Fogo Charitable Trust, the Cohen Charitable Trust, supported the study. The imaging was conducted in the Brain Research Imaging Center, University of Edinburgh (www.bric.ed.ac.uk), a center in the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) Collaboration. Dr. A Del Bene received research support from PhD Program University of Florence and from Health Targeted Research Programme, Italian Ministry of Health, 2008
Footnotes
Disclosure: None.
Contributor Information
Alessandra Del Bene, NEUROFARBA Department, Neuroscience section, University of Florence, Italy.
Stephen DJ Makin, Division of Neuroimaging Sciences, Western General Hospital, Crewe Rd, Edinburgh, EH4 2XU, Uk.
Fergus N Doubal, Division of Geriatric Medicine, Western General Hospital, Crewe Rd, Edinburgh, EH4 2XU, Uk.
Dominico Inzitari, NEUROFARBA Department, Neuroscience section, University of Florence, Italy.
Joanna M Wardlaw, Division of Neuroimaging Sciences, Western General Hospital, Crewe Rd, Edinburgh, EH4 2XU, UK.
References
- (1).Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- (2).Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989 Sep;39(9):1246–50. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- (3).Ryu DW, Shon YM, Kim BS, Cho AH. Conglomerated beads shape of lacunar infarcts on diffusion-weighted MRI: what does it suggest? Neurology. 2012 May 1;78(18):1416–9. doi: 10.1212/WNL.0b013e318253d62f. [DOI] [PubMed] [Google Scholar]
- (4).Takase K, Murai H, Tasaki R, Miyahara S, Kaneto S, Shibata M, et al. Initial MRI findings predict progressive lacunar infarction in the territory of the lenticulostriate artery. Eur Neurol. 2011;65(6):355–60. doi: 10.1159/000327980. [DOI] [PubMed] [Google Scholar]
- (5).Del Bene A, Palumbo V, Lamassa M, Saia V, Piccardi B, Inzitari D. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke. 2012;7(4):321–9. doi: 10.1111/j.1747-4949.2012.00789.x. [DOI] [PubMed] [Google Scholar]
- (6).Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–9. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- (7).Horowitz DR, Tuhrim S, Weinberger JM, Rudolph SH. Mechanisms in lacunar infarction. Stroke. 1992 Mar;23(3):325–7. doi: 10.1161/01.str.23.3.325. [DOI] [PubMed] [Google Scholar]
- (8).Lee PH, Oh SH, Bang OY, Joo IS, Huh K. Pathogenesis of deep white matter medullary infarcts: a diffusion weighted magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2005 Dec;76(12):1659–63. doi: 10.1136/jnnp.2005.066860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ohara T, Yamamoto Y, Oiwa K, Hayashi M, Nakagawa M. [Clinical classification for lacunar infarct. An investigation of 130 consecutive cases of lacunar infarctions] Rinsho Shinkeigaku. 2005 Jan;45(1):6–12. [PubMed] [Google Scholar]
- (10).Yamamoto Y, Ohara T, Hamanaka M, Hosomi A, Tamura A, Akiguchi I, et al. Predictive factors for progressive motor deficits in penetrating artery infarctions in two different arterial territories. J Neurol Sci. 2010 Jan 15;288(1-2):170–4. doi: 10.1016/j.jns.2009.08.065. [DOI] [PubMed] [Google Scholar]
- (11).Yonemura K, Kimura K, Minematsu K, Uchino M, Yamaguchi T. Small centrum ovale infarcts on diffusion-weighted magnetic resonance imaging. Stroke. 2002 Jun;33(6):1541–4. doi: 10.1161/01.str.0000016961.01086.94. [DOI] [PubMed] [Google Scholar]
- (12).Doubal FN, MacGillivray TJ, Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology. 2009 May 19;72(20):1773–8. doi: 10.1212/WNL.0b013e3181a60a71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339(20):1415–25. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- (14).Donnan GA, Bladin PF, Berkovic SF, Longley WA, Saling MM. The stroke syndrome of striatocapsular infarction. Brain. 1991 Feb;114(Pt 1A):51–70. [PubMed] [Google Scholar]
- (15).Wardlaw JM, Doubal FN, Eadie E, Chappell F, Shuler K, Cvoro V. Little association between intracranial arterial stenosis and lacunar stroke. Cerebrovasc Dis. 2011;31(1):12–8. doi: 10.1159/000319773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lammie GA, Wardlaw JM. Small centrum ovale infarcts - a pathological study. Cerebrovasc Dis. 1999 Mar;9(2):82–90. doi: 10.1159/000015903. [DOI] [PubMed] [Google Scholar]
- (17).Ashdaghi N, Pearce L, Nakajima M, Bazan C, Cermeno F, Lewis B, et al. Abstract WP187: Correlation between Infarct Shape and Volume and Ischemic Risk Factors and Recurrent Ischemic Rates in Small Subcortical Stroke; Data from the SPS3 Randomized Controlled Trial. Stroke. 2013 Feb 6;44(2) [Google Scholar]
- (18).Herve D, Mangin JF, Molko N, Bousser MG, Chabriat H. Shape and volume of lacunar infarcts: a 3D MRI study in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2005 Nov;36(11):2384–8. doi: 10.1161/01.STR.0000185678.26296.38. [DOI] [PubMed] [Google Scholar]