Abstract
Background
The method of loci (MoL) is a complex visuospatial mnemonic strategy. Previous research suggests older adults could potentially benefit from using the MoL, but that it is too attentionally demanding for them to use in practice. We evaluated the hypotheses that training can increase the use of MoL, and that MoL use is associated with better memory.
Methods
We analyzed skip patterns on response forms for the Auditory Verbal Learning Test (AVLT) in the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE, n=1,401) trial using 5 years of longitudinal follow-up.
Results
At baseline, 2% of participants skipped spaces. Fewer than 2% of control participants skipped spaces at any visit across 5 years, but 25% of memory-trained participants, taught the MoL, did so. Participants who skipped spaces used more serial clustering, a hallmark of the MoL (p<0.001). Trained participants who skipped spaces showed greater memory improvement after training than memory-trained participants who did not skip spaces (Cohen's d=0.84, P=0.007), and did not differ in the subsequent rate of long-term memory decline through up to 5 years of follow-up.
Conclusion
Despite being attentionally demanding, this study suggests that after training, the MoL is used by up to 25% of older adults, and that its use is associated with immediate memory improvement that was sustained through the course of follow-up. Findings are consistent with the notion that older adults balance complexity with novelty in strategy selection, and that changes in strategies used following memory training result in observable qualitative and quantitative differences in memory performance.
Keywords: Method of loci, strategy use, memory training, gerontology, older adults
Introduction
Memory is an important concern among older adults and essential for performing daily activities (Verhaeghen et al., 2000). Memory training interventions train certain memory strategies that are more effective than other strategies given a task. Memory strategies are cognitive mechanisms that enable individuals to better process information from the environment, thus saving time and mental resources necessary for memory performance to exceed expectations (Bruner et al., 1957; Lemaire & Siegler, 1999). Memory training programs differ in the types of strategies trained (Rebok et al., 2007). Because the ultimate goal of training is to teach strategies that will translate into improved everyday functioning, it is also essential to train strategies that older adults are likely to actually use in their daily lives.
Common mnemonic strategies include rehearsal, association, categorization, and imagery. Common combinations of these are formal mnemonic strategies in their own right, and include techniques for face-name recognition and name-learning, number mnemonics, and story mnemonics. The method of loci is a more effortful, formal mnemonic strategy that involves complex visuo-spatial mnemonics to remember information (Bower, 1970; Yates, 1966). The strategy takes advantage of known locations, which can be parts of the body or landmarks along a path, and pairs each location with a to-be-remembered item from a list. This list could be of grocery items or points in a speech. This structured sequence of images provides memory cues to facilitate recall, leading to improved memory performance for older adults (Hill et al., 1991; Kliegl, Smith, & Baltes, 1989; Kliegl, Smith, & Baltes, 1990; Rebok & Balcerak, 1989; Verhaeghen & Marcoen, 1996; Yesavage & Rose, 1984).
Besides improved memory, older adults experience changes in neurochemistry, brain activation, and even macrostructural increases in cortical thickness after being trained to use the method of loci, underscoring the plasticity of the brain to new experiences (Engvig et al., 2010; Kondo et al., 2005; Nyberg et al., 2003; Valenzuela et al., 2003). One study showed that use of this strategy in experimental settings is associated with increased activation in the occipital brain region in particular (Nyberg et al., 2003). The occipital region is the brain's visual processing center and the method of loci is a visuo-spatial mnemonic, suggesting that use of the method of loci is associated with meaningful changes in brain activation. In another study, cortical thickening was observed in the fusiform and lateral orbitofrontal cortices after an 8-week method of loci training program (Engvig et al., 2010). These brain regions are used during visual memory encoding (Stern et al., 1996) and episodic memory (Rugg et al., 2002; Wagner et al., 1998).
Although it is clear that the method of loci is efficacious for older adults who use it, it is unclear whether enough older adults would use it to make it worth teaching. Research suggests older adults do not show as much training-related memory improvement as do younger-old adults after being trained in the method of loci, potentially because older adults choose more elemental strategies such as semantic grouping or story mnemonics despite training in other methods (Baltes & Kliegl, 1992; Nyberg et al., 2003; Singer, Lindenberger, & Baltes, 2003; Verhaegen & Marcoen, 1996). Other research suggests that strategy execution for complex or attentionally demanding strategies declines with age, and that this disparity increases with increasing strategy complexity (Allen et al., 1992; Arnaud et al., 2008; Dunlosky & Hertzog, 1998; Dunlosky, Hertzog, & Powell-Moman, 2005; Geary & Wiley, 1991; Geary, French, & Wiley, 1993; Kausler, 1994; Siegler & Lemaire, 1997; St. Clair-Thompson, 2007). Thus, there is a trade-off between teaching potentially highly effective but complex strategies that are known to improve performance versus simpler strategies that are less attentionally demanding.
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study is to date the largest study of cognitive training in older adults (Willis et al., 2006). The memory training program emphasized strategies for learning and remembering. Memory strategies were practiced in individual and group sessions with exemplar problems and immediate individual feedback about performance (Jobe et al., 2001). ACTIVE training in memory improved memory outcomes up to five years following training (Willis et al., 2006). In method of loci training, participants practiced locating items disbursed throughout their living room, and that order of recall mattered in order to facilitate retracing the items. To provide a common set of locations in learning the method of loci, participants were introduced to Memory Man, a drawing of a man that highlighted specific areas on the human body (hair, forehead, eye, ear, nose, mouth, shoulder, chest, elbow, forearm, hand, thigh, knee, ankle, toes) to use as locations for target material to be remembered.
Importantly, the method of loci was the only strategy taught in the ACTIVE memory training intervention that emphasized the importance of the order of encoded material. Enhanced serial recall is a hallmark of the method of loci (Baltes & Kliegl, 1992; Kliegl, Smith, & Baltes, 1989; Kliegl, Smith, & Baltes, 1990; Singer, Lindenberger, & Baltes, 2003). When taught the method of loci strategy during ACTIVE memory training, participants were trained to skip an item and return to it later in order to maximize recall if they could not remember the target item in a sequence. No other strategy taught during ACTIVE memory training emphasized serial recall, and long-term follow-up assessments of word list recall following training included instructions to not worry about the order of recall. As described in the Methods, in ACTIVE we were able to objectively measure serial clustering, a marker of serial recall on word list recall tasks, administered during follow-up study visits (Gross & Rebok, 2011).
The present study is based on the premise that changes in strategies used as a consequence of memory training should result in qualitative differences in how information is stored and recalled from memory (Light, 1991). We evaluated the hypotheses that training can increase the use of method of loci, and that this strategy is associated with improvement memory that is maintained over time. We used a novel approach to identify method of loci use during ACTIVE study assessments that does not rely on self-report. We report the prevalence of the method of loci by memory training status. We hypothesized that memory-trained older adults would show more skipping behavior than untrained older adults on a word list-learning task. We also explored the association between method of loci use and serial recall on a word list-learning task. Consistent with research that serial recall is a marker of the method of loci, we hypothesized that older adults who skip spaces would show elevated levels of serial clustering. Finally, we hypothesized that use of the method of loci anytime during follow-up in ACTIVE, as indicated by skipping behavior, is associated with improved memory performance relative to no-contact control participants or memory-trained participants who never skipped spaces during the word list-learning task.
Methods
Study design
The ACTIVE study is a large, multi-center, longitudinal, and randomized trial of cognitive training among older adults that sought to characterize the effect of training beyond proximal cognitive outcomes to everyday functioning outcomes (Jobe et al., 2001). ACTIVE's target population consisted of community-living adults age 65 and older living in the United States in the late 1990s. Eligible participants had to be living in a non-institutional setting at baseline and be available in the study area throughout the study's duration. Individuals were excluded who, at baseline, were younger than 65, reported disabilities in common activities of daily living, were on chemotherapy or had been diagnosed with cancer in the last five years, had been diagnosed with Alzheimer's disease, had a stroke in the past 12 months, scored lower than 23 on the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), had any substantial sensory (e.g., vision) impairment that would interfere with training, or had received cognitive training similar to ACTIVE within the past two years. Additional features of the study design and recruitment strategy are described in detail elsewhere (Ball et al., 2002; Jobe et al., 2001; Willis et al., 2006).
Participants were randomized to one of three cognitive interventions: memory, reasoning, or speed of information processing training (Ball et al., 2002; Jobe et al., 2001). A no-contact control group constituted a fourth group. The memory training intervention was administered in 10 small-group sessions (three to five participants) offered over six weeks after the baseline assessment. Each session lasted from 60 to 75 minutes. Participants were provided a manual. Certified trainers used posters and handouts. The first training session was an educational overview of how memory works and how to benefit from training. Participants were told in the manual that the goals of memory training were to increase knowledge of the importance of memory in daily activities, increase confidence and provide social reinforcement, improve verbal list-learning and prose memory through relevant memory strategies and recall for gists rather than verbatim story recall, and to enhance awareness of the importance of memory for everyday functioning tasks including remembering items from a grocery list.
The next four training sessions consisted of guided memory strategy instruction, focusing on the method of loci, organization, association, and visualization. Formal instruction and supervised practice with the method of loci was covered in 11 pages of the 109 page trainer's manual following instruction in other visualization methods including sentence imagery. The method of loci was introduced during the third training session as one type of strategy to use to visualize target material. During the remaining five training sessions, participants practiced the strategies they were taught using different individual and group practice exercises; no new strategies were taught during these sessions (Jobe et al., 2001). Sessions for inductive reasoning and speed of processing training followed a similar pattern. Immediately prior to the first and third annual study visits, trained participants who completed at least 8 of the initial 10 training sessions were randomized to receive booster training. Booster training consisted of up to four 75-minute refresher sessions designed to reinforce strategies from the initial training program.
Participants
Participants were recruited from six metropolitan sites across the United States (University of Alabama at Birmingham, Johns Hopkins University, Wayne State University, Hebrew Rehabilitation Center for the Aged in Boston, Indiana University School of Medicine, and Pennsylvania State University). In total, 2,802 participants were correctly randomized to one of the four intervention groups. Participants were assessed with a variety of measures at baseline, immediate post-training, as well as one, two, three, five, and ten years after training concluded. Data in the present study are taken from 1,401 no-contact control (N=698) and memory-trained (N=703) participants' data from study visits through the fifth year wave. The trial was monitored by a data safety monitoring board, and the study protocol was approved by institutional review boards at each study site. Written informed consent was obtained from all participants.
Ascertainment of the use of method of loci
In ACTIVE memory training, the method of loci was introduced to participants as one of several strategies for remembering lists of unrelated items. When learning the method of loci, participants were instructed to skip a word and come back to it later if they did not remember the word in the sequence in which it was presented. This particular instruction was not part of other strategies taught in the ACTIVE memory training arm.
During study visits before and after training, participants were free to use any technique they preferred on standardized memory tests, which included the Auditory Verbal Learning Test (AVLT), Hopkins Verbal Learning Test (HVLT), and Rivermead Behavioral Memory Test. Some participants skipped spaces on the answer sheets used to write down words recalled in each trial of the AVLT. The AVLT response sheet consisted of a page with 15 lines, and so a skipped space was recorded when a participant did not write on a line but wrote a word on a subsequent line under that space during any trial of the test. An example response sheet that clearly demonstrates skipping behavior is provided in Figure 1. A participant was designated a skipper who skipped at least one space on any trial of the AVLT during a study visit. Spaces skipped because of erasures were not counted as skips. This behavior was hypothesized to indicate that the method of loci strategy was used to encode information because participants in memory training were told when learning the method of loci that, if they could not recall a word that goes with a location, to skip it and return to it later.
Figure 1.
Example AVLT response sheet used to collect recall for each trial in ACTIVE
Legend. Photocopy of an AVLT response sheet for a selected participant's trial 1 recall at the ACTIVE immediate post-training visit. Spaces between some words were left blank on the form. In this example, recalled words are written in the same serial order as presented.
Variables
AVLT
The AVLT is a word list-learning task consisting of five immediate recall trials of a 15-word list of unrelated words, followed by an interference trial using a different word list and a short-delay recall with the initial word list (Rey, 1964; Schmidt, 2004). Thus, the maximum AVLT recall score is 75 words across 5 immediate recall trials. In a modification to the test's standard clinical administration which made the present study possible, participants wrote down their recall on a special AVLT response sheet rather than repeat words aloud to the interviewer (Figure 1). The following directions were played by audiotape to all participants (emphasis added):
“The first thing I will do is read you a list of words one at a time. Listen carefully, because when I stop, I want you to write down as many of the words as you can remember on the form. It doesn't matter in what order you write them... Please write down as many words as you can remember, in any order.”
AVLT serial clustering
Serial clustering is a measure of the degree to which a respondent recalls words in the same serial order in which they were presented (Delis, Kramer, Kaplan, & Ober, 1987; Fisher et al., 1997; Stricker et al., 2002). It is highest when the order of recalled items matches the serial order presented. Serial clustering on the AVLT was calculated as a chance-adjusted number of observed pairs of adjacent words in the recalled list that were beside each other during the original list presentation. A correction for chance, based on the number of correctly recalled words, was subtracted from the observed number of serially recalled sets of words using an equation described by Stricker and colleagues (2002) and shown below.
Here, X is the total number of observed pairs of adjacent words in the recalled list that were also beside each other in the original list presentation, r is the total number of correct words recalled in the trial excluding intrusions and perseverations, and N is the total number of words presented in an AVLT list (N=15). The amount of serial clustering expected by chance on a single AVLT trial using the equation is a fraction less than one, suggesting that serial clustering is not highly expected by chance. The overall serial clustering score for a participant at one assessment used in the present study was calculated as the mean clustering score across all AVLT immediate recall trials for a visit. For example, given a list of words “horse river parent hat rich,” recalling horse and river together during recall would contribute 1 point for serial clustering and, if those were the only two words recalled on the list, the expected term would be (2–1)/5=0.2.
High levels of serial clustering on an unrelated list of words indicate excellent rote recall (Fisher & DeLuca, 1997; Harnadek & Rourke, 1994), facilitated by reliable associations between words formed using a mnemonic strategy like imagery or association, or a more effortful strategy like the method of loci.
Memory performance composite
We constructed a memory performance composite at each assessment occasion from three measures of memory: sum of trial recall on the AVLT, sum of trial recall on the HVLT (Brandt & Benedict, 2001), and paragraph recall from the Rivermead Behavioral Memory Test (Wilson, Cockburn, & Baddeley, 1985). This composite variable has been used previously to represent memory ability in the ACTIVE study (Ball et al., 2002; Parisi et al., 2011; Willis et al., 2006). We placed the composite on a t-score metric (mean 50, standard deviation 10 in the full sample) at the baseline study visit, and scaled follow-up visits to that reference. Parallel but nonequivalent forms for each memory test were used at each study visit, complicating inferences about within-person change over time. We adjusted composite scores at each follow-up visit to the baseline visit using an equipercentile equating approach (Kolen & Brennan, 1995) tailored to studies of longitudinal change (Gross et al., 2012).
Demographic variables
Demographic variables include participant age (coded continuously in years), years of education, self-rated health status, sex, and ethnicity (white and nonwhite). These demographic variables were selected a priori. Self-rated health status was measured on a scale of 1–5, but dichotomized into 1 (Excellent, Very Good) and 0 (Good, Fair, Poor). Those who rated their health as good were included with the latter group because they were more similar to those who rated their health as Fair or Poor in terms of everyday functional abilities.
Analysis plan
Baseline descriptive statistics were used to characterize the ACTIVE memory-trained and no-contact control sample. Logistic regression models with random effects for participant and time and fixed effects for serial clustering and control variables were used to examine whether serial clustering is associated with higher odds of skipping (Laird & Ware, 1982). We included random effects to minimize bias in parameter estimates and standard errors given the longitudinal repeated measures study design. It is a more appropriate method of modeling than repeated measures ANOVA given the long period of follow-up because the latter assumes equal variances in the outcome over time, and excludes participants who have missing data for any visit (Schuster & von Eye, 2001). Demographic and health predictors were included as covariates to assess whether any participant characteristics were associated with higher odds of skipping. Interactions between training and time were included as covariates to assess longitudinal effects of training on skipping. Because low levels of trial recall restrict the range of values for serial clustering and thus could prevent valid measures of strategy use (see Equation), we conducted a sensitivity analysis by excluding observations in the lowest tenth percentile of AVLT trial sum recall (32 words).
To characterize the association between skipping behavior and memory performance, we used a multiple-group latent growth model to estimate baseline level and change in memory performance over time (McArdle & Bell, 2000; Muthén, 1997; Muthén & Curran, 1997). The model independently estimated growth processes for three groups of participants: memory-trained participants who ever exhibited skipping behavior at any follow-up visit, memory-trained participants who never skipped spaces during follow-up visits, and no-contact control participants. The small number of control group participants who skipped spaces (n=33, 4.7%) were combined with the rest of the control participants. In this model, baseline memory function and the annual pace of change in memory were represented by latent variables constructed from observed scores at each study visit. Factor loadings from baseline to the observed memory variables for each assessment were fixed at 1. Factor loadings from the latent trajectory variable were fixed to values corresponding to linear time steps between ACTIVE assessments. A second intercept factor was included to accommodate immediate training gains between baseline and post-training for the two groups of memory-trained participants (Bollen & Curran, 2006). Because this parameter captures change between two time points, it has no random variability across persons, and its factor loadings were fixed to 0 at the baseline visit and to 1 at follow-up visits. To facilitate interpretability of group differences, we calculated effect sizes for standardized group differences in means for each parameter of interest using Cohen's d (Cohen, 1988).
Logistic regression analyses were conducted using Stata software, version 12 (StataCorp, 2011). Fit of the logistic regression model was assessed with deviance residuals. Variance inflation factors were used to assess multi-collinearity. Residuals versus predicted plots, variance inflation factors, and other model checking tools were employed. Latent growth curve analyses were conducted using MPLUS (version 6.2) software (Muthen & Muthen, 1998–2010). We used commonly accepted indices of model fit including the root mean square error of approximation (RMSEA; Steiger, 1989) and comparative fit index (CFI; Hu & Bentler, 1999). An RMSEA around or below 0.05 and CFI greater than 0.90 are indicators of excellent model fit (Hu & Bentler, 1999).
Results
Table 1 summarizes baseline demographic and health characteristics of the sample. The sample mean age at baseline was 74 years, and most participants were white, female, and had at least a high school education prior to the baseline study visit (Table 1). The memory composite score, AVLT recall, and serial clustering on the AVLT did not differ by intervention group at the baseline study visit, but the memory-trained group demonstrated significantly better memory performance and higher serial clustering scores at all subsequent study visits through five years (see Gross & Rebok, 2011). The group of memory-trained participants who ever skipped spaces on the AVLT at any study visit tended to be younger, more educated, more likely to be White, and had better memory at baseline than non-skipping memory-trained participants or control group participants (Table 1).
Table 1.
Baseline Characteristics and Test Scores of the ACTIVE Sample (N=1,401)
| Memory-trained group |
||||
|---|---|---|---|---|
| AVLT Skippers* (n=174) | AVLT Non-skippers (n=529) | Control group (n=698) | p-value for differences† | |
| AVLT skipping, n % | 174 (100) | 0 (0) | 33 (4.7) | < 0.001 |
| Age, mean (SD) | 71.8 (5.2) | 74.1 (6.2) | 74.0 (6.0) | < 0.001 |
| Years of Education, mean (SD) | 14.3 (2.7) | 13.4 (2.7) | 13.4 (2.7) | < 0.001 |
| MMSE score, mean (SD) | 27.8 (1.9) | 27.1 (2.1) | 27.3 (2.0) | < 0.001 |
| Health status, n % | 0.05 | |||
| Excellent | 16 (9.2) | 41 (7.9) | 64 (9.3) | |
| Very Good | 78 (45.1) | 169 (32.6) | 232 (33.7) | |
| Good | 65 (37.6) | 218 (42.0) | 287 (41.7) | |
| Fair | 13 (7.5) | 85 (16.4) | 100 (14.5) | |
| Poor | 1 (0.6) | 6 (1.2) | 6 (0.9) | |
| Sex, n % female | 128 (73.6) | 409 (77.3) | 514 (73.6) | 0.30 |
| Ethnicity, n % white | 139 (79.9) | 382 (72.2) | 500 (71.6) | 0.08 |
| Baseline memory composite, mean (SD) | 53.7 (9.3) | 49.3 (10.1) | 49.6 (9.9) | < 0.001 |
| AVLT, mean (SD) | ||||
| Sum of trials 1–5 | 52.2 (9.6) | 47.6 (10.8) | 48.1 (10.8) | < 0.001 |
| Serial clustering score | 0.3 (1.0) | 0.0 (1.0) | 0.0 (1.0) | < 0.01 |
Legend. Demographic characteristics and baseline strategy clustering scores are provided for control and memory-trained participants in ACTIVE.
In this study, skipping spaces on AVLT response forms are used as a surrogate for use of the method of loci; see Methods.
Pairwise comparisons of groups revealed significant difference between the AVLT skipping group and both other groups for all variables except sex and self-rated health status; AVLT non-skippers and control group participants did not differ on any covariates (P>0.1).
SD: standard deviation
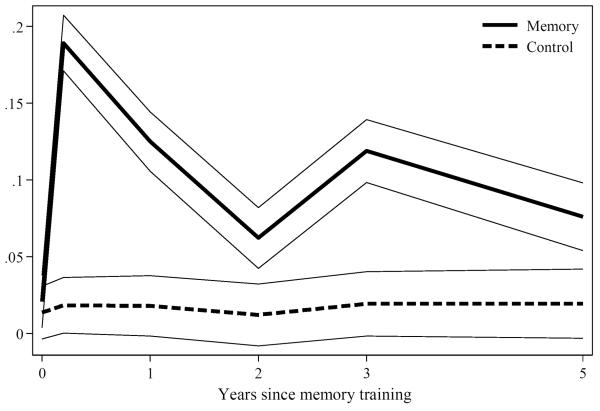
Figure 2 graphically shows proportions of participants who skipped spaces on AVLT forms by visit through the fifth year study visit. The ordinate shows the proportion of participants at each study visit who skipped a space on the AVLT during that visit. Fewer than 2% of all participants skipped spaces at the baseline visit, and no more than 2% of control participants skipped spaces at any follow-up visit. By contrast, 19% of memory-trained participants at immediate post-training showed skipping behaviors, and between 7% and 13% skipped spaces during subsequent study visits. Across any study visit, 25% of memory-trained skipped spaces and 5% of control participants missed spaces on an AVLT response form. The notable increase in skipping odds for the memory-trained group in year 3 is attributable to booster training that was offered to a random sample of memory-trained participants.
Figure 2.
Proportion of Participants Who Skipped Spaces on AVLT for Each Assessment by Training Status: Results from ACTIVE (N=1,401)
Legend. Time trend plots of the proportion of participants who skipped spaces on the AVLT in the memory-trained (solid line) and control (dashed line) groups. The percentage of participants who skipped a space on an AVLT trial is shown on the ordinate. 95% confidence bands are shown for each group.
Table 2 provides results of a logistic regression with random effects for participant predicting the odds of skipping spaces on the AVLT. Estimates are odds ratios comparing the odds of skipping spaces for each unit difference in the level of the covariate, controlling for other variables in the model. Consistent with inferences from Figure 2, memory-trained participants were 16 times more likely to skip spaces on AVLT forms than control participants immediately after training, and between three and seven times more likely during later study waves up to five years after training. After adjusting for covariates, these group differences were significant at all visits through the third annual study wave. AVLT serial clustering was greater among participants who skipped spaces, after controlling for covariates (Table 2). When observations in the lowest decile of AVLT recall were excluded in a sensitivity analysis, these associations strengthened and the odds of skipping was significantly higher in the memory trained group during all study visits except the fifth year visit, at which the association was marginally significant (P=0.06).
Table 2.
Associations Between Odds of Skipping Spaces on AVLT Forms and Demographic Characteristics: Results from ACTIVE (N=1,401)
| Odds ratio | 95% CI | |
|---|---|---|
| Effect Sizes of Training on skipping spaces on the AVLT | ||
| Baseline to Immediate post-training | 16.0 | (4.6, 55.2) |
| Baseline to Year 1 | 7.1 | (2.0, 25.7) |
| Baseline to Year 2 | 4.1 | (1.0, 16.6) |
| Baseline to Year 3 | 5.8 | (1.6, 21.0) |
| Baseline to Year 5 | 3.3 | (0.8, 12.9) |
| Predictors of skipping spaces on the AVLT | ||
| AVLT serial clustering | 2.0 | (1.7, 2.3) |
| Age | 0.6 | (0.4, 0.9) |
| Sex (Female) | 0.5 | (0.3, 0.8) |
| Ethnicity (White) | 1.7 | (0.9, 3.0) |
| Self-rated Health Status | 1.2 | (0.8, 1.9) |
| Education | 1.1 | (1.0, 1.2) |
Legend. Results from random effects logistic regression models of AVLT skipping regressed on time, training status, interactions between time and training status, AVLT serial clustering, age, sex, ethnicity, self-rated health status, and education. Interactions between treatment and time are interpreted as standardized effect sizes for the difference in odds of AVLT skipping between memory-trained and control groups.
95% CI: 95% Confidence interval.
For descriptive purposes, among memory-trained participants we identified high strategy users as those with the highest 25% of serial clustering scores at immediate post-test. The proportion of high strategy users who skipped spaces at immediate post-training was 43% (sensitivity of skipping behavior). The proportion of lower strategy users who did not skip spaces at immediate post-training was 89% (specificity of skipping behavior).
Results of a multiple-group latent growth model of memory performance are summarized in Table 3. Model fit statistics suggested an excellent fit of this model to the data. At baseline, estimated memory performance among memory-trained participants who ever skipped a space (53.7 points) was significantly greater than either memory-trained participants who never skipped a space on the AVLT (49.3 points; Cohen's d effect size: 2.4, P<0.001) or control group participants (49.8 points; d=2.1, P<0.001). For both of the memory-trained groups, the immediate pre-post training change in memory performance was significantly greater than that in the control group. Additionally, the average immediate training boost for participants who skipped spaces on the AVLT was greater than among memory-trained participants who never skipped spaces (d=0.84, P=0.007). All groups showed some age-related decline in memory performance, but the annual pace of memory decline did not differ between groups over the five year study period (Table 3).
Table 3.
Effects of skipping behavior on memory performance: Results from ACTIVE (N=1,401)
| Memory-trained participants who ever skipped spaces* on AVLT (n=174) |
Memory-trained never-skippers (n=529) |
Memory-trained ever-skippers vs never-skippers |
Control group (n=698) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | Cohen's d effect size (vs control) | P value (vs control) | Estimate (SE) | Cohen's d effect size (vs control) | P value (vs control) | Cohen's d effect size | P value | Estimate (SE) | |
| Means | |||||||||
| Baseline | 53.7 (0.7) | 2.1 | <0.001 | 49.3 (0.4) | 0.3 | 0.322 | 2.4 | <0.001 | 49.8 (0.4) |
| Immediate pre-post change | 3.6 (0.5) | 2.0 | <0.001 | 2.0 (0.3) | 1.1 | <0.001 | 0.8 | 0.007 | Fixed at 0 |
| Post-training pace of change | −1.0 (0.1) | 0.4 | 0.415 | −1.0 (0.1) | 0.5 | 0.142 | 0.1 | 0.869 | −0.9 (0.1) |
| Variances | |||||||||
| Initial level | 82.4 (3.3) | -- | 82.4 (3.3) | -- | -- | 82.4 (3.3) | |||
| Post-training pace of change | 0.4 (0.1) | -- | 0.4 (0.1) | -- | -- | 0.4 (0.1) | |||
| Model fit statistics | |||||||||
| RMSEA | 0.048 | ||||||||
| CFI | 0.987 | ||||||||
Legend. Results of a latent growth model of memory performance stratified by AVLT skipping behavior and training status. The memory composite outcome was standardized to the baseline measurement and standardized to a T scale (mean 50, standard deviation 10).
In this study, skipping spaces on AVLT response forms are used as a surrogate for use of the method of loci; see Methods.
SE: standard error
Discussion
We evaluated the hypothesis that changes in strategies used following memory training result in long-term, observable qualitative differences in memory performance (Light, 1991). Skipping patterns on the AVLT are consistent with the hypothesis that up to 25% of memory-trained older adults used the method of loci after training because participants who skipped spaces had higher serial clustering scores, whereas few control participants demonstrated such behavior. Participants who skipped spaces showed a greater gain in memory after training than memory-trained participants who did not skip spaces and control group participants. ACTIVE memory training taught several strategies, but only the method of loci emphasized both serial recall and skipping items to return to later. The ability to measure skipping on the AVLT was only possible because ACTIVE used a modified administration of the word list learning task, with participants writing down their recalled words. Replication of our findings would require another study that used a similar modification to the standard AVLT administration and that tracks serial order of recall. The present study is significant because it provides evidence using a non-self-report method that the method of loci can be used by a considerable proportion of older adults. Further, the strategy is associated with greater immediate benefits from memory training that are maintained over the five-year study period.
Skipping behavior is an indicator of the use of the method of loci, a highly effective but difficult memory strategy. The method of loci is one of the oldest, most celebrated mnemonic devices in modern history (Worthen & Hunt, 2011; Yates, 1966). Underscoring the potential clinical benefit of the method of loci, a recent randomized training study found that instruction in this strategy helped people with depression remember more on average than members of a control group that practiced categorization and rehearsal (Dalgleish et al., 2013). Although it is conceivable that memory-trained participants in our study used some strategy other than the method of loci, such as association or categorization, and skipped spaces as a consequence of using that strategy, those who skipped spaces on AVLT response sheets used considerably more serial clustering strategies even after adjusting for demographic characteristics (Table 2). This finding indicates use of the method of loci because serial recall is a hallmark of the strategy (Nyberg et al., 2003). Although use of other mnemonic techniques also entails serial recall, notably the pegword method in which imagery is used to link information to a known set of words or numbers (pegs; Worthen & Hunt, 2011), no other such strategies were taught during ACTIVE training. Other well-known psychological factors, notably recency and primacy effects, are likely responsible for some serial clustering. However, these effects are presumed to operate to similar extents in both memory-trained and control groups and thus cannot explain the large differences in serial recall by training status at follow-up visits (Gross & Rebok, 2011) or differences in serial recall by skipping status.
Despite its rich history, the method of loci is an attentionally demanding, effortful task. This study's findings suggest that the method of loci is used by as many as 18% of memory-trained participants immediately after training and by 25% of memory-trained participants across all visits. It is curious why a strategy requiring so much effort is so common. A variety of factors contribute to intra-individual variability in decisions to use strategies, including personal preferences, metamemory, and the strategy's novelty (Bjorklund & Coyle, 1995; Hertzog & Dunlosky, 2004; Touron & Hertzog, 2004).
A strategy's novelty might make it more likely to be chosen despite others in the repertoire until it has been practiced and gathers strength through experience. The method of loci is a novel technique to someone unfamiliar with it, and so this strategy might still be practiced despite stronger strategies in one's repertoire. Novelty of a strategy was part of Siegler's (1988) strategy choice model. This model explains how children choose among strategies in their repertoire, but is applicable to older age groups as well. Older adults select strategies specific to tasks and goals that lead to optimal combinations of speed and accuracy (Duverne & Lemaire, 2005; Saczynski et al., 2004). Because people approach tasks in multiple ways and by using multiple strategies (Siegler, 1987; Saczynski et al., 2007), a strategy in one's cognitive repertoire must compete with other strategies to be used. The probability of using a strategy includes perceived benefits in speed and accuracy that have been achieved in prior applications of the strategy, and so is a function of an individual's experience. Importantly, the novelty component in Siegler's (1988) strategy choice model supplements a new strategy temporarily until it has been practiced enough to gather strength of its own. A strategy's novelty deteriorates with experience using the strategy. Thus, the desire to use novel strategies explains why individuals might use an effortful strategy such as the method of loci.
Although participants who skipped spaces demonstrated greater training-related gains in memory performance, the rate of age-related cognitive decline following the post-test training visit did not differ by intervention group or by skipping status among memory-trained participants. The lack of training-related differences in long-term rate of memory decline has been reported previously (Gross & Rebok, 2011; Parisi et al., 2011). This finding suggests that initial gains in memory performance attributable to training are not lost at a faster rate later (Jones et al., 2012; Salthouse, 2006). The lack of differences in the rate of memory decline between ever skippers and never skippers in the memory-trained group further suggests that those who use a strategy as complex as the method of loci are not at risk of losing their boost in memory performance faster than those who used other strategies, which were possibly less complex.
Use of strategies is pervasive in any human activity (Worthen & Hunt, 2011). An important qualification of this study is that participants who showed no evidence of using the method of loci probably were not necessarily using an inappropriate or ineffective strategy during ACTIVE study visits. Although some memory strategies are better than others, their selection and use is heavily influenced by personal preferences and abilities. Procedural metamemory refers to implicit knowledge of a strategy's appropriateness as well as memory monitoring during task execution (Brown et al., 1983; Paris & Lindauer, 1982). Further, if the method of loci is a new strategy for someone and is being tried for its novelty, a utilization deficiency may prevent realization of improved memory performance. A utilization deficiency is defined as when a person is able to spontaneously and effectively use a strategy but realizes no performance gain (Bjorklund et al., 1997; Miller, 1990).
An important study limitation is that skipping spaces on AVLT response forms is likely neither a sensitive nor a specific indicator of method of loci. It is not our intention to propose a valid method of measuring the prevalence of this strategy, but to point out that many participants do use it when shown it. Participants using the method of loci who have perfect recall on a trial, who compensate with intrusion errors, or who skip lines initially but then fill in the space later during a trial, might not leave any skipped spaces. Additionally, not all participants may have taken their training instructions to skip spaces literally, so may not have actually skipped spaces on test forms. It is even likely that some participants inadvertently skipped spaces. However, if the prevalence of such a behavior is systematically higher among trained participants than control participants, and if no other strategy taught in ACTIVE can explain elevated levels of serial clustering strategies among such skippers, we believe the assumption that skipping spaces indicates the method of loci is the only reasonable explanation. An alternative explanation for the increased level of skipping behavior in the memory-trained group is that memory training relaxed a preconceived inclination to not skip spaces. However, given the degree to which the standardized audio-taped directions in ACTIVE were clear in stating that the order of recall is not important, it is unlikely that individuals are naturally reticent to skip around during recall. A final caveat of the present study is, as is evident from Table 1, subsetting the memory-trained group as we have done loses benefits of randomization at baseline between control and memory-trained participants. Thus, causal inferences about the effect of the method of loci on trajectory of memory performance are limited.
Strategy use behaviors are not commonly reported in training programs with older adults (Schmitt, Murphy, & Sanders, 1981). Among more than 400 studies of memory training published in the last 40 years, we are aware of only 13 that reported data about strategic knowledge or behaviors before and after memory training (Gross & Rebok, 2011). The present study showed older adults' strategy use is modifiable through intervention, as seen from patterns of missed spaces on AVLT response sheets. Skipping patterns on the AVLT are consistent with the hypothesis that the method of loci is used by older adults, and that it is a worthwhile strategy to learn that leads to quantitative gains in memory performance.
Acknowledgements
The ACTIVE intervention trials are supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), the University of Alabama at Birmingham (U01 AG14289), and the University of Florida (U01AG14276). Dr. Gross was supported by a National Institutes of Health Translational Research in Aging fellowship (T32AG023480-07) and the Johns Hopkins Sommer Fellowship. Dr. Rebok is an investigator with Compact Disc Incorporated for the development of an electronic version of the ACTIVE memory intervention. He has received no financial support from them for ACTIVE. Dr. Brandt receives royalty income from Psychological Assessment Resources, Inc., on sales of the Hopkins Verbal Learning Test-Revised. Drs. Rebok's and Brandt's relationships are managed by the Johns Hopkins University according to its established conflict of interest policies.
References
- Allen PA, Ashcraft MH, Weber TA. On mental multiplication and age. Psychology and Aging. 1992;7:536–545. doi: 10.1037//0882-7974.7.4.536. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Lemaire P, Allen P, Michel BF. Strategic aspects of young, healthy older adults' and Alzheimer patients' arithmetic performance. Cortex. 2008;44:119–130. doi: 10.1016/j.cortex.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Kliegl R. Further testing of limits of cognitive plasticity: Negative age differences in a mnemonic skill are robust. Developmental Psychology. 1992;28:121–125. [Google Scholar]
- Bjorklund DF, Coyle TR. Utilization deficiencies in the development of memory strategies. In: Weinert FE, Scheider W, editors. Memory performance and competencies: Issues in growth and development. Lawrence Erlbaum Associates; Mahwah, NJ: 1995. pp. 161–180. [Google Scholar]
- Bjorklund DF, Douglas RN. The development of memory strategies. In: Cowan N, editor. The development of memory in childhood. Psychology Press; Hove East Sussex: 1997. pp. 201–246. [Google Scholar]
- Bollen KA, Curran PJ. Latent curve models: A structural equation approach. Wiley; Hoboken, NJ: 2006. [Google Scholar]
- Bower GH. Analysis of a mnemonic device. American Scientist. 1970;58:496–510. [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test–Revised: Professional manual. Psychological Assessment Resources; Odessa, FL: 2001. [Google Scholar]
- Brown AL, Bransford JD, Ferrara RA, Campione JC. Learning, remembering, and understanding. In: Mussen PH, editor. Handbook of child psychology. 4th ed. Vol. 3. Wiley; New York: 1983. pp. 77–166. [Google Scholar]
- Bruner JS, Brunswik E, Festinger L, Heider F, Muenzinger KF, Osgood GE, Rapaport D. Contemporary approaches to cognition. Harvard University Press; Cambridge: 1957. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. second ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dalgleish T, Navrady L, Bird E, Hill E, Dunn BD, Golden A. Method-of-Loci as a mnemonic device to facilitate access to self-affirming personal memories for individuals With depression. Clinical Psychological Science. 2013 epub ahead of print. DOI 10.1177/2167702612468111. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Dunlosky J, Hertzog C. Aging and deficits in associative memory: What is the role of strategy production? Psychology and Aging. 1998;13:597–607. doi: 10.1037//0882-7974.13.4.597. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, Hertzog C, Powell–Moman A. The contribution of five mediator–based deficiencies to age–related differences in associative learning. Developmental Psychology. 2005;41:389–400. doi: 10.1037/0012-1649.41.2.389. [DOI] [PubMed] [Google Scholar]
- Duverne S, Lemaire P. Arithmetic split effects reflect strategy selection: an adult age comparative study in addition comparison and verification tasks. Canadian Journal of Experimental Psychology. 2005;59:262–278. doi: 10.1037/h0087479. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, Walhovd KB. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52(4):1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Fisher NJ, DeLuca JW. Verbal learning strategies of adolescents and adults with the syndrome of Nonverbal Learning Disabilities. Child Neuropsychology. 1997;3:192–198. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini–mental state”: A practical guide for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geary DC, Wiley JG. Cognitive addition: Strategy choices and speed–of–processing differences in young and elderly adults. Psychology and Aging. 1991;6:474–483. doi: 10.1037//0882-7974.6.3.474. [DOI] [PubMed] [Google Scholar]
- Geary DC, Frensch PA, Wiley JG. Simple and complex mental subtraction: strategy choice and speed–of–processing differences in younger and older adults. Psychology and Aging. 1993;8:242–256. doi: 10.1037//0882-7974.8.2.242. [DOI] [PubMed] [Google Scholar]
- Gross AL, Inouye SK, Rebok GW, Brandt J, Crane PK, Parisi JM, Tommet D, Bandeen-Roche K, Carlson MC, Jones RN. Parallel but not equivalent: Challenges and solutions for repeated assessment of cognition over time. Journal of Clinical and Experimental Neuropsychology. 2012;34(7):758–772. doi: 10.1080/13803395.2012.681628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Rebok GW. Memory training and strategy use in older adults: Results from the ACTIVE cognitive intervention trial. Psychology and Aging. 2011;26:503–517. doi: 10.1037/a0022687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnadek MCS, Rourke BP. Principal identifying features of the syndrome of nonverbal learning disabilities in children. Journal of Learning Disabilities. 1994;27:144–154. doi: 10.1177/002221949402700303. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dunlosky J. Aging, metacognition, and cognitive control. In: Ross BH, editor. The psychology of learning and motivation: Advances in research and theory. Academic Press; San Diego, CA: 2004. pp. 215–251. [Google Scholar]
- Hill RD, Allen C, McWhorter P. Stories as a mnemonic aid for older learners. Psychology and Aging. 1991;6:484–486. doi: 10.1037//0882-7974.6.3.484. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Marsiske M, Ball K, Rebok G, Willis SL, Morris JN, Tennstedt SL. The ACTIVE cognitive training interventions and trajectories of performance among older adults. Journal of Aging Health. 2012 doi: 10.1177/0898264312461938. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausler DH. Learning and memory in normal aging. Academic Press; San Diego, CA: 1994. [Google Scholar]
- Kliegl R, Smith J, Baltes PB. Testing the limits and the study of adult age differences in cognitive plasticity of a mnemonic skill. Developmental Psychology. 1989;25:247–256. [Google Scholar]
- Kliegl R, Smith J, Baltes PB. On the locus and process of magnification of age differences during a mnemonic training. Developmental Psychology. 1990;26:894–904. [Google Scholar]
- Kolen M, Brennan R. Test equating: Methods and practices. Springer; New York: 1995. [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Iijima T, Fujii T. Changes in brain activation associated with use of a memory strategy: a functional MRI study. Neuroimage. 2005;24:1154–1163. doi: 10.1016/j.neuroimage.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data: an overview of recent results. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lemaire P, Siegler R. Four aspects of strategic change: Contributions to children's learning of multiplication. Journal of Experimental Psychology: General. 1995;124:83–97. doi: 10.1037//0096-3445.124.1.83. [DOI] [PubMed] [Google Scholar]
- Light L. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Miller PH. The development of strategies of selective attention. In: Bjorklund DF, editor. Children's strategies: Contemporary views of cognitive development. Erlbaum; Hillsdale. NJ: 1990. pp. 157–184. [Google Scholar]
- McArdle JJ, Bell RQ. Recent trends in modeling longitudinal data by latent growth curve methods. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multiple–group data: Practical issues, applied approaches, and scientific examples. Lawrence Erlbaum; Mahwah, NJ: 2000. pp. 69–108. [Google Scholar]
- Muthén BO. Latent variable modeling with longitudinal and multilevel data. In: Raftery A, editor. Sociological methodology. Blackwell Publishers; Boston: 1997. pp. 453–480. [Google Scholar]
- Muthén BO, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods. 1997;2:371–402. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide: Sixth Edition. Muthén & Muthén; Los Angeles, CA: 1998–2010. [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, Bäckman L. Neural correlates of training–related memory improvement in adulthood and aging. Proceedings of the National Academy of Sciences. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris SG, Lindauer BK. The development of cognitive skills during childhood. In: Wolman BW, editor. Handbook of developmental psychology. Prentice Hall; Englewood Cliffs, N.J.: 1982. pp. 333–349. [Google Scholar]
- Parisi JM, Gross AL, Langbaum JB, Saczynski JS, Cleary L, Cook S, Rebok GW. Modeling change in memory performance and perceptions: Findings from the ACTIVE study. Psychology and Aging. 2011;26:518–524. doi: 10.1037/a0022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Balcerak LJ. Memory self–efficacy and performance differences in young and old adults: Effect of mnemonic training. Developmental Psychology. 1989;25:714–721. [Google Scholar]
- Rebok GW, Carlson MC, Langbaum JBS. Training and maintaining memory abilities in healthy older adults: Traditional and novel approaches. Journal of Gerontology: Psychological Sciences. 2007;62B:53–61. doi: 10.1093/geronb/62.special_issue_1.53. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Presses Universitaires de France; Paris, France: 1964. [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philosophical Transactions of the Royal Society B: Biological Sciences. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczynski JS, Rebok GW. Strategies for memory improvement in older adults. Topics in Advanced Practice Nursing eJournal. 2004;4 [Google Scholar]
- Saczynski JS, Rebok GW, Whitfield KE, Plude DL. Spontaneous production and use of mnemonic strategies in older adults. Experimental Aging Research. 2007;33:273–294. doi: 10.1080/03610730701318899. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory and Verbal Learning Test: A Handbook. Western Psychological Services; Los Angeles, CA: 2004. [Google Scholar]
- Schmitt FA, Murphy MD, Sanders RE. Training older adult free recall rehearsal strategies. Journal of Gerontology. 1981;36:329–37. doi: 10.1093/geronj/36.3.329. [DOI] [PubMed] [Google Scholar]
- Schuster C, Von Eye A. The relationship of ANOVA models with random effects and repeated measurement designs. Journal of Adolescent Research. 2001;16:205–220. [Google Scholar]
- Siegler RS. The perils of averaging data over strategies: an example from children's addition. Journal of Experimental Psychology: General. 1987;116:250–264. [Google Scholar]
- Siegler RS. Individual differences in strategy choices: Good students, not–so–good students, and perfectionists. Child Development. 1988;59:833–51. [PubMed] [Google Scholar]
- Siegler RS, Lemaire P. Older and younger adults' strategy choices in multiplication: Testing predictions of ASCM using the choice/no–choice method. Journal of Experimental Psychology: General. 1997;126:71–92. doi: 10.1037//0096-3445.126.1.71. [DOI] [PubMed] [Google Scholar]
- Singer T, Lindenberger U, Baltes PB. Plasticity of memory for new learning in very old age: A story of major loss? Psychology and Aging. 2003;18:306–317. doi: 10.1037/0882-7974.18.2.306. [DOI] [PubMed] [Google Scholar]
- St. Clair–Thompson HL. The effects of cognitive demand upon relationships between working memory and cognitive skills. The Quarterly Journal of Experimental Psychology. 2007;60:1378–1388. doi: 10.1080/17470210601025505. [DOI] [PubMed] [Google Scholar]
- StataCorp . Stata Statistical Software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- Steiger JH. EZPATH: A supplementary module for SYSTAT and SYGRAPH. Systat; Evanston, IL: 1989. [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker JL, Brown GB, Wixted J, Delis DC. New semantic and serial clustering indices for the California Verbal Learning Test–Second Edition: Background, rationale, and formulae. Journal of the International Neuropsychological Society. 2002;8:425–435. doi: 10.1017/s1355617702813224. [DOI] [PubMed] [Google Scholar]
- Touron DR, Hertzog C. Distinguishing age differences in knowledge, strategy use, and confidence during strategic skill acquisition. Psychology and Aging. 2004;19:452–466. doi: 10.1037/0882-7974.19.3.452. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Jones M, Wen W, Rae C, Graham S, Shnier R, Sachdev P. Memory training alters hippocampal neurochemistry in healthy elderly. NeuroReport. 2003;14:1333–1337. doi: 10.1097/01.wnr.0000077548.91466.05. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Geraerts N, Marcoen A. Memory complaints, coping, and well-being in old age: A systemic approach. The Gerontologist. 2000;40:540–548. doi: 10.1093/geront/40.5.540. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A. On the mechanisms of plasticity in young and older adults after instruction in the method of loci: Evidence for an amplification model. Psychology and Aging. 1996;11:164–178. doi: 10.1037//0882-7974.11.1.164. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long–term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. Thames Valley Test Company; Bury St. Edmunds, England: 1985. [Google Scholar]
- Worthen JB, Hunt RR. Mnemonology: Mnemonics for the 21st century. Psychology Press; Hove East Sussex: 2011. [Google Scholar]
- Yates FA. The art of memory. University of Chicago Press; Chicago: 1966. [Google Scholar]
- Yesavage JA, Rose TL. The effects of a face-name mnemonic in young, middle-aged, and elderly adults. Experimental Aging Research. 1984;10:55–57. doi: 10.1080/03610738408258543. [DOI] [PubMed] [Google Scholar]