Abstract
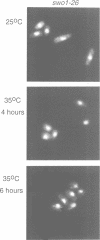
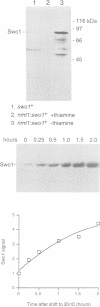
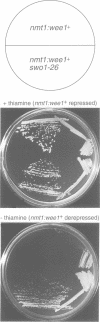
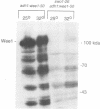
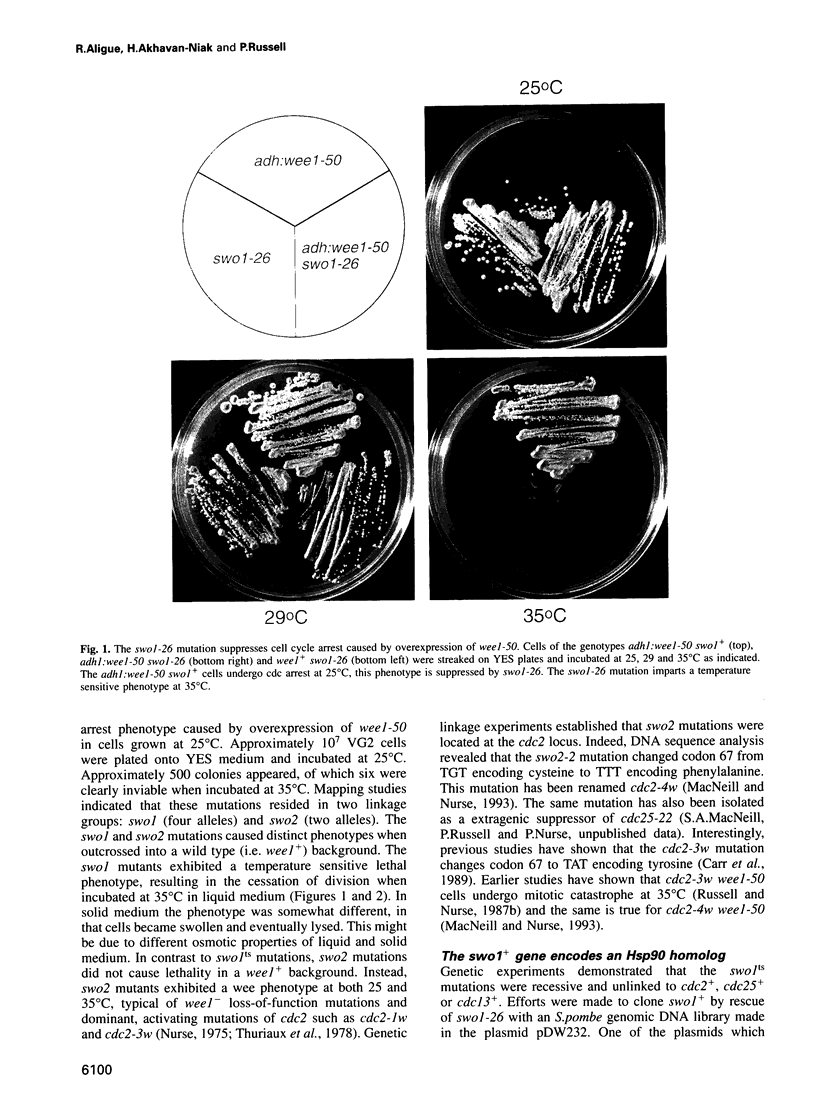
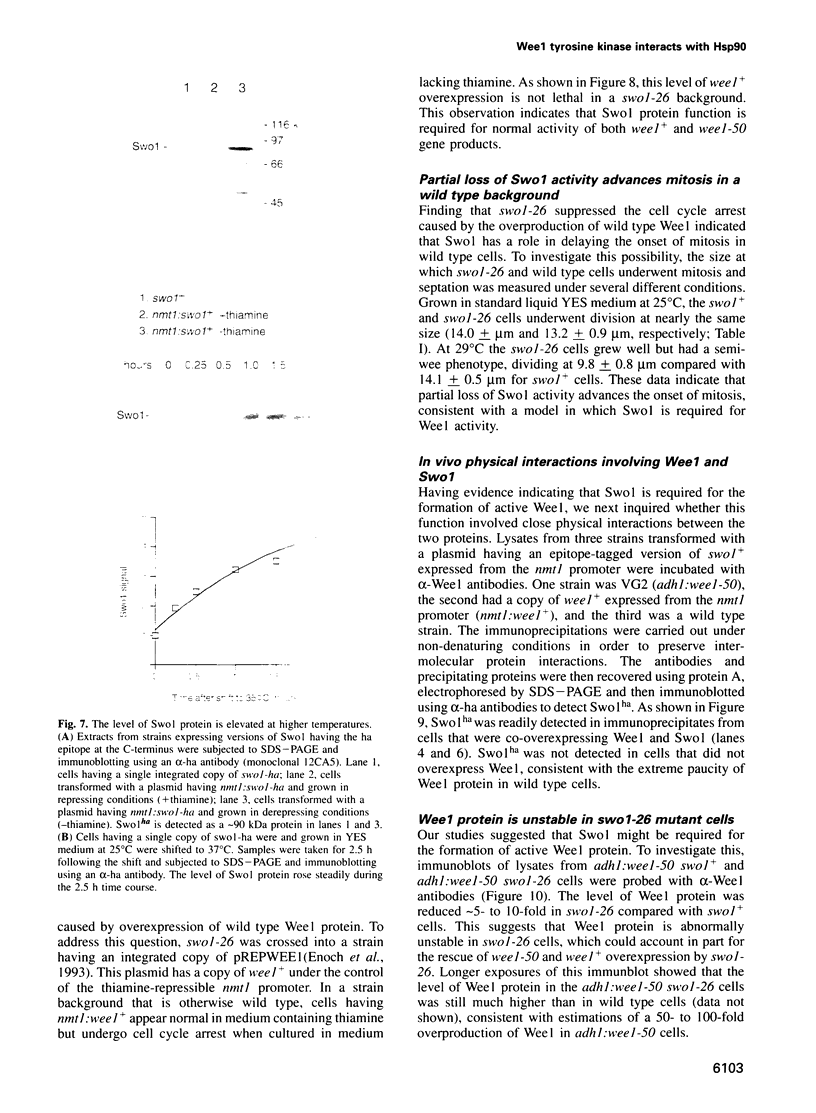
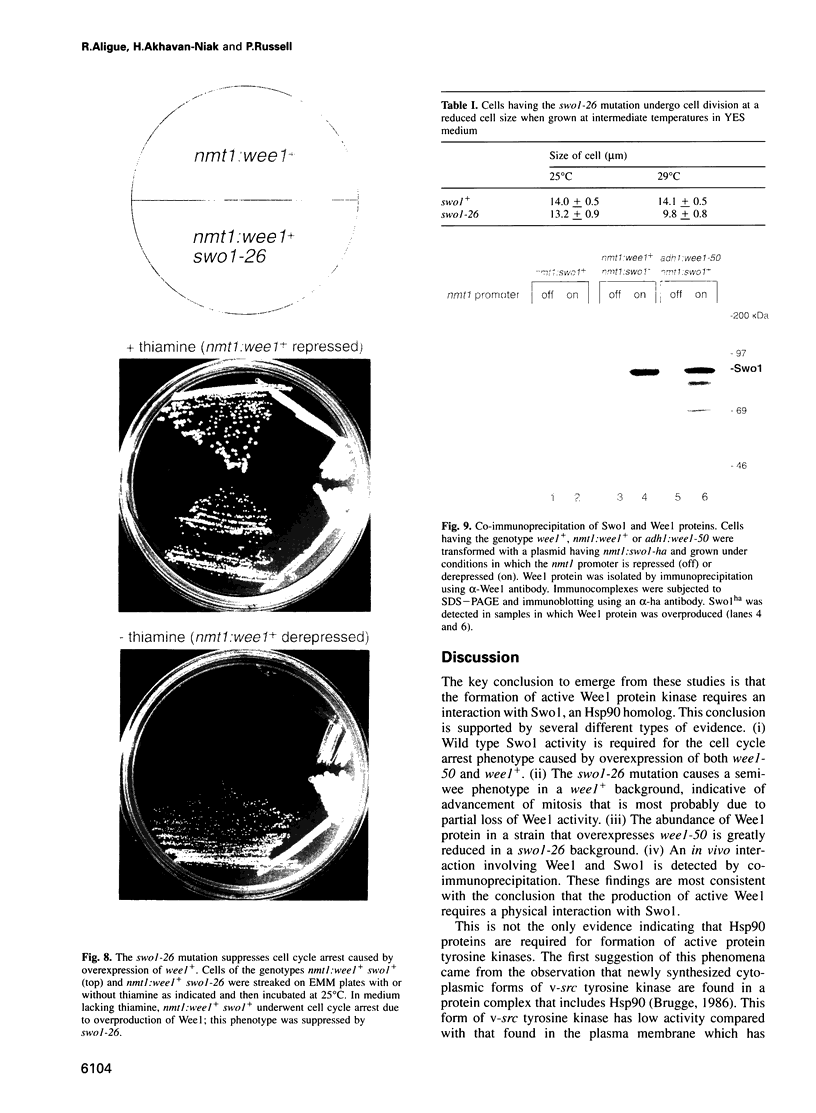
Wee1 protein kinase regulates the length of G2 phase by carrying out the inhibitory tyrosyl phosphorylation of Cdc2-cyclin B kinase. Mutations were isolated that suppressed the G2 cell cycle arrest caused by overproduction of Wee1. One class of swo (suppressor of wee1 overproduction) mutation, exemplified by swo1-26, also caused a temperature sensitive lethal phenotype in a wee1+ background. The swo1+ gene encodes a member of the Hsp90 family of stress proteins. Swo1 is essential for viability at all temperatures. Swo1 coimmunoprecipitates with Wee1, showing that the two proteins interact. The swo1-26 mutant undergoes premature mitosis when grown at a semi-permissive temperature. These data strongly indicate that formation of active Wee1 tyrosine kinase requires interaction with Swo1, perhaps in a manner analogous to the previously demonstrated interaction between Hsp90 and v-src tyrosine kinase. These observations demonstrate a unexpected role for Hsp90 in cell cycle control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfa C. E., Booher R., Beach D., Hyams J. S. Fission yeast cyclin: subcellular localisation and cell cycle regulation. J Cell Sci Suppl. 1989;12:9–19. doi: 10.1242/jcs.1989.supplement_12.2. [DOI] [PubMed] [Google Scholar]
- Alfa C. E., Ducommun B., Beach D., Hyams J. S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990 Oct 18;347(6294):680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
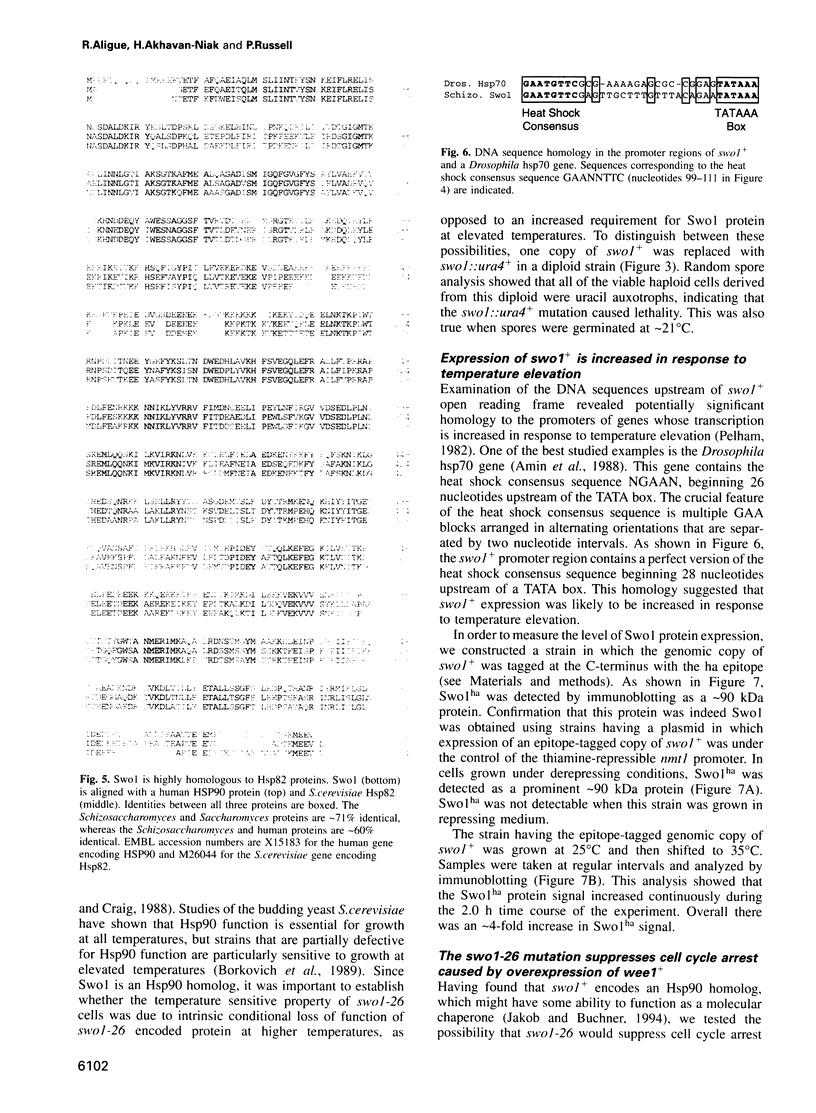
- Amin J., Ananthan J., Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988 Sep;8(9):3761–3769. doi: 10.1128/mcb.8.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Carr A. M., MacNeill S. A., Hayles J., Nurse P. Molecular cloning and sequence analysis of mutant alleles of the fission yeast cdc2 protein kinase gene: implications for cdc2+ protein structure and function. Mol Gen Genet. 1989 Jul;218(1):41–49. doi: 10.1007/BF00330563. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Tang Z., Dunphy W. G. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell. 1993 Mar 26;72(6):919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- Cutforth T., Rubin G. M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994 Jul 1;77(7):1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G. The decision to enter mitosis. Trends Cell Biol. 1994 Jun;4(6):202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Enoch T., Carr A. M., Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 1992 Nov;6(11):2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Fikes J. D., Becker D. M., Winston F., Guarente L. Striking conservation of TFIID in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Nature. 1990 Jul 19;346(6281):291–294. doi: 10.1038/346291a0. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Jakob U., Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994 May;19(5):205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Ogg S., Xu M., Parker L. L., Donoghue D. J., Maller J. L., Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992 Jan;3(1):73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- MacNeill S. A., Nurse P. Mutational analysis of the fission yeast p34cdc2 protein kinase gene. Mol Gen Genet. 1993 Jan;236(2-3):415–426. doi: 10.1007/BF00277142. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993 Jan 15;123(1):127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McGowan C. H., Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993 Jan;12(1):75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991 Dec;10(13):4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell-cycle control: turning on mitosis. Curr Biol. 1993 May 1;3(5):291–293. doi: 10.1016/0960-9822(93)90182-n. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975 Aug 14;256(5518):547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L. L., Walter S. A., Young P. G., Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature. 1993 Jun 24;363(6431):736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987 May 22;49(4):569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Shaknovich R., Shue G., Kohtz D. S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84). Mol Cell Biol. 1992 Nov;12(11):5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Coleman T. R., Dunphy W. G. Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J. 1993 Sep;12(9):3427–3436. doi: 10.1002/j.1460-2075.1993.tb06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuriaux P., Nurse P., Carter B. Mutants altered in the control co-ordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1978 May 3;161(2):215–220. doi: 10.1007/BF00274190. [DOI] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Wu L., Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993 Jun 24;363(6431):738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Akaogi K., Miwa T., Imai T., Soeda E., Yokoyama K. Nucleotide sequence of a full-length cDNA for 90 kDa heat-shock protein from human peripheral blood lymphocytes. Nucleic Acids Res. 1989 Sep 12;17(17):7108–7108. doi: 10.1093/nar/17.17.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]