Abstract
Hepatocellular accumulation of free fatty acids (FFAs) in the form of triglycerides constitutes the metabolic basis for the development of nonalcoholic fatty liver disease (NAFLD). Recent data demonstrate that excess FFA hepatocyte storage is likely to lead to lipotoxicity and hepatocyte apoptosis. Hence, FFA-mediated hepatocyte injury is a key contributor to the pathogenesis of nonalcoholic steatohepatitis (NASH). Nonalcoholic steatohepatitis, obesity, type 2 diabetes, essential hypertension, and other common medical problems together comprise metabolic syndrome. Evidence suggests that peptide hormones from the L cells of the distal small intestine, which comprise the core of the enteroendocrine system (EES), play two key roles, serving either as incretins, or as mediators of appetite and satiety in the central nervous system. Recent data related to glucagon-like peptide-1 (GLP-1) and other known L-cell hormones have accumulated due to the increasing frequency of bariatric surgery, which increase delivery of bile salts to the hindgut. Bile acids are a key stimulus for the TGR5 receptor of the L cells. Enhanced bile-salt flow and subsequent EES stimulation may be central to elimination of hepatic steatosis following bariatric surgery. Although GLP-1 is a clinically relevant pharmacological analogue that drives pancreatic β-cell insulin output, GLP-1 analogues also have independent benefits via their effects on hepatocellular FFA metabolism. The authors also discuss recent data regarding the role of the major peptides released by the EES, which promote satiety and modulate energy homeostasis and utilization, as well as those that control fat absorption and intestinal permeability. Taken together, elucidating novel functions for EES-related peptides and pharmacologic development of peptide analogues offer potential far-ranging treatment for obesity-related human disease.
Keywords: nonalcoholic fatty liver disease, enteroendocrine cells, glucagon-like peptide 1 (GLP-1), lipotoxicity, neuropeptide YY (NPY)
Hepatic Lipid Metabolism, NAFLD, and Gut Peptides in the Era of Metabolic Syndrome
Obesity is a growing and costly pandemic in industrialized countries. In the United States alone, a recent study published by the Robert Wood Johnson Foundation (Princeton, NJ) found that 40% of Americans are obese or extremely obese, with half of all Americans expected to be obese (defined as having a body mass index [BMI] ≥ 30) by 2030. Moreover, the medical expenditures associated with obesity-related diseases in 2008 were estimated to be $147 billion.1 Obesity is also associated with > 111,000 excess deaths in the United States as compared with persons of normal weight2–4 as determined by analysis of the NHANES III survey data.4 The most closely associated feature of obesity in the liver is nonalcoholic fatty liver disease (NAFLD). Nonalcoholic fatty liver disease is a spectrum of liver diseases defined on both clinical and histopathologic grounds. Hepatic lesions may include simple steatosis, or NAFL; steatohepatitis, or nonalcoholic steatohepatitis (NASH); nonalcoholic steatofibrosis (NASF). Nonalcoholic fatty liver disease also carries a potential risk for the development of cirrhosis, end-stage liver disease, as well as a synergistic risk for hepatocellular carcinoma (HCC). Along with obesity, NAFLD is now recognized as a common feature of the metabolic syndrome (MetS), for which diagnostic algorithms typically include at least three of the following: essential hypertension, elevated triglycerides (TGs), low high-density lipoproteins (HDLs), type 2 diabetes mellitus (T2DM) or glucose intolerance, polycystic ovarian disease (PCOD), waist circumference greater than 40 inches (102 cm) in men or more than 35 inches (88 cm) in women. We have included here several major features of metabolic syndrome, but refer the reader to a review of the diagnostic criteria established by several published studies.5–7
A common feature of MetS-related disease is both hepatic and systemic insulin resistance. Mammalian insulin resistance occurs in three cell types: adipocytes, skeletal myocytes, and hepatocytes. In this review, we will focus on hepatocyte insulin resistance, more specifically as it relates to triglyceride and FFA metabolism. The reader is referred, however, to several recent reports for a comprehensive review of systemic insulin resistance.8,9 Upon binding of insulin to the hepatic insulin receptor and subsequent insulin receptor substrate activation, a well-defined pathway ultimately resulting in activation of Akt (or protein kinase B—PKB) occurs that permits storage of glycogen. For the purpose of this discussion when hepatocyte insulin resistance develops and glycogen synthesis is exhausted, de novo lipogenesis (DNL) of free fatty acids (FFAs) becomes the primary pathway to store excess glucose, or energy, in the liver. Key enzymes associated with DNL include, fatty acid synthase (FAS) and acylCoA carboxylase (ACC). Most FFAs become associated with TG assembly, and as TGs, are considered to be relatively nontoxic. However, FFAs per se and their metabolites are more likely to result in hepatocyte injury, in part by exacerbating inflammation, invoking the unfolded protein response (UPR), and disrupting orderly electron transport in the mitochondria. Taken together these interrelated FFA-induced organelle and cell membrane perturbations conceptually define lipotoxicity. Lipotoxicity will be briefly reviewed in the context of recent work reported on the role of glucagon-like peptide-1 (GLP-1) and its analogues.
It is important to recognize the accumulation of both FFAs and TGs in the setting of hepatocyte insulin resistance is exacerbated as a result of regulatory changes of enzymes related to the normal handling of FFA storage and disposal by hepatocytes. For example, carnitine palmityl transferase I (CPT-1), the rate-limiting enzyme for delivery of FFA to mitochondria for β-oxidation, is downregulated.10 Apolipoprotein biosynthesis and the secretion of very low-density lipoproteins (VLDLs) are also impaired. Hence, in the setting of hepatocyte insulin resistance, FFA metabolism is dynamically altered by increased production (DNL), impaired secretion, and decreased β (β), delta (δ), and omega (ω) oxidation. The role of FFA oxidation as a means of a safe and effective elimination of hepatocyte FA/TG stores is controversial, however. Nonetheless, perturbations in normal FFA hepatocyte metabolism will result in excess storage of hepatocyte FFAs, ceramides, and other lipid intermediates, and increase the likelihood of hepatocyte lipotoxicity while perpetrating hepatocyte insulin resistance, which only serves to further lipid accumulation.
Hepatocyte Lipotoxicity
Hepatocyte lipotoxicity, the result of FFA accumulation and respective intermediates leads not only to cellular dysfunction, but ultimately to apoptotic cell death. For an in-depth discussion the reader is referred to a recent review by Trauner and colleagues11; however, injury and inflammation that result as a consequence of lipotoxicity and hepatocyte apoptosis are the most likely culprits in driving the progression of simple steatosis to NASH, NASF, and cirrhosis. Investigational efforts to dissect the molecular mechanisms accounting for hepatocyte lipotoxicity are ultimately critical for intervention to prevent progression of NAFLD and chronic liver disease. Recent investigational efforts have focused on FFA saturation in NAFLD, which can exacerbate progression of disease. In vitro studies indicate that the degree of FFA saturation, e.g., palmitic acid (PA) is more likely to provoke hepatocyte injury and death, when compared with monounsaturated fatty acids, for example, oleic acid (OA).12–14 The accumulation of saturated FFAs, and hepatocyte insulin resistance—seminal risk factors for NAFLD progression—has intensified the investigation of these biochemical features with respect to endoplasmic reticular stress (ER stress)—or the UPR—as the major player in lipotoxicity and lipoapoptosis associated with NAFLD progression. Endoplasmic reticular stress is reviewed in greater detail elsewhere in this issue.15 Briefly, ER stress suppresses translation of mRNA by inhibiting eukaryotic initiation factor 2 α (eIF2-α).16–18 The consequence of such actions in the ER is accumulation of misfolded proteins and cell cycle arrest; however, if the capacity to handle the stress is exceeded, induction of transcription factors including c-β homologous protein (CHOP) sets off a series of molecular events that inhibit the Bcl-2 family of cell survival proteins, which leads to impaired mitochondrial function and ultimately apoptosis.11,12 Another mechanism associated with NASH-related injury is autophagy, a lysosomal-mediated mechanism that degrades specific cell organelle components. The degradation of cellular fat plays a significant role in preventing hepatocyte-mediated lipid injury. This process, also called lipophagy, ultimately results in the degradation of cellular fats; however, the homeostatic role of lipophagy can be significantly altered when excess hepatocyte FFAs are present. A recently detailed review by Czaja and colleagues is referenced for the reader.19
Endoplasmic reticular stress and lipophagy are just two factors that can modulate liver injury when FFAs—and not triglycerides—change the metabolic dynamics in hepatocytes. In this review, we will summarize the growing importance of gut peptide hormones with respect to obesity, NAFLD, and T2DM. We have become interested in these peptides following extensive investigation of GLP-1 and its relationship to hepatocyte FFA metabolism. Moreover, we will summarize how gut peptide hormones have the potential to have clinical relevance based on recent basic and clinical observations. Biological observation of GLP proteins and glucose-dependent insulinotropic peptide (GIP) has resulted from the fundamental metabolic studies by Drucker and colleagues.20–23 We will briefly review work we have conducted in vitro and in vivo specifically regarding mechanisms that account for how GLP-1 analogs protect hepatocytes from excess FFA stores.24 Clinically, the increased utility of bariatric surgery for obesity and other MetS indications has greatly facilitated a renewed interest in the beneficial role of gut peptides, the intestinal cells responsible for their production, as well as the relationship between the enterohepatic circulation of bile salts and the key receptor on these cells, the TGR5 receptor. Following a brief review of the EES, we will update the reader on potential roles for gut hormones with a focus on lipid and FFA metabolism in the liver associated with hepatocyte insulin resistance.
The Enteroendocrine System
The enteroendocrine system (EES) is considered the largest endocrine system in mammals.25,26 Enteroendocrine cells (EEC) of the gut are specialized epithelial cells that undergo rapid mitosis, but represent only a small fraction of gut epithelial cells. These cells secrete a vast array of peptide hormones that include somatostatin, gastrin, cholecystokinin (CCK), GIP, glucagon-like peptides (GLP), oxyntomodulin (OXM), neuropeptide Y (NPY), peptide YY (PYY), and pancreatic peptide (PP).25,26 Found primarily in the mucosa, EEC arise from pluripotent intestinal stem cells, and are primarily characterized by morphology. They are preferentially labeled α, D, F, G, I, K, L, P, or X cells, and enterochromaffin cells (►Table 1).26–30 The core function of the EEC is to serve as the nutritional sensor of the gut, secreting hormones that are widely known to affect intestinal motility, insulin secretion, appetite, and metabolism.
Table 1.
Cells of the enteroendocrine system
| Cell type | Hormone/peptide | Cell location |
|---|---|---|
| α | Glucagon | Pancreas |
| D | Somatostatin | Pancreas |
| F or PP cells | Pancreatic polypeptide | Pancreas, small intestine, and large intestine |
| G | Gastrin | Stomach |
| I | Cholecystokinin | Small intestine |
| K | GIP | Small intestine |
| L | GLP-1, GLP-2, oxyntomodulin, PYY | Small intestine, large intestine, and colon |
| P or X | Ghrelin | Stomach and small intestine |
| Enterochromaffin cells | Serotonin | Pancreas, stomach, small and large intestine |
Morphologically, EEC are either “open cells,” exposed to luminal contents, with apical cytoplasmic processes and microvilli extending into the luminal space; or “closed cells,” which are not exposed to luminal contents, and therefore signal indirectly through neural circuits or humoral mechanisms.26,31,32 Open EEC contain chemoreceptors that respond directly to nutrients and other luminal contents. Their products are stored in secretory vesicles located near capillaries and nerve endings, and are released by exocytosis at the basolateral membrane, thereby having the capacity to affect both local and distal targets.26,32,33
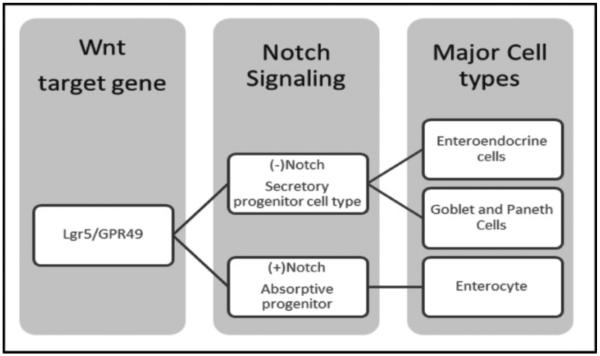
Current lineage data suggest that EEC differentiation is controlled by both Notch and Wnt signaling (►Fig. 1).28,34 Notch signaling mediates lateral inhibition of adjacent cells, thereby preventing adjacent cells from adopting the same phenotype.25,35 Recent data from Barker et al demonstrated that a Wnt target gene Lgr5/GPR49 is present and required in all EEC types (►Fig. 1).35,36 The vast array of cell types (►Table 1) makes the EEC extremely versatile in its ability to handle various nutritional signals presented in the gastrointestinal (GI) lumen. Initially described by the peptide they secreted, recent studies have demonstrated that these different cells types are capable of secreting a variety of peptides, with considerable overlap.35 Indeed, mouse studies by Habib et al demonstrated that L cells from the upper portion of the small intestine more closely resembled K cells from the same region of the small intestine than colonic L cells.27 One current hypothesis suggests that L and K cells within the small intestine comprise a single cellular subtype in which changes are mediated by location and exposure to dietary nutrients. The concept of “geographic” and nutrient specialization typifies the dynamic capacity of phenotypic function of the EEC cells, mediated primarily by intraluminal contents. This plasticity may also account for how Roux-en-Y gastric bypass results in the resolution of T2DM when such a bariatric surgical procedure is performed for the treatment of obesity and MetS.
Fig. 1.
Key signaling elements in the differentiation of enteroendocrine cells. Enteroendocrine cells are derived from gut epithelial stem cell lineage in which Wnt signaling, via the LGR5 receptor, activates Notch negative progenitor cells. These ultimately become secretory cells at maturation. By contrast, Notch positive progenitor cells follow a differentiation pathway that maintains absorptive capacity in the gastrointestinal lumen.
Bariatric Surgery: The Role of Gut Peptides in the Cure for Diabetes
The concept that EEC hormones play a pivotal role in FFA metabolism has gained significant attention due to effects clinicians have observed in patients undergoing bariatric surgery. Various surgical procedures for obesity result in appetite suppression and improved fuel utilization, which has been extensively reported elsewhere.37–40 The most common bariatric procedures performed include laparoscopic adjustable gastric banding, which accounts for 42% of all bariatric procedures worldwide, and the Roux-en-Y gastric bypass, accounting for 47%.41,42 Roughly 10% of bariatric surgical procedures performed include sleeve gastrectomy and biliopancreatic diversion (with or without duodenal switch).42,43 A review of these procedures is summarized by O'Brien41 and Hng et al.43 The Roux-en-Y procedure creates a small gastric pouch by removing a small portion of the stomach, which is then connected to the proximal jejunum forming the Roux limb, which is anastomosed to the duodenal limb forming a Y configuration.41,43 This procedure more than all others has yielded significant information and biological insights into gut peptide regulation related to fatty acid metabolism. Postoperatively patients who undergo the Roux-en-Y procedure have a marked reduction in hepatic lipid content and improved hepatic insulin sensitivity well before significant weight loss occurs.44–46 These benefits to the liver are directly related to at least two EEC-synthesized gut peptides GLP-1, and peptide YY (PYY).
Glucagon-Like Peptide
Of the gut peptides discussed here, the most widely studied to date has been GLP-1. Glucagon-like peptide-1 is secreted by the L cells of the distal small intestine and proximal colon. It was initially discovered in the saliva of the Gila monster, Heloderma suspectum.47 In fact, part of the reason GLP-1 was discovered and characterized was based on the cardinal feature of human bites by the Heloderma monsters that result in hypoglycemia. Over the span of mammalian evolution, however, GLP-1 peptide secretion by the L cells was thought to have limited biological relevance because it is immediately cleaved by dipeptidyl peptidase IV (DPPIV), which prevents the peptide from stimulating pancreatic insulin secretion following feeding48–51—the incretin function for which GLP-1 was originally described. Today, pharmacological development of GLP-1 analogues renders such analogues impervious to DDPIV enzyme cleavage. Conversely, pharmacological use of DPPIV inhibitors results in the prolonged half-life of naturally occurring GLP-1, an alternative in restoring its incretin activity. Taken together, these agents have been an important advancement in the treatment of T2DM because their use may delay the administration of exogenous insulin to patients with insulin resistance. Until recently, the primary functions of GLP-1 and related synthetic protein analogues were limited to the incretin function. Also GLP-1 analogues were thought to improve systemic and hepatic insulin sensitivity (or decrease insulin resistance) indirectly via insulin release from the endocrine pancreas. However, recent published data have challenged these paradigms
The L cells responsible for secreting GLP-1 possess TGR5 receptors, which are proving to be of intense clinical interest following bariatric surgery. This is because, for example, creation of Roux limbs associated with bariatric surgery is highly beneficial in eliminating hepatic fat stores shortly after the procedure, resulting in a significant increase in the intraluminal bile acid (bile salt) concentrations. TGR5 is a G-protein-coupled receptor (GPCR) that is also known as a membrane-type receptor for bile acids (M-BAR) or G-protein-coupled bile acid receptor 1 (GBAR1). Therefore, it is well-established the TGR5 receptor avidly bind bile salts, with lithocholic acid being its most potent ligand (EC50 35 nM).52 A consequence of increased bile acids following bariatric surgery is a sharp increase in the concentration of serum GLP-1, far more than is typical following ingestion of a meal. Hence, excess GLP-1 secretion increases β–cell output of insulin, but excess GLP-1 itself may have direct beneficial effects on liver and adipose tissue independent of insulin. Admittedly, although GLP-1 receptors are known to be present in brain, kidney, and the enteric nervous system—with known physiologic function—their presence on other metabolic tissues, including the liver, has been disputed.
Glucagon-like peptide-1 receptors on pancreatic β cells have been cloned and are also identified as G-protein-coupled receptors (GPCR type II).22,53,54 Glucagon-like peptide-1 receptors function by activating G proteins, allowing for recruitment of signaling (and active) GS proteins that in turn activate adenylate cyclase, generating cAMP. Over the span of 10 years following the initial discovery of these receptors, many investigators searched for additional receptor isoforms for GLP-1 in various organs including the CNS, kidney, and GI tract. As a consequence, the GLP-1 receptor has been identified with functional GPCR capacity and physiological function in the satiety center of the brain, kidney, the enteric nervous system of the GI tract, and bile duct cells. Glucagon-like peptide-1 is also considered to be an anorexigenic hormone.54–56 Furthermore, physiological studies in rodents demonstrate that GLP-1 suppresses appetite in the satiety center, delays gastric emptying, and increases insulin secretion.22,56–59 In short, GLP-1 has key pleotropic functions in addition to aiding in serum glucose utilization indirectly via insulin action. That is, these effects of GLP-1 on fundamental metabolic functions are independent of insulin, have been demonstrated in humans,60 and are not solely dependent on improving insulin resistance by GLP-1-enhanced insulin output.61 These physiological control mechanisms, however, are also known to be regulated following identi-fication of the identical GPCR in the pancreatic β cells.54,61,62 For these reasons, the GLP-1 analogues and DPPIV inhibitors are such attractive agents in the treatment of T2DM because many patients are not only insulin resistant, but are obese. The clinical benefits for GLP-1 therapies are significant: appetite suppression, improved systemic insulin resistance, and reduced gastric emptying with increased satiety. Hence, these systemic physiological benefits have been thought to be responsible for improved hepatic lipid metabolism—that the benefit of GLP-1 in reducing FFA and TG liver stores was an incidental and not a direct consequence of GLP-1 pharmacological action.
There are recent studies, however, which do not dispute the indirect beneficial actions of GLP-1 proteins, but rather provide additional evidence that there are independent features of GLP-1 proteins that directly reduce hepatic FFA and triglyceride stores, and thereby directly improve hepatocyte insulin resistance. These studies imply a direct role for GLP-1 proteins in hepatic lipid metabolism in addition to—and not because of—suppression of appetite, delayed gastric emptying, and improved insulin sensitivity.
Before reviewing these putative independent GLP-1 functions, several laboratories, including our own, have reported identifying a GLP-1 receptor on hepatocytes in vitro.61,63–65 Recent data indicate that the GLP-1 receptor may also exist on adipocytes.66 We and others have demonstrated that the GLP-1 receptor, initially cloned in the 1990s in the endocrine pancreas, is present on the hepatocyte.53,54,61,62,67 Drucker and colleagues still report their inability to identify the GLP-1R on hepatocytes, but were able to identify receptor in whole mouse liver protein lysates. This group also confirmed data that we and others have reported, showing that the GLP-1 analogue exendin-4 results in hepatocyte enzyme expression that favors FFA depletion through increased β oxidation.68
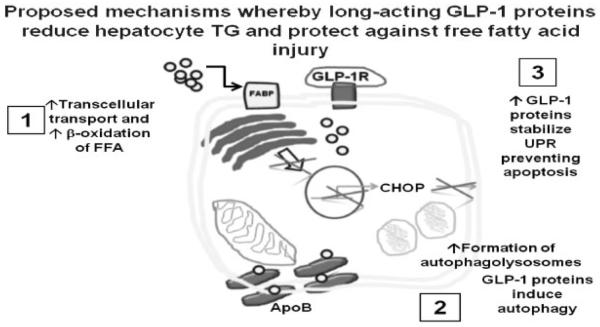
Our laboratory has used a variety of means to demonstrate that the presence of GLP-1R on hepatocytes functions as a GPCR, and invokes several protective mechanisms that are metabolically active with respect to insulin sensitivity (►Fig. 2).61,63,67 In vitro, even in the absence of insulin, fat-loaded primary human hepatocytes and established human cell lines, e.g., Huh7, all demonstrate that the direct effect of GLP-1 reduces FFA stores and directly affects FFA and triglyceride metabolism.61,63,69 Importantly, in addition to proving enhanced elimination of FFA and triglycerides in vitro in human hepatocytes, we have also demonstrated that GLP-1 analogues reduce hepatocyte lipotoxicity and enhance lipophagy (►Fig. 2). Although experts in the field all now agree that GLP-1 peptides enhance FFA elimination from hepatocytes, exactly how this occurs remains controversial. Because the use of PPAR agonists and other agents have not proven to be sufficient to treat NAFLD over a sustained period, the use of GLP-1 analogues in clinical medicine for the indication of treating some patients with aggressive NASH has become quite attractive.
Fig. 2.
Multiple potential roles for glucagon-like peptide 1 (GLP-1) and GLP-1 analogues in modulating hepatocyte fatty acid and lipid metabolism. Although there is controversy as to the specificity of a G-protein-coupled receptor (GPCR) for GLP-1 in hepatocytes (see text), it is now well-established that GLP-1 proteins enhance β oxidation of free fatty acids (FFAs) in steatotic hepatocytes. We have also demonstrated that GLP-1 analogues exendin-4 and liraglutide can stabilize the unfolded protein response (UPR) and mitigate the death pathway via apoptosis when endoplasmic reticulum stress overload occurs: iGLP-1 can protect hepatocytes from lipotoxicity. We have also demonstrated that liraglutide can increase macroautophagy (or lipophagy) to eliminate FFA stores by lysosomal degradation as assessed by autophagic flux.
Although beyond the scope of this review, our co-investigators have shown that the GLP-1 analogue exendin-4 (Exenatide®) improves hepatocyte survival in rodent liver grafts with higher percentages of hepatocyte steatosis than control substances (unpublished communication, N. K. Gupta, MD, Emory University). These observations are potentially significant for liver transplantation because many donor livers have higher percentages of steatotic hepatocytes, limiting viable donor organ availability—a major problem facing transplant surgeons in the United States.70,71 Additional published data also indicate that GLP-1 proteins can interfere with both physiological and cellular mechanisms that inhibit FFA storage as TG in the liver. However, dietary restriction alone in the mouse model cohorts in the study failed to result in identical hepatic endpoints even though the mice lost significant body weight.72 In short, studies with GLP-1 peptide analogues in vitro and in rodent models, as well as recent data from the bariatric surgery literature, suggest possible benefits for use in reducing hepatic steatosis.
Gastric Inhibitory Peptide: Structure and Action
Gastric inhibitory peptide (GIP) is a 42 amino acid polypeptide secreted by K cells found in the proximal small intestine and duodenum. There is significant amino acid sequence homology (> 90%) between human, porcine, bovine, mouse, and rat GIP.73,74 The active form is a 42 amino acid circulating peptide GIP-(1–42); and similar to GLP-1, GIP is rapidly degraded by DPPIV to an inactive form, GIP-(3–42), with a half-life of ~ 5 to 7 minutes.23,32,75 The primary role of GIP, like GLP-1, is that of an incretin, stimulating insulin secretion upon food ingestion. Hence, GIP is also known as glucose-dependent insulinotropic peptide. Gastric inhibitory peptide has also been shown to simulate pancreatic β-cell proliferation, and increase β-cell survival.23,73,74,76 Both glucose and dietary fat are potent stimulators of GIP secretion.32,77,78 Rodent studies, using high fat diets, demonstrate significant increases in GIP mRNA and circulating plasma GIP73,79; however, there still remains considerable debate about the role of GIP in obesity and T2DM. Gastric inhibitory peptide levels in many patients are elevated in both T2DM and obesity, but the incretin effect of GIP is blunted.80 The GIP receptor (GIP-R) belongs to the GPCR glucagon receptor superfamily.23,81 The seven-peptide-member transmembrane receptor is expressed in the pancreas, stomach, small intestine, adipose tissue, adrenal cortex, pituitary, heart, testis, endothelial cells, bone, brain, and central nervous system.73,74,76 The GIP receptor has not been identified in the liver.
GIP and Lipid Metabolism: Controversy about GIP Therapeutics
In addition to its role in stimulating glucose uptake, GIP may play a key role in lipid homeostasis. Gastric inhibitory peptide has been shown in rodents to increase plasma triglyceride clearance following meals, increase fatty acid uptake, synthesis, re-esterification, and fat storage by adipocytes.23,73,76,79 Indeed, disruption of GIP signaling in mice reduced fat accumulation in adipocytes, and GIP receptor knockout mice showed improved insulin sensitivity.23,73,76,79,82 However, several recent studies raise more questions concerning the benefits of pursuing GIP therapeutics. Studies by Nie et al using 3T3-L1 CAR adipocytes, and Timper et al using human primary adipocyte cultures, both revealed that GIP treatment induced production of inflammatory cytokines and chemokines, and GIP-induced inflammation activated the Jun N-terminal kinase (JNK) pathway leading to disruption of insulin signaling in vitro.83,84 Recent studies by Asmar et al in humans, found evidence of enhanced FFA re-esterification in adipose tissue, but found no evidence of lipid clearance with GIP administration.85,86 Further studies revealed that GIP administration only affected triglyceride uptake in adipose tissue when combined with hyperinsulinemia and hyperglycemia—and that these effects were only demonstrated in lean subjects—indicating that GIP may be one of the pro-obesogenic stimuli mediating obesity-induced insulin resistance.83,85–87 Finally, a recent clinical study indicates that GIP in nondiabetic NASH patients may exacerbate fatty acid accumulation and biosynthesis, and that GIP antagonism would be clinically beneficial.88
GLP-2 Biology and Structure
Glucagon-like peptide-2 (GLP-2) is a 33-amino acid peptide produced as a cleavage product of proglucagon and is released in conjunction with GLP-1 from L cells following ingestion of a meal.56,89 GLP-2 is regulated in a nutrient-dependent manner, with carbohydrate and lipid serving as potent stimulators for its secretion.22,89 Glucagon-like peptide-2 is also degraded by DPPIV; however, the half- life of GLP-2 is longer than GLP-1 (~ 7–8 min in humans). The cleavage product GLP-2 (3–33) serves as a competitive antagonist to its cognate receptor, glucagon-like receptor-2 (GLP-2R).22,59,90 Glucagon-like peptide-2 release has been demonstrated to stimulate intestinal mucosal growth, increase glucagon secretion, inhibit gastric emptying and gastric acid secretion, and stimulate intestinal blood flow.22,59,89–91 Glucagon-like peptide-2 has recently been approved for the treatment of adult patients with short bowel syndrome (SBS).
GLP-2: The Counter Agent to GLP-1
Because GLP-2 invokes significant changes in intestinal absorption and mucosal growth, the major focus of GLP-2 research has been related to its induction of intestinal crypt cell proliferation; however, the majority of the GLP-2 receptors do not actually reside in intestinal crypt cells. Rather GLP-2 has been identified in enteric neurons, sub-epithelial myofibroblasts, and other EEC. Hence, the effects of GLP-2 via its receptor on the intestine are indirect.
There are limited data regarding the role of GLP-2 and lipid metabolism.92 A clinical study by Meier et al demonstrates that following a test meal, GLP-2 administration in humans leads to an increase in postprandial plasma concentrations of triglycerides and free fatty acids compared with placebo controls.93,94 Hsieh et al evaluated GLP-2-mediated effects on lipid metabolism utilizing the Syrian golden hamster, a model with similarities to humans with respect to lipoprotein assembly and metabolism in which apolipoproteinB (ApoB)-48 is secreted exclusively by the intestine.94–98 They found that GLP-2 stimulates intestinal ApoB-48 lipoprotein secretion via the scavenger receptor CD36 or fatty acid translocase (FAT).94,98 Although GLP-1 and GLP-2 are secreted and released by the same EEC in the fed-state in identical concentrations, evidence suggests that they have opposing effects with respect to postprandial FFA handling. As noted previously, GLP-1 appears to inhibit lipid accumulation and DNL, whereas GLP-2 appears to increase lipoprotein secretion and absorption.63,69,94,99–102
The physiological relationship that exists between GLP-1 and GLP-2 is undoubtedly complex, and further studies are needed to clarify regulation, molecular signaling, interplay, and balance between these two gut hormones, particularly if novel therapeutic combinations are developed by industry. It would be of interest to determine the potential synergistic benefit of increased GLP-1 and GLP-2 in obese humans following bariatric surgery because increased flow of bile acids is known to stimulate the GPCR TGR5 in L cells where both peptides are synthesized and released. One attractive feature of GLP-2 therapy for SBS is restoration of the structural and functional integrity of the intestinal epithelium, which will reduce intestinal permeability. Because one hypothesis accounting for hepatic steatosis and NAFLD progression implicates intestinal dysbiosis that ultimately serves to impair intestinal barrier function with enhanced intestinal permeability—the recently approved pharmacological agent (teduglutide, or Gattex®) may be an attractive addition in clinical treatment trials for NAFLD.
Biology of Oxyntomodulin: A Bridge between Glucagon and GLP-1
Oxyntomodulin (OXM), a cleavage product of proglucagon, is a 37 amino acid peptide also secreted by L cells of the small intestine.103 Oxyntomodulin is comprised of 29 amino acids of glucagon, with an additional eight amino acid carboxy-terminal extension, which results from preproglucagon processing.103,104 Oxyntomodulin is released after a meal, in proportion to nutrient and calorie intake,42,103 slowing gas tric emptying, increasing glucose uptake, inhibiting gastric and pancreatic exocrine secretion, increasing energy expenditure, and ultimately, reducing food intake and body weight in humans as well as in animal models.105,106 There is considerable excitement about the potential benefits of OXM as both an antiobesity agent and a potential T2DM treatment. Oxyntomodulin is a dual agonist, acting on both GLP-1R and glucagon receptors.105 It has reduced affinity for each receptor as compared with their respective cognate proteins; however, OXM is considered to be an equipotent agonist.103,104,107 Consequently, although OXM has reduced affinity, upon binding to either receptor it can activate the signaling cascades with the same strength as the cognate protein. Oxyntomodulin, like both glucagon and GLP-1, has been shown to induce hepatic cAMP accumulation.108–110 However, research by Koole et al demonstrated that OXM, as opposed to GLP-1, differs in that OXM is more likely to invoke ERK1/2 activation than GLP-1, suggesting that OXM may have a different, as yet unknown role in vivo.103,108,111 Very little information is known regarding OXM and lipid metabolism. Even less is known about how or whether OXM affects hepatocyte handling of FFA and TG. Most current research related to OXM has focused on weight loss observed in patients, and to a lesser extent its role in glucose homeostasis.
Biology of Neuropeptide Y: An Orexigenic Neuropeptide
Originally isolated from pig brain, NPY is a member of the pancreatic peptide family, which includes peptide YY (PYY) and pancreatic polypeptide (PP).112,113 Neuropeptide Y is a regulator of several key physiological processes and is rigidly conserved through evolution with respect to sequence homology and structure.114 Neuropeptide Y mediates vasoconstriction and blood pressure, energy homeostasis, ethanol consumption (in rodents), anxiety reduction, and chronic pain.115,116 Neuropeptide Y is secreted by the hypothalamus and adrenal medulla and is released from both sympathetic and parasympathetic nerves. Within the GI tract the enteric neurons are the major source for NPY secretion.112,117 Neuropeptide Y is considered the most powerful central enhancer of appetite. It has been demonstrated to play a critical role in the control of food intake and body weight within the hypothalamus.118 Within hypothalamic feeding circuits, NPY is located primarily in the arcuate nucleus, the orexigenic region of the hypothalamus.112 Neuropeptide Y and agouti-related protein (AgRP) extend into the lateral hypothalamus and upon stimulation increase feeding.116,118 Several feeding studies in rodent models demonstrate that central administration of NPY into different hypothalamic sites result in a robust, dose-dependent feeding response.118 Interestingly, food deprivation is a major stimulus for hypothalamic NPY secretion, and NPY expression and activity are increased in strains of genetically obese mice.116,118–120 Taken together, these data provide a physiologic explanation for why long-term calorie restriction (dieting) may fail in attempting to provoke weight reduction in humans.118
All of the PP (NPY, PYY) interact with a family of GPCRs that belong to the rhodopsin-superfamily (class I). The four functional NPY receptors are Y1, Y2, Y4, and Y5.112,116,121,122 The cloned receptors have all been shown to couple to inhibitory G-proteins (Gi) and thus inhibit cyclic AMP synthesis.112,121,122 Elucidating the exact role of each NPY receptor's role remains incomplete. Seminal studies by Herzog et al demonstrated that the receptor and cell type determine which second messenger system is primarily utilized. In general, the G-protein is dependent on phosphatidylinositol 3-kinase (PI3-K) activity, independent of protein kinase C (PKC) activity, and inhibited by Pertussis toxin (PTX).112,115,123 Unlike NPY, which is ubiquitously expressed, NPY receptor subtypes appear to be restricted to specific tissues. In this manner, NPY is able to elicit a wide range of effects115; more importantly, it would appear that some NPY receptors have opposing effects (as was discovered in at least two receptor subtypes Y1 and Y4).115 This type of expression and regulation appears to serve as a unique mechanism for adapting to an individual's behavioral response to chronic stress.124
NPY in Obesity and NAFLD
Several studies have been conducted in rodent models to determine NPY's role in obesity, but there is limited direct data regarding NPY and NAFLD presently. As previously mentioned, central injection of NPY into the hypothalamus of rodents increases appetite and feeding. Additional studies by van den Hoek, demonstrated acute intracerebroventricular (ICV) infusion of NPY in mice signaled through the sympathetic nervous system125; and subsequent effects of ICV infusion included hyperinsulinemia, hyperglycemia, and dyslipidemia; moreover, VLDL production was also significantly higher.125,126 Neuropeptide Y neuronal activity also appears to be governed by nutrient availability.118,125 Furthermore, NPY-mediated acute metabolic changes also appear to be regulated in the hypothalamus, whereas prolonged exposure to NPY may be under the control of the pituitary gland and adrenal medulla network.
Although a recent study implies that NPY receptors are upregulated in human NAFLD, the sample size was very small, and the focus of the experimental work using human tissue samples was hepatic stellate cells and not hepatocytes.127 Patients with NAFLD had a positive correlation between increased saturated FFA intake and higher NPY levels, even after following a 12-month life-style/diet intervention, indicating that some individuals with NAFLD display hypersensitivity, or alternatively have abnormal stimulation of orexigenic NPY.128
Future studies will need to focus on the interrelationship between saturated FFA consumption and liver on the one hand, and visceral fat on the other because both organs share a common sympathetic pathway129,130; hence, NPY represents an important link between the sympathetic nervous system, liver, and adipose tissue. Furthermore, the role of NPY stimulation in the CNS and the sympathetic nervous system may be an attractive strategy to promote weight reduction.
The Role of Peptide YY: Appetite Suppressor
Peptide YY, a 36 amino acid peptide, is another member of the pancreatic peptide family. It shares a close structural homology to both NPY and PP117,131,132. Like the other gut peptides reviewed, PYY is secreted and synthesized primarily by small intestinal L cells.117,132,133 Peptide YY immunoreactive cells have also been found within the colon and rectum.131,132,134,135 Similar to GLP-1, PYY upon secretion is cleaved by DPPIV, which removes the N-terminal tyrosine and proline residues resulting in production of PYY (3–36), the major circulating form.131,132,136 Peptide YY release is stimulated by a host of luminal nutrients, including short- and medium-chain fatty acids, amino acids, bile acids, and glucose.132,136 Peptide YY is a powerful inhibitor of GI motility, gastric emptying, and absorption of water and electrolytes. Peptide YY has also been shown to inhibit both pancreatic exocrine secretion and pancreatic insulin secretion.117,131,132,136
To date, the bulk of PYY research has focused on appetite and feeding suppression albeit with potential therapeutic use for weight management and morbid obesity. Peptide YY (3–36), a selective NPY Y2 agonist, has been shown in both humans and animal models to reduce food intake,42,137,138 by suppressing appetite through prohibiting NPY release in the arcuate nucleus.42,117,136 Clinical use in pharmacological studies with both intranasal and oral preparations of PYY (3–36), however, has resulted in significant side effects that are dose-dependent, and include severe nausea and vomiting.42,137,139 These side effects may represent a significant obstacle to what is otherwise promising preliminary studies of PYY (3–36] in the management of obesity.
PYY Signaling and Effect within the Liver
Despite the vast amount of research detailing the effect of PYY on appetite and satiation, there is very little data on its effects in the liver, particularly with respect to lipid homeostasis. Initial studies, demonstrated that both medium- and long-chain fatty acids stimulated PYY release, and PYY treatment increased both intestinal and liver fatty acid binding protein (LFABP) expression.140–142 New research insights into how PYY modulates fatty acid metabolism is limited, and recent reports present conflicting data. Experiments in mice by Pedersen et al using PYY (3–36) analogues reported a reduction in nonesterified FAA and TG.143 Conversely, experiments by Shi et al using transgenic PYY mice revealed that PYY overexpression resulted in increased fatty acid synthesis capacity, due to a reduction in phosphorylated acetyl-CoA carboxylase, and hepatic lipogenic capacity.144 Consequently, more studies are needed to clarify the role of PYY in NAFLD and T2DM.
Biology of Pancreatic Polypeptide: Gut Sensor of Satiety
Pancreatic polypeptide (PP) is a 36 amino acid hormone, that like NPY and PYY, has a tertiary hairpin fold, amidated C-terminal ends, and an extended polyproline helix and α helix connected by a β turn, giving it a characteristic U-shape.117,145,146 Originally isolated from chicken pancreatic extracts, PP is produced in the islets of Langerhans, and is released by the F cells postprandially via vagal-(cholinergic-) dependent mechanisms.42,117,145–147 Unlike PYY's wide gut distribution, PP is primarily expressed in the pancreas, with smaller populations of EEC in the small and large intestine146,148. Endogenous PP has a short half-life (~ 7 min) due to cleavage by DPPIV.149 Pancreatic polypeptide release in response to a meal is in direct proportion to caloric intake with levels remaining elevated for up to 6 hours.149,150 Studies of PP acute administration reveal reduced food intake in rodents and humans145,151; while chronic administration has been shown to reduce weight gain and decrease energy expenditure.145 Additionally, PP functions to inhibit pancreatic exocrine secretion, gallbladder motility, and gastric emptying.42,149,152 Pancreatic polypeptide is a high-affinity Y4 agonist153 and experiments by Lin et al in Y4 receptor knockout mice demonstrated that PP inhibits food intake primarily through the anorexigenic α-melanocyte stimulating hormone signaling pathway within the arcuate nucleus of the hypothalamus.154 There is very little data on PP in relation to lipid metabolism and homeostasis. However, circulating PP levels have been found in obese patients to be reduced, while the postprandial PP response in patients with anorexia nervosa is hyperresponsive.149,155–157 The plasticity of Y receptors provides a plausible mechanism whereby PP could have direct action related to intermediary and lipid metabolism.
Summary and Conclusions
Gut hormones are products of the EEC system, a collection of cells arising from GI tissue for which the L cell is the best studied and a major source for synthesis and secretion. Recent research demonstrates nearly all of these peptides have pleotropic effects on key organs and can modulate satiety, suppress appetite, and alter GI motility. Furthermore, important data are emerging with respect to gut peptide modulation of FFA and TG metabolism in the liver. Finally, there are relevant relationships between gut-peptide control of appetite, obesity, and MetS—of which NAFLD is a central clinical feature. As we have described in this review, and outlined in ►Tables 1 and 2, and in ►Fig. 3, there are several ways to classify gut peptide hormones: the EEC type, for example, α which synthesizes glucagon; or anatomically in the GI tract, such as the pancreas. For our purposes, physiological function of the gut peptides, both known and novel, is probably the most important aspect for liver biologists and clinicians to become familiar with this class of hormones. We anticipate that nearly all of these peptides could be synthesized as pharmacological analogues and will become more important clinically in the near future. As a final note, we did not discuss glucagon, somatostatin, gastrin, cholecystokinin, and serotonin primarily because little is known at present with respect to their respective effects on hepatic lipid metabolism.
Table 2.
Summary of key enteroendocrine hormones with potential effect on hepatic lipid metabolism
| Enteroendocrine hormone | Target organ(s) | Known biological function | Novel lipid biological function | Clinical/pharmacological use |
|---|---|---|---|---|
| GLP-1 | Pancreas, stomach, liver, small intestine | Incretin | Increases elimination of hepatocyte fatty acid stores through increased FFA flux, enhanced oxidation and autophagy | Synthesized as longer acting incretins for type 2 diabetes mellitus including Exenatide®, Byattea®, Victoza®; also DPPIV inhibitors serve to prolong t1/2 of naturally occurring GLP-1 peptides in vivo. |
| GLP-2 | Stomach, small intestine, liver | Intestinal mucosal growth, reduces intestinal permeability and improves barrier function | Stimulates apoB-48 secretion and absorption | Treatment of adult patients with short bowel syndrome |
| Oxyntomodulin | Stomach, pancreas, small intestine, liver | Increase glucose uptake, slows gastric emptying | Dual agonist for both glucagon and GLP-1 receptors | None at present |
| GIP | Pancreas | Incretin | Increases lipid absorption and re-esterification | None at present |
| NPY | Brain, liver | Appetite stimulator | Increases VLDL production | None at present |
| PYY | Stomach, small and large intestine, liver | Appetite suppressor | Reduces nonesterified FFA and increases respiratory exchange ratio | None at present |
| Pancreatic polypeptide | Brain, pancreas, gallbladder | Satiety | Unknown at this time | None at present |
Abbreviations: FFA, free fatty acids; GIP, glucagon-like peptide; NPY, neuropeptide YY; PYY, peptide YY; VLDL, very low-density lipoprotein.
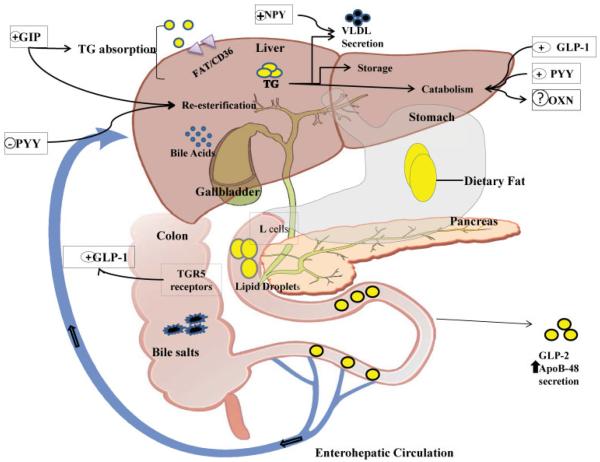
Fig. 3.
Bariatric surgical procedures modify L-cell peptide release and net bile salt absorption from the enterohepatic circulation: Implication for weight loss and increased L-cell function. Surgical procedures for weight reduction (but not restrictive procedures such as adjustable gastric banding or vertical banded gastroplasty) reduce the dipeptidyl peptidase IV (DPPIV) degradation of peptides secreted from the L-cells, increasing their ability to affect triglyceride metabolism and usage. These procedures reduce absorption of intestinal free fatty acids (FFAs), but can also lead to increased diarrhea and excess weight loss secondary to dumping syndrome. Conversely, L cells are stimulated by increased exposure to medium and long FFA, as well as increased presentation of bile acids to the distal small intestine and proximal colon. Bile acids augment release of glucagon-like peptide-1 (GLP-1), and the costimulated incretins GLP-2 and glucose-dependent insulinotropic peptide (GIP) by L cells because bile salts are known to avidly bind TGR-5—the principle receptor for bile salts on L cells. Increased ligand binding enhances incretin output and in turn increases insulin secretion by pancreatic β cells enhancing systemic insulin sensitivity. Additional changes in peptide YY (PYY) secretion after surgery appear to blunt the significant release of GLP-2, GIP, and neuropeptide YY (NPY).
Here we have reviewed in some detail seven EEC-related gut peptide hormones. Glucagon-like peptide 1 and GIP are historically known as incretins, whereas GLP-2 promotes intestinal mucosal growth and ultimately improves intestinal barrier function. Recently, GLP-1 analogues have been shown to play a key role in reducing hepatocyte FFA stores through a variety of mechanisms—though controversy still remains over whether these effects are direct (receptor mediated in hepatocytes) or indirect (through the actions of decreased absorption or action on adipocytes). Glucose-dependent insulinotropic peptide appears to increase lipid absorption and re-esterification of FFA and, thus, despite being an incretin, does not share the beneficial effects of GLP-1 with respect to fat metabolism. Taken together, however, GLP-1 and GLP-2 may be beneficial in the treatment of NAFLD because of respective complementary mechanisms reviewed. The other four EEC gut peptides discussed—NPY, OXM, PYY, and PP—at present seem to have more potential in the treatment of obesity as opposed to directly impacting hepatic FFA metabolism; however the reader is referred to ►Table 2 and ►Fig. 3 for notable exceptions. Although these data are limited, an attractive component to this group of peptides, nonetheless, is their potential to suppress appetite, increase satiety, and slow GI motility. The role of neurogenic regulatory events in the liver that are controlled centrally will be critical to using gut peptides that control satiety and modulate energy homeostasis since data suggesting that protective effects of gut peptide hormones in the liver are via hepatic nerve endings.95,96,158
In conclusion, the epidemic of obesity and its treatment with more frequent use of bariatric surgery has fueled great interest in EEC-synthesized gut peptides, pharmacological analogues, and their respective contributions to satiety, energy partitioning, as well as novel functions with respect to lipid handling. On the other hand, recent data have raised concerns that long-term administration of GLP-1 analogues may increase the risk of pancreatitis, and certain types of pancreatic tumors in rats. Because GLP-1 is a mitogen for pancreatic tissues, these concerns warrant further study. Undoubtedly, the next several years will be crucial for NASH therapeutic development. Although preliminary data offer tantalizing results, more work needs to be done to understand exactly how EEC-related hormones modulate hepatocyte lipid metabolism and how gut hormone analogues can be exploited to treat MetS, and in particular NAFLD, safely and effectively.
Acknowledgments
This work was supported by Public Health Service Grant NIH DK062092 and Department of Veterans' Affairs Grant I01BX001746 both to FAA. Jaime Eugene Mells is supported by the NIH K12 GM000680 NIGMS Fellowship in Research and Science Teaching (FIRST) Institutional Research and Academic Career Development Award.
References
- 1.O'Grady MJ, Capretta JC. Campaign to End Obesity. Robert Wood Johnson Foundation; Princeton, NJ: 2012. Assessing the Economics of Obesity and Obesity Interventions; pp. 1–40. [Google Scholar]
- 2.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86(5):599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM. Excess deaths associated with obesity: cause and effect. Int J Obes (Lond) 2006;30(8):1171–1172. doi: 10.1038/sj.ijo.0803313. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 5.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Lindsey JB, Khera A, et al. Independent associations between metabolic syndrome, diabetes mellitus and atherosclerosis: observations from the Dallas Heart Study. Diab Vasc Dis Res. 2008;5(2):96–101. doi: 10.3132/dvdr.2008.016. [DOI] [PubMed] [Google Scholar]
- 7.Monda KL, North KE, Hunt SC, Rao DC, Province MA, Kraja AT. The genetics of obesity and the metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2010;10(2):86–108. doi: 10.2174/187153010791213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 9.Capeau J. Insulin resistance and steatosis in humans. Diabetes Metab. 2008;34(6 Pt 2):649–657. doi: 10.1016/S1262-3636(08)74600-7. [DOI] [PubMed] [Google Scholar]
- 10.Hiltunen JK, Autio KJ, Schonauer MS, Kursu VA, Dieckmann CL, Kastaniotis AJ. Mitochondrial fatty acid synthesis and respiration. Biochim Biophys Acta. 2010;1797(6–7):1195–1202. doi: 10.1016/j.bbabio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 13.Ricchi M, Odoardi MR, Carulli L, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 14.Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:20. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henkel A, Green RM. The unfolded protein response in fatty liver disease. Sem Liver Dis. 2013;33:321–329. doi: 10.1055/s-0033-1358522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 17.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291(2):E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 18.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 19.Czaja MJ, Ding WX, Donohue TM, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9(8):1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drucker DJ, Lovshin J, Baggio L, et al. New developments in the biology of the glucagon-like peptides GLP-1 and GLP-2. Ann N Y Acad Sci. 2000;921:226–232. doi: 10.1111/j.1749-6632.2000.tb06970.x. [DOI] [PubMed] [Google Scholar]
- 21.Baggio LL, Drucker DJ. Harnessing the therapeutic potential of glucagon-like peptide-1: a critical review. Treat Endocrinol. 2002;1(2):117–125. doi: 10.2165/00024677-200201020-00005. [DOI] [PubMed] [Google Scholar]
- 22.Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145(6):2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 23.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117(1):24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baggio LL, Holland D, Wither J, Drucker DJ. Lymphocytic infiltration and immune activation in metallothionein promoter-exendin-4 (MT-Exendin) transgenic mice. Diabetes. 2006;55(6):1562–1570. doi: 10.2337/db05-1502. [DOI] [PubMed] [Google Scholar]
- 25.Moran GW, Leslie FC, Levison SE, Worthington J, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1(1):51–60. doi: 10.1177/1756283X08093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of `taste' in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib AM, Richards P, Cairns LS, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153(7):3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab. 2011;13(Suppl 1):5–12. doi: 10.1111/j.1463-1326.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92(4):219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahlman H, Nilsson The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12(Suppl 2):S63–S68. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- 31.Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE. 2012;7(8):e42373. doi: 10.1371/journal.pone.0042373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran-Ramos S, Tovar AR, Torres N. Diet: friend or foe of enteroendocrine cells—how it interacts with enteroendocrine cells. Adv Nutr. 2012;3(1):8–20. doi: 10.3945/an.111.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez-Aguilar R, Woods SC. Nutrition and L and K-enteroendocrine cells. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):35–41. doi: 10.1097/MED.0b013e32834190b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 35.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323(1):70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 37.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 38.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621–632. doi: 10.1016/j.surg.2007.07.018. discussion 632–635. [DOI] [PubMed] [Google Scholar]
- 39.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 40.Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309(21):2250–2261. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358–1365. doi: 10.1111/j.1440-1746.2010.06391.x. [DOI] [PubMed] [Google Scholar]
- 42.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6(8):444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 43.Hng KN, Ang YS. Overview of bariatric surgery for the physician. Clin Med. 2012;12(5):435–440. doi: 10.7861/clinmedicine.12-5-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96(7):2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 45.Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101(2):368–373. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 46.Tiikkainen M, Bergholm R, Vehkavaara S, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52(3):701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 47.Göke R, Fehmann HC, Linn T, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268(26):19650–19655. [PubMed] [Google Scholar]
- 48.Pohl M, Wank SA. Molecular cloning of the helodermin and exendin-4 cDNAs in the lizard. Relationship to vasoactive intestinal polypeptide/pituitary adenylate cyclase activating polypeptide and glucagon-like peptide 1 and evidence against the existence of mammalian homologues. J Biol Chem. 1998;273(16):9778–9784. doi: 10.1074/jbc.273.16.9778. [DOI] [PubMed] [Google Scholar]
- 49.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214(3):829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 50.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80(3):952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 51.Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47(2):159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 52.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298(5):714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 53.Graziano MP, Hey PJ, Borkowski D, Chicchi GG, Strader CD. Cloning and functional expression of a human glucagon-like peptide-1 receptor. Biochem Biophys Res Commun. 1993;196(1):141–146. doi: 10.1006/bbrc.1993.2226. [DOI] [PubMed] [Google Scholar]
- 54.Dillon JS, Tanizawa Y, Wheeler MB, et al. Cloning and functional expression of the human glucagon-like peptide-1 (GLP-1) receptor. Endocrinology. 1993;133(4):1907–1910. doi: 10.1210/endo.133.4.8404634. [DOI] [PubMed] [Google Scholar]
- 55.Arnés L, González N, Tornero-Esteban P, et al. Characteristics of GLP-1 and exendins action upon glucose transport and metabolism in type 2 diabetic rat skeletal muscle. Int J Mol Med. 2008;22(1):127–132. [PubMed] [Google Scholar]
- 56.Baggio LL, Drucker DJ. Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2. Best Pract Res Clin Endocrinol Metab. 2004;18(4):531–554. doi: 10.1016/j.beem.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Brubaker PL, Drucker DJ. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels. 2002;8(3–4):179–188. [PubMed] [Google Scholar]
- 58.Baggio L, Kieffer TJ, Drucker DJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology. 2000;141(10):3703–3709. doi: 10.1210/endo.141.10.7720. [DOI] [PubMed] [Google Scholar]
- 59.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142(2):521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 60.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta NA, Mells J, Dunham RM, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51(5):1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42(11):1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 63.Mells JE, Fu PP, Sharma S, et al. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302(2):G225–G235. doi: 10.1152/ajpgi.00274.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31(9):1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 65.Pedersen J, Holst JJ. Glucagon like-peptide 1 receptor and the liver. Liver Int. 2011;31(9):1243–1245. doi: 10.1111/j.1478-3231.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 66.Vendrell J, El Bekay R, Peral B, et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology. 2011;152(11):4072–4079. doi: 10.1210/en.2011-1070. [DOI] [PubMed] [Google Scholar]
- 67.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43(1):173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panjwani N, Mulvihill EE, Longuet C, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE (−/−) mice. Endocrinology. 2013;154(1):127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 69.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS ONE. 2011;6(9):e25269. doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamanaka-Okumura H, Urano E, Kawaura A, et al. Treatment of rapid weight loss in a donor with hepatic steatosis in living donor liver transplantation: a case report. Hepatogastroenterology. 2012;59(115):869–871. doi: 10.5754/hge10237. [DOI] [PubMed] [Google Scholar]
- 71.Nativ NI, Maguire TJ, Yarmush G, et al. Liver defatting: an alternative approach to enable steatotic liver transplantation. Am J Transplant. 2012;12(12):3176–3183. doi: 10.1111/j.1600-6143.2012.04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trevaskis JL, Griffin PS, Wittmer C, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G762–G772. doi: 10.1152/ajpgi.00476.2011. [DOI] [PubMed] [Google Scholar]
- 73.Cho YM, Kieffer TJ. K-cells and glucose-dependent insulinotropic polypeptide in health and disease. Vitam Horm. 2010;84:111–150. doi: 10.1016/B978-0-12-381517-0.00004-7. [DOI] [PubMed] [Google Scholar]
- 74.McIntosh CH, Widenmaier S, Kim SJ. Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP) Vitam Horm. 2009;80:409–471. doi: 10.1016/S0083-6729(08)00615-8. [DOI] [PubMed] [Google Scholar]
- 75.Rao RS, Kini S. GIP and bariatric surgery. Obes Surg. 2011;21(2):244–252. doi: 10.1007/s11695-010-0305-x. [DOI] [PubMed] [Google Scholar]
- 76.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 77.Musso G, Gambino R, Cassader M. Emerging molecular targets for the treatment of nonalcoholic fatty liver disease. Annu Rev Med. 2010;61:375–392. doi: 10.1146/annurev.med.60.101107.134820. [DOI] [PubMed] [Google Scholar]
- 78.Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20(4):236–242. doi: 10.1016/j.idairyj.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 80.Paschetta E, Hvalryg M, Musso G. Glucose-dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity-associated disorders. Obes Rev. 2011;12(10):813–828. doi: 10.1111/j.1467-789X.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- 81.García-Jiménez C. Wnt and incretin connections. Vitam Horm. 2010;84:355–387. doi: 10.1016/B978-0-12-381517-0.00014-X. [DOI] [PubMed] [Google Scholar]
- 82.Miyawaki K, Yamada Y, Yano H, et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96(26):14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nie Y, Ma RC, Chan JC, Xu H, Xu G. Glucose-dependent insulinotropic peptide impairs insulin signaling via inducing adipocyte inflammation in glucose-dependent insulinotropic peptide receptor-overexpressing adipocytes. FASEB J. 2012;26(6):2383–2393. doi: 10.1096/fj.11-196782. [DOI] [PubMed] [Google Scholar]
- 84.Timper K, Grisouard J, Sauter NS, et al. Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am J Physiol Endocrinol Metab. 2013;304(1):E1–E13. doi: 10.1152/ajpendo.00100.2012. [DOI] [PubMed] [Google Scholar]
- 85.Asmar M. New physiological effects of the incretin hormones GLP-1 and GIP. Dan Med Bull. 2011;58(2):B4248. [PubMed] [Google Scholar]
- 86.Asmar M, Simonsen L, Madsbad S, Stallknecht B, Holst JJ, Bülow J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes. 2010;59(9):2160–2163. doi: 10.2337/db10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asmar M, Simonsen L, Arngrim N, Holst JJ, Dela F, Bülow J. Glucose-dependent insulinotropic polypeptide has impaired effect on abdominal, subcutaneous adipose tissue metabolism in obese subjects. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.73. [DOI] [PubMed] [Google Scholar]
- 88.Musso G, Gambino R, Pacini G, De Michieli F, Cassader M. Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr. 2009;89(2):558–567. doi: 10.3945/ajcn.2008.26720. [DOI] [PubMed] [Google Scholar]
- 89.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr. 2006;26:391–411. doi: 10.1146/annurev.nutr.26.061505.111223. [DOI] [PubMed] [Google Scholar]
- 90.Drucker DJ. Glucagon-like peptide 2. J Clin Endocrinol Metab. 2001;86(4):1759–1764. doi: 10.1210/jcem.86.4.7386. [DOI] [PubMed] [Google Scholar]
- 91.Wallis K, Walters JR, Forbes A. Review article: glucagon-like peptide 2–current applications and future directions. Aliment Pharmacol Ther. 2007;25(4):365–372. doi: 10.1111/j.1365-2036.2006.03193.x. [DOI] [PubMed] [Google Scholar]
- 92.Yusta B, Huang L, Munroe D, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119(3):744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 93.Meier JJ, Nauck MA, Pott A, et al. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130(1):44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Mellitzer G, Gradwohl G. Enteroendocrine cells and lipid absorption. Curr Opin Lipidol. 2011;22(3):171–175. doi: 10.1097/MOL.0b013e32834622a2. [DOI] [PubMed] [Google Scholar]
- 95.McCuskey RS. Anatomy of efferent hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(1):821–826. doi: 10.1002/ar.a.20087. [DOI] [PubMed] [Google Scholar]
- 96.Ding WG, Kitasato H, Kimura H. Development of neuropeptide Y innervation in the liver. Microsc Res Tech. 1997;39(4):365–371. doi: 10.1002/(SICI)1097-0029(19971115)39:4<365::AID-JEMT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 97.Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38(4):141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Hsieh J, Longuet C, Maida A, et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137(3):997–1005. e1–e4. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 99.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62(2):373–381. doi: 10.2337/db12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Degrace P, Moindrot B, Mohamed I, et al. Upregulation of liver VLDL receptor and FAT/CD36 expression in LDLR−/− apoB100/100 mice fed trans-10,cis-12 conjugated linoleic acid. J Lipid Res. 2006;47(12):2647–2655. doi: 10.1194/jlr.M600140-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Buqué X, Martínez MJ, Cano A, et al. A subset of dysregulated metabolic and survival genes is associated with severity of hepatic steatosis in obese Zucker rats. J Lipid Res. 2010;51(3):500–513. doi: 10.1194/jlr.M001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 103.Pocai A. Unraveling oxyntomodulin, GLP1's enigmatic brother. J Endocrinol. 2012;215(3):335–346. doi: 10.1530/JOE-12-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab. 2005;1(1):22–31. doi: 10.1038/ncpendmet0017. [DOI] [PubMed] [Google Scholar]
- 105.Santoprete A, Capitò E, Carrington PE, et al. DPP-IV-resistant, long-acting oxyntomodulin derivatives. J Pept Sci. 2011;17(4):270–280. doi: 10.1002/psc.1328. [DOI] [PubMed] [Google Scholar]
- 106.ThanThan S, Asada Y, Saito T, et al. Oxyntomodulin attenuates exendin-4-induced hypoglycemia in cattle. Domest Anim Endocrinol. 2013;44(2):70–80. doi: 10.1016/j.domaniend.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 107.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food in-take and energy expenditure. Gastroenterology. 2004;127(2):546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 108.Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE. Oxyntomodulin differentially affects glucagon-like peptide-1 receptor beta-arrestin recruitment and signaling through Galpha(s) J Pharmacol Exp Ther. 2007;322(1):148–154. doi: 10.1124/jpet.107.120006. [DOI] [PubMed] [Google Scholar]
- 109.Bataille D, Gespach C, Tatemoto K, et al. Bioactive enteroglucagon (oxyntomodulin): present knowledge on its chemical structure and its biological activities. Peptides. 1981;2(Suppl 2):41–44. doi: 10.1016/0196-9781(81)90008-5. [DOI] [PubMed] [Google Scholar]
- 110.Gespach C, Bataille D, Vauclin N, Rosselin G, Moroder L, Wünsch E. Secretin binding sites coupled with adenylate cyclase in rat fundic membranes. Peptides. 1981;2(Suppl 2):247–251. doi: 10.1016/0196-9781(81)90039-5. [DOI] [PubMed] [Google Scholar]
- 111.Koole C, Wootten D, Simms J, et al. Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol Pharmacol. 2010;78(3):456–465. doi: 10.1124/mol.110.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gehlert DR. Introduction to the reviews on neuropeptide Y. Neuropeptides. 2004;38(4):135–140. doi: 10.1016/j.npep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Chandarana K, Batterham R, Peptide YY. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):65–72. doi: 10.1097/MED.0b013e3282f3f4b1. [DOI] [PubMed] [Google Scholar]
- 114.Balasubramaniam AA. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18(3):445–457. doi: 10.1016/s0196-9781(96)00347-6. [DOI] [PubMed] [Google Scholar]
- 115.Mullins DE, Zhang X, Hawes BE. Activation of extracellular signal regulated protein kinase by neuropeptide Y and pancreatic polypeptide in CHO cells expressing the NPY Y(1), Y(2), Y(4) and Y(5) receptor subtypes. Regul Pept. 2002;105(1):65–73. doi: 10.1016/s0167-0115(01)00388-3. [DOI] [PubMed] [Google Scholar]
- 116.Lin S, Boey D, Herzog H. NPYand Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38(4):189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46(6):261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chee MJ, Colmers WF. Y eat? Nutrition. 2008;24(9):869–877. doi: 10.1016/j.nut.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 119.Lin S, Boey D, Couzens M, Lee N, Sainsbury A, Herzog H. Compensatory changes in [125I]-PYY binding in Y receptor knockout mice suggest the potential existence of further Y receptor(s) Neuropeptides. 2005;39(1):21–28. doi: 10.1016/j.npep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 120.Lin S, Boey D, Lee N, Schwarzer C, Sainsbury A, Herzog H. Distribution of prodynorphin mRNA and its interaction with the NPY system in the mouse brain. Neuropeptides. 2006;40(2):115–123. doi: 10.1016/j.npep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 121.Lindner D, Stichel J, Beck-Sickinger AG. Molecular recognition of the NPY hormone family by their receptors. Nutrition. 2008;24(9):907–917. doi: 10.1016/j.nut.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 122.Lindner D, van Dieck J, Merten N, et al. GPC receptors and not ligands decide the binding mode in neuropeptide Y multi-receptor/multiligand system. Biochemistry. 2008;47(22):5905–5914. doi: 10.1021/bi800181k. [DOI] [PubMed] [Google Scholar]
- 123.Herzog H, Hort YJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc Natl Acad Sci U S A. 1992;89(13):5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 125.van den Hoek AM, van Heijningen C, Schröder-van der Elst JP, et al. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57(9):2304–2310. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van den Hoek AM, Voshol PJ, Karnekamp BN, et al. Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes. 2004;53(10):2529–2534. doi: 10.2337/diabetes.53.10.2529. [DOI] [PubMed] [Google Scholar]
- 127.Sigala B, McKee C, Soeda J, et al. Sympathetic nervous system catecholamines and neuropeptide Y neurotransmitters are upregulated in human NAFLD and modulate the fibrogenic function of hepatic stellate cells. PLoS ONE. 2013;8(9):e72928. doi: 10.1371/journal.pone.0072928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Piano A, Tock L, Carnier J, et al. The role of nutritional profile in the orexigenic neuropeptide secretion in nonalcoholic fatty liver disease obese adolescents. Eur J Gastroenterol Hepatol. 2010;22(5):557–563. doi: 10.1097/MEG.0b013e3283346df2. [DOI] [PubMed] [Google Scholar]
- 129.Garruti G, Cotecchia S, Giampetruzzi F, Giorgino F, Giorgino R. Neuroendocrine deregulation of food intake, adipose tissue and the gastrointestinal system in obesity and metabolic syndrome. J Gastrointestin Liver Dis. 2008;17(2):193–198. [PubMed] [Google Scholar]
- 130.Garruti G, Giusti V, Nussberger J, et al. Expression and secretion of the atrial natriuretic peptide in human adipose tissue and preadipocytes. Obesity (Silver Spring) 2007;15(9):2181–2189. doi: 10.1038/oby.2007.259. [DOI] [PubMed] [Google Scholar]
- 131.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. The role of peptide YY in gastrointestinal diseases and disorders (review) (review)Int J Mol Med. 2013;31(2):275–282. doi: 10.3892/ijmm.2012.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bradley WD, Zwingelstein C, Rondinone CM. The emerging role of the intestine in metabolic diseases. Arch Physiol Biochem. 2011;117(3):165–176. doi: 10.3109/13813455.2011.578651. [DOI] [PubMed] [Google Scholar]
- 133.Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin Liver Dis. 2012;32(1):80–91. doi: 10.1055/s-0032-1306428. [DOI] [PubMed] [Google Scholar]
- 134.El-Salhy M, Falkmer S, Kramer KJ, Speirs RD. Immunocytochemical evidence for the occurrence of insulin in the frontal ganglion of a Lepidopteran insect, the tobacco hornworm moth. Manduca sexta L.. Gen Comp Endocrinol. 1984;54(1):85–88. doi: 10.1016/0016-6480(84)90202-8. [DOI] [PubMed] [Google Scholar]
- 135.El-Salhy M. Immunocytochemical investigation of the gastroentero-pancreatic (GEP) neurohormonal peptides in the pancreas and gastrointestinal tract of the dogfish Squalus acanthias. Histochemistry. 1984;80(2):193–205. doi: 10.1007/BF00679996. [DOI] [PubMed] [Google Scholar]
- 136.Dong CX, Brubaker PL. Ghrelin, the proglucagon-derived peptides and peptide YY in nutrient homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9(12):705–715. doi: 10.1038/nrgastro.2012.185. [DOI] [PubMed] [Google Scholar]
- 137.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 138.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 139.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 140.Maas MI, Hopman WP, Katan MB, Jansen JB. Release of peptide YY and inhibition of gastric acid secretion by long-chain and medium-chain triglycerides but not by sucrose polyester in men. Eur J Clin Invest. 1998;28(2):123–130. doi: 10.1046/j.1365-2362.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 141.Halldén G, Aponte GW. Evidence for a role of the gut hormone PYY in the regulation of intestinal fatty acid-binding protein transcripts in differentiated subpopulations of intestinal epithelial cell hybrids. J Biol Chem. 1997;272(19):12591–12600. doi: 10.1074/jbc.272.19.12591. [DOI] [PubMed] [Google Scholar]
- 142.Onaga T, Zabielski R, Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides. 2002;23(2):279–290. doi: 10.1016/s0196-9781(01)00609-x. [DOI] [PubMed] [Google Scholar]
- 143.Pedersen SL, Sasikumar PG, Chelur S, et al. Peptide hormone isoforms: N-terminally branched PYY3-36 isoforms give improved lipid and fat-cell metabolism in diet-induced obese mice. J Pept Sci. 2010;16(11):664–673. doi: 10.1002/psc.1281. [DOI] [PubMed] [Google Scholar]
- 144.Shi YC, Hämmerle CM, Lee IC, et al. Adult-onset PYY overexpression in mice reduces food intake and increases lipogenic capacity. Neuropeptides. 2012;46(4):173–182. doi: 10.1016/j.npep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 145.Asakawa A, Inui A, Yuzuriha H, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124(5):1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- 146.Stanley S, Wynne K, Bloom S. Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G693–G697. doi: 10.1152/ajpgi.00536.2003. [DOI] [PubMed] [Google Scholar]
- 147.Zac-Varghese S, Tan T, Bloom SR. Hormonal interactions between gut and brain. Discov Med. 2010;10(55):543–552. [PubMed] [Google Scholar]
- 148.Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007;133(1):76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 149.Simpson K, Parker J, Plumer J, Bloom S. CCK, PYY and PP: the control of energy balance. Handbook Exp Pharmacol. 2012;(209):209–230. doi: 10.1007/978-3-642-24716-3_9. [DOI] [PubMed] [Google Scholar]
- 150.Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz PH, Barnes AJ. Distribution and release of human pancreatic polypeptide. Gut. 1976;17(12):940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Asakawa A, Inui A, Ueno N, Fujimiya M, Fujino MA, Kasuga M. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides. 1999;20(12):1445–1448. doi: 10.1016/s0196-9781(99)00155-2. [DOI] [PubMed] [Google Scholar]
- 152.Kojima S, Ueno N, Asakawa A, et al. A role for pancreatic polypeptide in feeding and body weight regulation. Peptides. 2007;28(2):459–463. doi: 10.1016/j.peptides.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 153.Gerald C, Walker MW, Criscione L, et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382(6587):168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- 154.Lin S, Shi YC, Yulyaningsih E, et al. Critical role of arcuate Y4 receptors and the melanocortin system in pancreatic polypeptide-induced reduction in food intake in mice. PLoS ONE. 2009;4(12):e8488. doi: 10.1371/journal.pone.0008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lassmann V, Vague P, Vialettes B, Simon MC. Low plasma levels of pancreatic polypeptide in obesity. Diabetes. 1980;29(6):428–430. doi: 10.2337/diab.29.6.428. [DOI] [PubMed] [Google Scholar]
- 156.Uhe AM, Szmukler GI, Collier GR, Hansky J, O'Dea K, Young GP. Potential regulators of feeding behavior in anorexia nervosa. Am J Clin Nutr. 1992;55(1):28–32. doi: 10.1093/ajcn/55.1.28. [DOI] [PubMed] [Google Scholar]
- 157.Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res. 2012;2012:824305. doi: 10.1155/2012/824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Phillips JK, McLean AJ, Hill CE. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br J Pharmacol. 1998;124(7):1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]