Significance
Prevalence of chronic kidney disease (CKD) has reached epidemic proportions in the Western world in recent decades. Abnormally elevated blood insulin and impaired insulin action (insulin resistance) are common complications of CKD, and are associated with increased cardiovascular-related deaths in CKD patients. Therefore, novel therapies are required to treat insulin resistance in CKD. Abnormally elevated levels of glucocorticoids can cause insulin resistance. Here we demonstrate a crucial role for the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) predominantly in liver, which is essential for glucocorticoid production, in causing insulin resistance in CKD. Additionally, 11βHSD1 inhibition corrected insulin resistance in CKD rodent models. Taken together, this is strong evidence that selective inhibition of 11βHSD1 is a promising therapeutic target for treatment of insulin resistance in CKD.
Abstract
Insulin resistance and associated metabolic sequelae are common in chronic kidney disease (CKD) and are positively and independently associated with increased cardiovascular mortality. However, the pathogenesis has yet to be fully elucidated. 11β-Hydroxysteroid dehydrogenase type 1 (11βHSD1) catalyzes intracellular regeneration of active glucocorticoids, promoting insulin resistance in liver and other metabolic tissues. Using two experimental rat models of CKD (subtotal nephrectomy and adenine diet) which show early insulin resistance, we found that 11βHSD1 mRNA and protein increase in hepatic and adipose tissue, together with increased hepatic 11βHSD1 activity. This was associated with intrahepatic but not circulating glucocorticoid excess, and increased hepatic gluconeogenesis and lipogenesis. Oral administration of the 11βHSD inhibitor carbenoxolone to uremic rats for 2 wk improved glucose tolerance and insulin sensitivity, improved insulin signaling, and reduced hepatic expression of gluconeogenic and lipogenic genes. Furthermore, 11βHSD1−/− mice and rats treated with a specific 11βHSD1 inhibitor (UE2316) were protected from metabolic disturbances despite similar renal dysfunction following adenine experimental uremia. Therefore, we demonstrate that elevated hepatic 11βHSD1 is an important contributor to early insulin resistance and dyslipidemia in uremia. Specific 11βHSD1 inhibitors potentially represent a novel therapeutic approach for management of insulin resistance in patients with CKD.
The prevalence of chronic kidney disease (CKD) has increased dramatically in recent years causing substantial morbidity and mortality (1). Although diabetic patients with CKD sometimes develop recurrent hypoglycemia, possibly due to reduced renal catabolism of insulin, it is increasingly recognized that insulin resistance and associated hyperinsulinemia are common complications in patients with CKD (2, 3) with an insulin resistance-like syndrome occurring even at the earliest stage of renal dysfunction (4). CKD-induced insulin resistance is positively and independently associated with increased cardiovascular mortality (5, 6). Furthermore, mortality among patients treated with hemodialysis is higher in those with more severe insulin resistance (7). Despite this, the mechanisms responsible for the onset of insulin resistance in CKD are unclear.
Increased hepatic gluconeogenesis can cause hyperinsulinemia and hyperglycemia (8, 9). Expression of genes encoding key gluconeogenic enzymes such as phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (G-6pase) are transcriptionally induced in response to stimuli such as glucagon and glucocorticoids, and suppressed by insulin. This process is tightly regulated by transcription factors and cofactors, in particular peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC1α) (10). Hepatic gluconeogenesis is inappropriately elevated in rodent models and human patients with insulin resistance and type 2 diabetes mellitus (T2DM). Abnormal elevation of gluconeogenesis leading to insulin resistance can occur as a result of circulating glucocorticoid excess, as observed in Cushing syndrome (11, 12). However, the role of glucocorticoids in the pathophysiology of CKD-induced insulin resistance has not been described.
11β-Hydroxysteroid dehydrogenase (11βHSD) enzymes function to regulate intracellular glucocorticoid levels. 11βHSD type 1 (11βHSD1) catalyzes the conversion of intrinsically inactive cortisone to active cortisol (11-dehydrocorticosterone to corticosterone in rats), thus amplifying local glucocorticoid levels, whereas 11βHSD2 catalyzes the opposite reaction (11, 13) but is largely confined to the distal nephron. 11βHSD1 is expressed at high levels in the major organs underpinning metabolism such as liver and adipose tissue. Hepatic overexpression of 11βHSD1 leads to insulin resistance in mice with increased lipogenesis (14), consistent with increased intrahepatic glucocorticoid action, whereas 11βHSD1 inhibition or deficiency leads to decreased hepatic gluconeogenesis (and decreased PCK1), improved insulin sensitivity, and correction of hyperglycemia in rodent models of insulin resistance and patients with T2DM (15–18).
We investigated the hypothesis that 11βHSD1-induced glucocorticoid excess mediates abnormal elevation of gluconeogenesis and lipogenesis in uremia, using two experimental rodent models with entirely distinct mechanisms of development of renal failure; subtotal nephrectomy (SNx) and adenine feeding. To investigate a potential causal role for 11βHSD1 in uremia-induced insulin resistance, we used the 11βHSD1 inhibitors carbenoxolone (CBX) (16, 19) and UE2316 and investigated 11βHSD1−/− mice.
Results
Markers of Renal Failure in Models of Experimental Uremia.
Serum creatinine was elevated 3.6-fold in SNx and 8.1-fold in adenine-fed rats, and 3.5-fold in adenine-fed 11β-HSD1−/− mice, whereas serum urea was elevated 5.5-, 11.8-, and 4.5-fold, respectively. Further markers of chronic renal injury are shown in Tables S1–S3. Body weights, mean food intake and average heart rate were not significantly different between the uremic and sham groups. Mean blood pressure, although tending to be higher in CBX treated groups, was not significantly different because of high variability (Tables S4 and S5).
Hepatic 11βHSD1 Is Elevated in CKD.
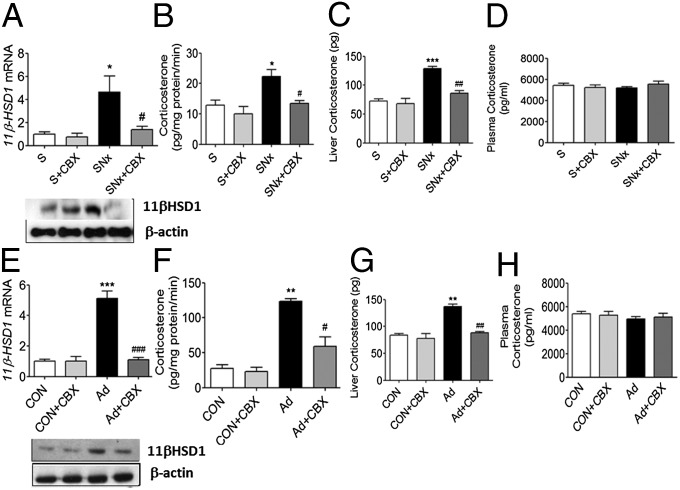
Hepatic 11βHSD1 mRNA and protein levels, together with 11βHSD1 reductase activity, were significantly elevated in SNx (Fig. 1 A and B) and adenine-fed rats (Fig. 1 E and F). Intrahepatic corticosterone levels were also markedly elevated in SNx and adenine-fed rats (Fig. 1 C and G) however, consistent with the notion that 11βHSD1 determines tissue-specific intracrine glucocorticoid levels, this occurred in the absence of elevated systemic serum corticosterone levels (Fig. 1 D and H). Administration of CBX (50 mg⋅kg−1⋅d−1; 2 wk) normalized hepatic 11βHSD1 mRNA and protein levels, suppressed 11βHSD1 reductase activity and lowered hepatic corticosterone levels in SNx and adenine-fed rats. In contrast, CBX had no effect on serum corticosterone levels, demonstrating that CBX selectively influences intrahepatic glucocorticoid levels. Similar to observations in liver, white adipose tissue levels of 11βHSD1 mRNA and protein were increased in uremia, an effect that was reversed by CBX (Fig. S1). In contrast, 11βHSD1 mRNA and protein levels in skeletal muscle were unchanged across all four experimental groups (Fig. S1).
Fig. 1.
Hepatic 11βHSD1 levels and acitivity are elevated in SNx and Ad rats. (A and E) 11βHSD1 mRNA and protein, (B and F) hepatic corticosterone production (pg⋅min−1⋅mg−1 liver protein), (C and G) hepatic corticosterone levels, and (D and H) plasma corticosterone levels. Data are expressed as mean ± SEM (n = 8 per group). Statistically significant differences between sham/control and Ad or SNx are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant effects of CBX treatment are indicated by #P < 0.05, ##P < 0.01, and ###P < 0.001.
Consistent with recent evidence demonstrating induction of 11βHSD1 transcription by proinflammatory cytokines (20–23) serum levels of IL-1β, TNFα, and IL-6 were elevated in the chronic phase of SNx and adenine-induced uremia (Fig. S2), but were unaffected by CBX, suggesting a possible uremia-dependent mechanism upstream of 11βHSD1 induction and hepatic glucocorticoid metabolism, involving elevated proinflammatory cytokine levels.
Uremic Rats Develop Impaired Glucose Tolerance and Reduced Insulin Sensitivity.
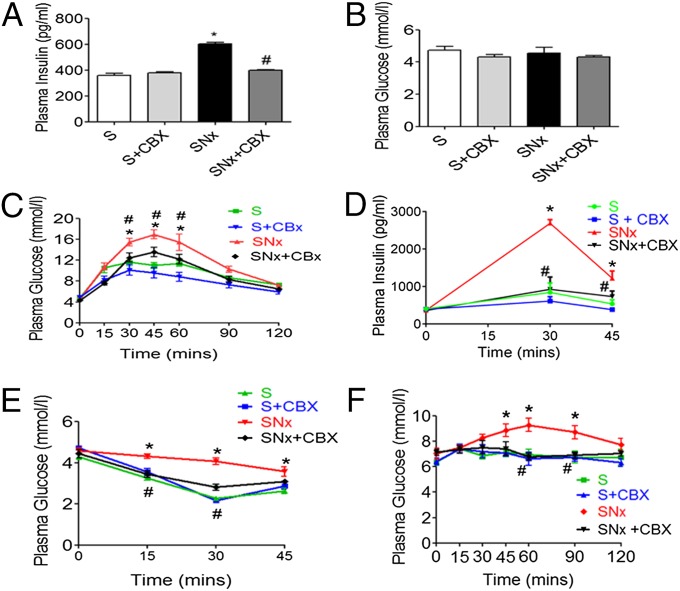
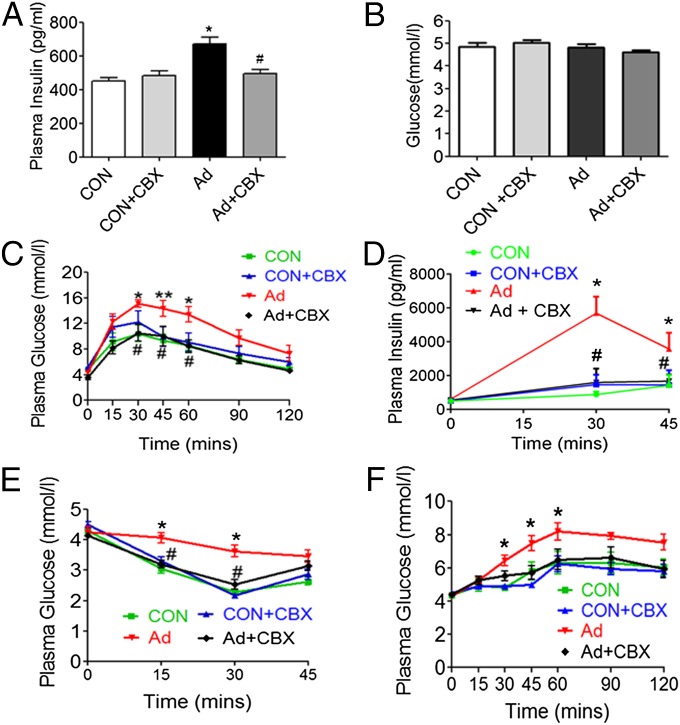
Serum insulin levels were markedly elevated in SNx (Fig. 2A) and adenine-fed rats (Fig. 3A). However, fasting serum glucose levels were unchanged (Figs. 2B and 3B), suggestive of uremia-induced insulin resistance. To further analyze potential uremia-induced changes in systemic glucose tolerance and insulin sensitivity, we conducted i.p. glucose and insulin tolerance tests (GTT and ITT). During the GTT, blood glucose levels were significantly higher in SNx and adenine-fed rats compared with sham up to 60 min post-glucose administration (Figs. 2C and 3C). In addition, serum insulin levels were elevated during a GTT, remaining elevated 45 min post-glucose administration (Figs. 2D and 3D) in SNx and adenine-fed rats compared with sham. Moreover, impairment in insulin’s ability to lower blood glucose levels in an ITT was observed in SNx and adenine-fed rats, with blood glucose levels remaining significantly elevated in both models 30 min post-insulin administration (Figs. 2E and 3E). Taken together, these data demonstrate the presence of insulin resistance in both models of uremia. To determine whether uremia-induced insulin resistance may be linked to elevated hepatic glucose production, we conducted a pyruvate tolerance test (PTT). Consistent with abnormally elevated hepatic glucose production, SNx and adenine-fed rats displayed significantly increased blood glucose levels up to 90 min post-pyruvate administration (Figs. 2F and 3F).
Fig. 2.
11βHSD1 inhibition ameliorates insulin resistance in SNx rats. (A) Fasting plasma insulin, (B) fasting plasma glucose, (C) plasma glucose response to GTT, (D) plasma insulin response to GTT, (E) plasma glucose response to ITT, and (F) plasma glucose response to PTT. Data are expressed as mean ± SEM (n = 8 per group). Statistically significant differences between sham and SNx are indicated by *P < 0.05. Statistically significant effects of CBX treatment are indicated by #P < 0.05.
Fig. 3.
11βHSD1 inhibition ameliorates insulin resistance in adenine-fed rats. Experimental uremia was induced in rats by administration of 0.75% Ad (n = 8 per group). CBX (50 mg⋅kg−1⋅d−1) or vehicle was administered by oral gavage for 2 wk in both groups. (A) Fasting plasma insulin, (B) fasting plasma glucose, (C) plasma glucose response to 2 g per kg dextrose injected i.p. (GTT), (D) plasma insulin response to glucose load following i.p. GTT, (E) plasma glucose response to i.p. ITT, and (F) plasma glucose response to i.p. PTT. Data are expressed as mean ± SEM. Statistically significant differences between control and Ad are indicated by *P < 0.05 and **P < 0.01. Statistically significant effects of CBX treatment are indicated by #P < 0.05.
Collectively, these data demonstrate significant insulin resistance associated with abnormally elevated hepatic glucose production in both SNx and adenine-feeding models of CKD.
11βHSD1 Inhibition Improves Systemic Glucose Tolerance and Insulin Sensitivity in CKD Rats.
To assess the potential role of increases in 11βHSD1 levels and activity in mediating insulin resistance in uremia, we analyzed rats treated with CBX. Administration of CBX did not improve markers of renal failure in any group (Tables S1 and S2). Despite this, CBX completely prevented uremia-induced increases in serum insulin levels in both models (Figs. 2A and 3A). Moreover, 11βHSD1 inhibition with CBX resulted in a significant improvement in insulin sensitivity and glucose tolerance (Figs. 2 A–F and 3 A–F), as well as reduced hepatic glucose production following a PTT. Taken together, these data demonstrate that abnormally elevated 11βHSD1 plays a crucial role in mediating insulin resistance in uremia.
11βHSD1 Inhibition Suppresses Hepatic Gluconeogenic Gene Expression and Markers of Impaired Insulin Signaling in Uremia.
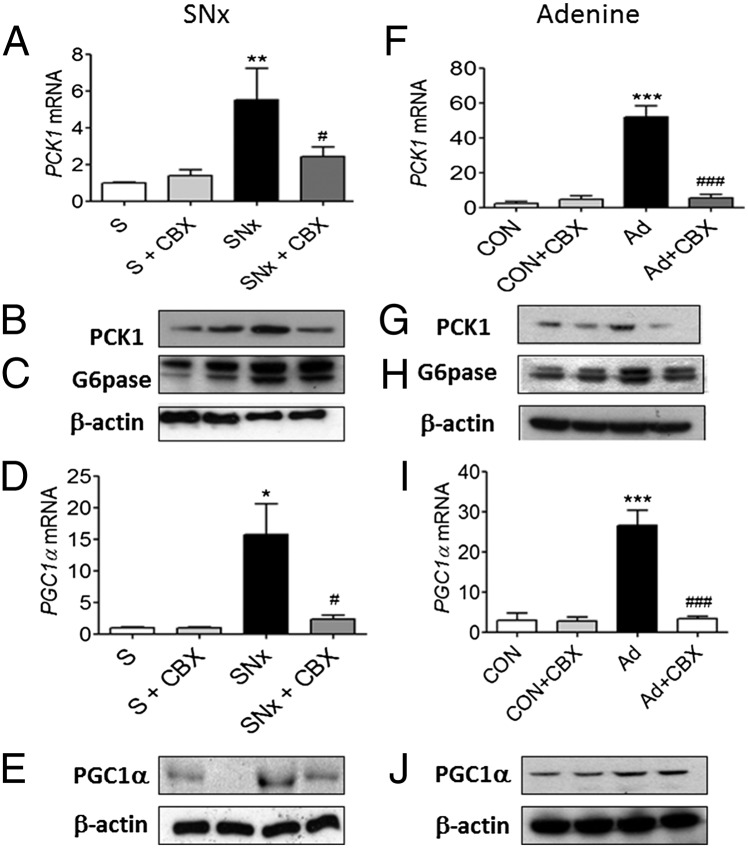
Because uremia-induced insulin resistance was associated with elevated blood glucose levels following a PTT, we examined alterations in hepatic gluconeogenic enzymes. Levels of PCK1 mRNA and protein and G6Pase protein were elevated in SNx (Fig. 4 A–C) and adenine-fed rats (Fig. 4 F–H). PGC1α mRNA and protein levels were also increased in SNx (Fig. 4 D and E) and adenine-fed rats (Fig. 4 I and J). Similar to effects observed on systemic insulin resistance, CBX administration reversed these effects on gluconeogenic enzymes in both models of uremia. To assess whether increased hepatic gluconeogenesis occurs in association with impaired insulin signaling, we measured phosphorylation of AKT at serine 473. Protein levels of phospho-(Ser473)-AKT were reduced in liver and also in the skeletal muscle and white adipose tissue of uremic rats. In all three tissues, this effect was reversed by CBX administration (Fig. S1). Taken together, these data suggest that 11βHSD1 mediates insulin resistance in uremia through abnormal elevation of hepatic gluconeogenesis and dysregulation insulin signaling.
Fig. 4.
CBX corrects uremia-mediated insulin resistance in association with suppressed hepatic gluconeogenesis. Experimental uremia was induced in rats by SNx (n = 8 per group) or Ad (n = 8 per group). CBX (50 mg⋅kg−1⋅d−1) or vehicle was administered by oral gavage for 2 wk. (A and B) SNx, PCK1 mRNA and protein; (C) SNx, G-6pase protein; (D and E) SNx, PGC1α mRNA and protein; (F and G) Ad, PCK1 mRNA and protein; (H) Ad, G-6pase protein; and (I and J) Ad, PGC1α mRNA and protein. Data are expressed as mean ± SEM. Statistically significant differences between sham and Ad or SNx are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant effects of CBX treatment are indicated by #P < 0.05 and ###P < 0.001.
Uremia-Induced Dyslipidemia Is Corrected by 11βHSD1 Inhibition.
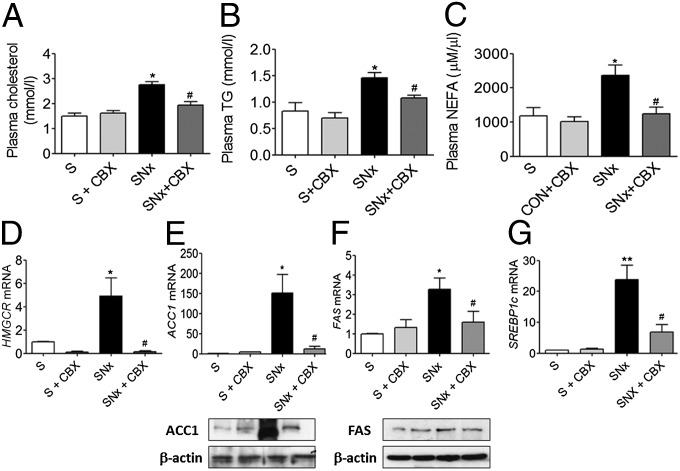
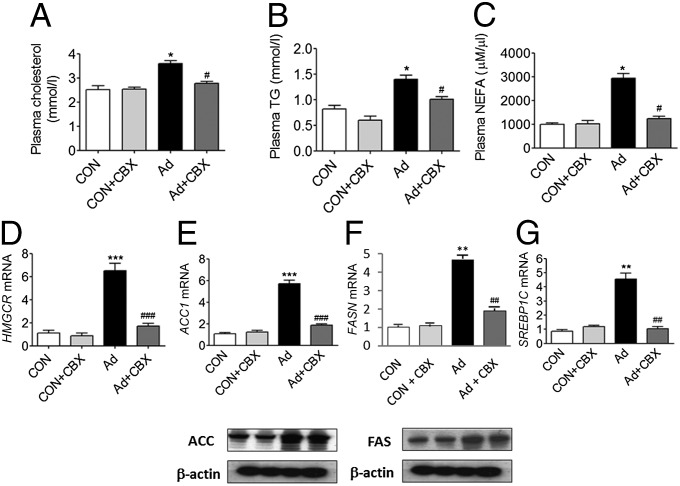
Dyslipidemia manifested by raised serum levels of triglycerides, free fatty acids, and cholesterol is observed in CKD patients and rodent models of uremia (5, 6, 24). Dyslipidemia can cause insulin resistance (25, 26). The de novo synthesis of fatty acid and cholesterol is controlled through transcriptional regulation of several key genes, in particular acetyl CoA carboxylase (ACC), fatty acid synthase (FASN), and 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR) (27). Transcriptional regulation of these genes is controlled by sterol regulatory element-binding protein 1 (SREBP1) (28), which is induced by insulin (29).
Consistent with previous reports in experimental models of uremia (30, 31), SNx and adenine-fed rats showed elevated serum levels of cholesterol, triglycerides, and nonesterified fatty acids (NEFAs), (Figs. 5 A–C and 6 A–C). Hepatic mRNA expression of HMGCR was elevated in both uremic models (Figs. 5D and 6D) suggesting increased de novo hepatic cholesterol biosynthesis. Moreover, mRNA and protein levels of ACC (Figs. 5E and 6E) and FASN (Figs. 5F and 6F) and mRNA levels of SREBP1c (Figs. 5G and 6G) were markedly increased in SNx and adenine-fed rats, indicative of increased hepatic de novo lipogenesis. Importantly, in both CKD models, abnormally elevated serum levels of cholesterol, triglycerides, and NEFAs were ameliorated following administration of CBX, demonstrating that uremia-induced dyslipidemia, like gluconeogenesis, may occur in part through an 11βHSD1-mediated mechanism (Figs. 5 A–C and 6 A–C). Consistent with this, mRNA and protein levels of ACC1, FASN, SREBP1c, and HMGCR were also partially normalized by CBX in SNx and adenine-fed rats (Figs. 5 D–G and 6 D–G).
Fig. 5.
CBX suppresses total and hepatic lipogenesis in SNx animals. Experimental uremia was induced in rats by SNx (n = 8 per group). CBX (50 mg⋅kg−1⋅d−1) or vehicle was administered by oral gavage for 2 wk. (A) Plasma cholesterol, (B) plasma triglycerides, (C) plasma NEFA, (D) HMGCR mRNA, (E) ACC mRNA and protein, (F) FAS mRNA and protein, and (G) SREBP1 mRNA. Data are expressed as mean ± SEM. Statistically significant differences between sham and SNx are indicated by *P < 0.05 and **P < 0.01. Statistically significant effects of CBX treatment are indicated by #P < 0.05.
Fig. 6.
CBX suppresses total and hepatic lipogenesis in Adenine-fed animals. Experimental uremia was induced in rats by Ad (n = 8 per group). CBX (50 mg⋅kg−1⋅d−1) or vehicle was administered by oral gavage for 2 wk. (A) Plasma cholesterol, (B) plasma triglycerides, (C) plasma NEFA, (D) HMGCR mRNA, (E) ACC mRNA and protein, (F) FAS mRNA and protein, (G) SREBP1 mRNA. Data are expressed as mean ± SEM. Statistically significant differences between sham and Ad are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant effects of CBX treatment are indicated by #P < 0.05, ##P < 0.01, and ###P < 0.001.
We also assessed liver and skeletal muscle lipid content by measuring triglyceride levels in SNx rats. Despite observed changes in lipogenic gene and protein levels, hepatic triglyceride content was unchanged between sham and uremic rats (Fig. S3). In contrast, uremia increased skeletal muscle triglyceride levels, but these increases were not reversed by CBX.
Taken together, these data demonstrate that uremia-induced dyslipidemia occurs through an 11βHSD1-dependent mechanism and may contribute to insulin resistance in these models.
Specific Inhibition of 11βHSD1 with UE2316 Protects Uremic Rats from Insulin Resistance.
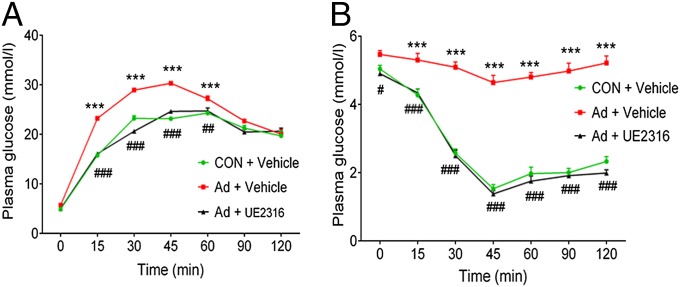
Finally, to confirm a specific role for 11βHSD1 in uremia-induced insulin resistance and rule out potential nonspecific CBX effects, we examined the effects of specific 11βHSD1 inhibitor (UE2316) (32) on insulin resistance in uremic (adenine-fed) rats. In agreement with data obtained from CBX treatment, UE2316 (20 mg⋅kg−1⋅d−1) significantly improved glucose tolerance and insulin sensitivity in adenine-fed rats (Fig. 7 A and B), without affecting parameters of renal failure (Table S6), confirming a role for 11βHSD1 in mediating uremia-induced insulin resistance.
Fig. 7.
Specific inhibition of 11βHSD1 corrects uremia-induced insulin resistance in adenine-fed rats. Experimental uremia was induced in rats by Ad (n = 8 per group). UE2316 (20 mg⋅kg−1⋅d−1) or vehicle was administered by osmotic minipump for 2 wk. (A) plasma glucose response to 2 g per kg dextrose injected i.p. (GTT) and (B) plasma glucose response to 2 units per kg of body weight porcine insulin injected i.p. (ITT). Data are expressed as mean ± SEM. Statistically significant differences between CON and Ad are indicated by ***P < 0.001. Statistically significant effects of CBX treatment are indicated by #P < 0.05, ##P < 0.01, and ###P < 0.001.
11βHSD1−/− Mice Are Protected from Uremic Insulin Resistance.
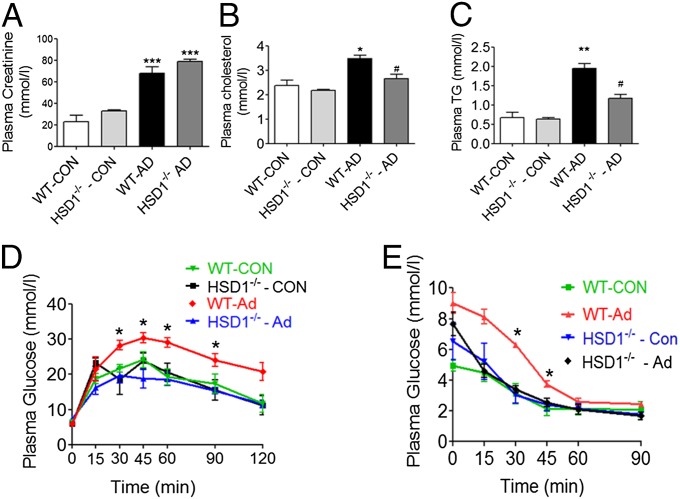
To further establish the role of 11βHSD1 in uremia-induced insulin resistance we used 11βHSD1−/− mice (33). Following consumption of an adenine-diet, both 11βHSD1−/− and wild-type (WT) mice developed similar levels of renal dysfunction (Fig. 8A and Table S3). Similar to the phenotype observed in rats, uremic WT mice developed dyslipidemia (raised systemic triglyceride levels), impaired glucose tolerance, and reduced insulin sensitivity (Fig. 8 B–E). In marked contrast, 11βHSD1−/− mice were protected against uremia-induced insulin resistance and dyslipidemia, displaying a lipid profile similar to control animals, as well as improved glucose tolerance and insulin sensitivity comparable with that in WT controls.
Fig. 8.
11βHSD1−/− mice have improved insulin sensitivity and glucose tolerance and a favorable lipid profile despite uremia. Experimental uremia was induced in mice by administration of 0.25% Ad (n = 8 per group). (A) Plasma creatinine, (B) total plasma cholesterol, (C) plasma triglycerides, (D) plasma glucose response to 2 g per kg dextrose injected i.p. (GTT), and (E) plasma glucose response to i.p. PTT. Data are expressed as mean ± SEM. Statistically significant differences between sham and Adenine diets are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant differences effects of HSD1−/− are indicated by #P < 0.05.
Discussion
We report here that elevated hepatic 11βHSD1 mediates impaired glucose tolerance, reduced insulin sensitivity, hyperinsulinemia, and dyslipidemia in two distinct male rat models of CKD. Importantly, increased 11βHSD1 elevated hepatic corticosterone content without changes in systemic corticosterone, suggesting a key role for hepatic 11β-HSD1, the major source of local corticosterone regeneration in liver, in development of insulin resistance in CKD. Although these studies have yet to be confirmed in female rats, improvements in metabolic profiles following 11βHSD1 inhibition or ablation occurred without corrections in renal dysfunction, indicating that selective inhibitors of 11βHSD1 may be a plausible therapeutic approach to insulin resistance and its complications in CKD.
Previous studies have reported elevated hepatic glucose production in chronically uremic patients (34). Furthermore, increased hepatic gluconeogenesis in acute experimental uremia is reversed by the glucocorticoid receptor antagonist RU 38486 (35). Elevated hepatic gluconeogenesis can cause insulin resistance and hyperinsulinemia (8, 9), whereas knockdown of gluconeogenic genes and cofactors have resulted in the correction of insulin resistance and improvement in insulin sensitivity in murine models (36–39). This suggests that 11βHSD1-mediated increases in hepatic gluconeogenesis via up-regulation of PCK1 and PGC1α may represent a likely cause of hyperinsulinemia and insulin resistance in uremia.
Dyslipidemia is also a common complication of CKD (5, 6, 24). We observed abnormally elevated plasma lipids and cholesterol in uremic rats, with parallel increases in lipogenic gene and protein expression. 11βHSD1 inhibition corrected systemic dyslipidemia, in association with reduced hepatic lipogenic gene and protein expression. These observations are consistent with glucocorticoid induction of triglyceride synthesis and fatty liver in rats, whereas mice overexpressing hepatic 11βHSD1 display increased hepatic lipogenesis (14). However, it is unclear in our model whether increased hepatic lipogenic gene expression occurs via direct induction by glucocorticoids or through elevated insulin-mediated SREBP1c induction, as would be anticipated because of undesirable metabolic consequence of hyperinsulinemia (28, 29).
Although our data points to a crucial role for hepatic 11βHSD1 as a key mediator of insulin resistance in uremia, we also observed increases in 11βHSD1 levels and parallel impairment of insulin signaling in adipose tissue, changes which were reversed by CBX. These changes may also contribute to uremia-induced insulin resistance, for example via glucocorticoid-mediated increases in circulating NEFA levels, as observed in our models, which may account for the impaired insulin signaling observed in uremic liver, through ectopic lipid deposition in liver and skeletal muscle. This may be particularly plausible given the lack of increased triglyceride levels in uremic liver. Interestingly, studies have suggested that CBX may not act directly on adipose tissue (15), raising the possibility that a secreted hepatokine produced during hepatic CBX metabolism may have impact on adipose tissue 11βHSD1. Similar to adipose tissue and liver, insulin signaling was also impaired in uremic skeletal muscle, whereas skeletal muscle lipid levels are increased, suggesting a potential contributory role for muscle ectopic lipid deposition in uremia-induced insulin resistance. However, uremia-induced increases in skeletal muscle triglyceride were not reversed by CBX, while we did not observe changes in skeletal muscle 11βHSD1 levels across all four experimental groups. Despite this, skeletal muscle insulin signaling was improved by CBX. These data suggest that the insulin-sensitizing effects of CBX in skeletal muscle are indirect, and are not mediated through reversal of skeletal muscle triglyceride levels, instead occurring through direct CBX effects on liver and adipose tissue. Thus, both hepatic and adipose tissue 11βHSD1 contribute to onset of insulin resistance in uremia. However, without conducting longitudinal studies of uremia, it is not possible to deduce in which tissue insulin resistance initially manifests.
CBX also inhibits 11βHSD2. However, 11βHSD2 is not expressed in the liver and its renal expression is confined to the distal nephron which alone is unlikely to account for the substantial metabolic impacts observed. Importantly, a crucial, specific role for 11βHSD1 is underlined by the protective metabolic phenotype observed in the uremic 11βHSD1−/− mice and the fact that a specific 11βHSD1 inhibitor (UE2316) (32), which has no activity against 11βHSD2, also improved glucose tolerance and insulin sensitivity in uremic rats.
The mechanism underlying elevated 11βHSD1 in uremia has not been fully elucidated, but may involve inflammation. Consistent with observations in CKD patients, serum levels of IL-6, TNFα, and IL-1β were elevated in both models of uremia, likely as a result of decreased renal cytokine clearance and increased systemic oxidative stress (40, 41). TNFα and IL-1β induce transcription of hepatic and adipose 11βHSD1 via a mechanism mediated by p38 and C/EBPα signaling (21, 42), suggesting a potential mechanism for up-regulation of 11βHSD1 in uremia. Proinflammatory cytokines can also impair insulin signaling. However, because CBX corrects insulin resistance without changes in plasma cytokine levels, this rules out a direct role for proinflammatory cytokine-induced insulin resistance.
In conclusion, we provide evidence that hyperinsulinemia and insulin resistance in CKD can occur through a mechanism mediated by elevated 11βHSD1. These changes occurred without increased systemic corticosterone levels, suggesting that an intrahepatic Cushing-type syndrome mediates insulin resistance in CKD. Selective inhibitors of 11βHSD1, already progressed to clinical trials for type 2 diabetes and obesity, are potential therapeutic strategies for management of the common metabolic abnormalities in CKD.
Methods
Rat Models of Uremia.
Chronic experimental uremia was studied using two distinct rat models. For subtotal SNx, uremia was induced in male Wistar rats (7 wk old, eight per group; Charles River) using an established two-stage surgical procedure for a period of 4 wk (43). Controls were sham operated by removing the renal capsule and replacing the intact kidney. Separately, uremia was induced using dietary manipulation. Male Wistar rats (7 wk old, eight per group) were fed a high-adenine (0.75% in standard rat chow) diet or standard rodent diet for 2 wk (Lillico Biotechnology). CBX (50 mg⋅kg−1⋅d−1; 2 wk in saline) or vehicle (saline) was administered to both uremic models by oral gavage, giving four SNx model groups [(i) SNx, (ii) SNx + CBX, (iii) sham operated (S), and (iv) S + CBX] and four adenine-fed model groups [(i) adeinine (Ad), (ii) Ad + CBX, (iii) control (CON), and (iv) CON + CBX]. For specific 11βHSD1 inhibitor studies, 2-wk adenine-fed rats were administered UE2316 (20 mg⋅kg−1⋅d−1) or vehicle (50:50 DMSO/PEG-400) equivalent for 2 wk by s.c. minipump (Alzet) to create three groups [(i) CON + vehicle, (ii) Ad + vehicle, and (iii) Ad + UE2316].
For all experimental models, after 4 wk, animals were fasted overnight and killed between 0800 and 1000 hours. Blood was obtained immediately via cardiac puncture and centrifuged to obtain plasma, whereas liver, skeletal muscle, and epididymal white adipose tissue were snap-frozen in liquid nitrogen for protein and mRNA analyses. For all rodent studies, animals were maintained on standard chow on a 12 h light/12 h dark cycle. All animal experiments were conducted in accordance with the UK Home Office Animals (Scientific Procedures), 1986 (44), with Queen Mary Ethics of Research Committee approval.
11βHSD1−/− Mice.
Male progeny of mice with targeted global disruption of the 11βHSD1−/− gene congenic on C57BL/6J were derived as described previously (33). Controls were WT, C57BL/6J, age-matched males. Adult 8-wk old WT and 11βHSD1−/− (six to eight per group) were fed a control or 0.25% adenine diet for 4 wk. Mice were fasted overnight and killed at approximately 9:00 AM, within 1 min of disturbing each cage, or used in dynamic physiological studies.
Quantification of Renal Injury.
Plasma and urine levels of urea, creatinine, Na+, and K+ along with urinary protein were measured commercially using a dry-slide automated analyzer (Idexx Laboratories).
Glucose, Pyruvate, and Insulin Tolerance Test.
For GTT and PTT, animals were fasted overnight and injected i.p. with 2 g per kg body weight of 25% (wt/vol) dextrose (Sigma) or sodium pyruvate (Sigma), respectively. Blood glucose (tail vein) was measured (Accucheck) at 0–120 min and additional blood was collected in a heparinized tube at 0–45 min for measurement of insulin concentration. For ITT, animals were fasted overnight and injected i.p. with 2 units per kg body weight of porcine insulin (Intervet). Blood glucose (tail vein) was measured (Accucheck) at 0–45 min.
Metabolite Measurements.
Serum glucose was measured using a colorimetric glucose oxidase assay based on the method described (45). Plasma insulin (Crystal Chem), corticosterone (Cambridge Biosciences), and IL-6, TNFα, and IL-1β (all R&D Systems) levels were determined by specific ELISA. Plasma levels of cholesterol and triglycerides were measured commercially using a dry-slide automated analyzer (Idexx Laboratories). Plasma NEFA levels were measured by colorimetric assay kit (ZenBio, Inc.). Liver and skeletal muscle triglyceride levels were determined using a Fluorometric Triglyceride Assay kit (Cayman Chemical).
Quantitative RT-PCR.
All gene expression was measured using quantitative RT-PCR (qRT-PCR), according to the procedure previously described (46) via Taqman or Sybr green methodology using specific primers (all Eurogentec; Table S7) designed with Primer Express 2.0 Software (Applied Biosystems). Relative changes in gene expression were determined by standard curve methodology normalized against 18S RNA (Applied Biosystems).
Immunoblotting.
Immunoblotting was conducted as previously described (47). Primary antibodies against 11βHSD1, PCK1, G6Pase (Abcam), ACC, FASN, phospho(Ser473)-AKT, total AKT (Cell Signaling Technologies), and PGC1α (SCBT) were used in this study. Reference protein measurements were made with mouse monoclonal anti–β-actin (clone AC-15; Sigma).
Hepatic Glucocorticoid Levels and 11βHSD1 Reductase Activity.
The Hepatic glucocorticoid levels and 11βHSD1 reductase activity were measured as previously described (48). Full methodology is discussed in SI Methods.
Statistical Analysis.
Results are expressed as mean ± SEM. Statistical comparisons were obtained using GraphPad Prism Version 5. Statistical differences were calculated using either an unpaired Student t test or one-way ANOVA followed by a Fisher’s posttest, where appropriate.
Supplementary Material
Acknowledgments
The authors thank Dr. S. W. Brouilette (Queen Mary University of London) for his help with qRT-PCR. This work was partly funded by the Barts and the London National Institute of Health Research Cardiovascular Biomedical Research Unit. P.W.C. is the recipient of a European Foundation for the Study of Diabetes/Lilly research fellowship. S.P.W., B.R.W., and J.R.S. are recipients of a Wellcome Trust Seeding Drug Discovery Award for development of UE2316 and acknowledge the support of the British Heart Foundation Centre of Research Excellence.
Footnotes
Conflict of interest statement: S.P.W., B.R.W., and J.R.S. are inventors on patents owned by the University of Edinburgh, which claim therapeutic use of compounds, including UE2316.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312436111/-/DCSupplemental.
References
- 1.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: Common, harmful, and treatable—World Kidney Day 2007. Clin J Am Soc Nephrol. 2007;2(2):401–405. doi: 10.2215/CJN.04041206. [DOI] [PubMed] [Google Scholar]
- 2.Kaysen GA. Disorders in high-density metabolism with insulin resistance and chronic kidney disease. J Ren Nutr. 2007;17(1):4–8. doi: 10.1053/j.jrn.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Stefanović V, Nesić V, Stojimirović B. Treatment of insulin resistance in uremia. Int J Artif Organs. 2003;26(2):100–104. doi: 10.1177/039139880302600202. [DOI] [PubMed] [Google Scholar]
- 4.Fliser D, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53(5):1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 5.Shinohara K, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(7):1894–1900. doi: 10.1097/01.asn.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura M, et al. Insulin resistance and impaired myocardial fatty acid metabolism in dialysis patients with normal coronary arteries. Kidney Int. 2006;69(3):553–559. doi: 10.1038/sj.ki.5000100. [DOI] [PubMed] [Google Scholar]
- 7.Bodlaj G, Berg J, Pichler R, Biesenbach G. Prevalence, severity and predictors of HOMA-estimated insulin resistance in diabetic and nondiabetic patients with end-stage renal disease. J Nephrol. 2006;19(5):607–612. [PubMed] [Google Scholar]
- 8.Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 1994;91(19):9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, et al. Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem. 2002;277(26):23301–23307. doi: 10.1074/jbc.M200964200. [DOI] [PubMed] [Google Scholar]
- 10.Yoon JC, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 11.Wamil M, Seckl JR. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov Today. 2007;12(13-14):504–520. doi: 10.1016/j.drudis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997;349(9060):1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 13.Chapman KE, Seckl JR. 11beta-HSD1, inflammation, metabolic disease and age-related cognitive (dys)function. Neurochem Res. 2008;33(4):624–636. doi: 10.1007/s11064-007-9504-9. [DOI] [PubMed] [Google Scholar]
- 14.Paterson JM, et al. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci USA. 2004;101(18):7088–7093. doi: 10.1073/pnas.0305524101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingstone DE, Walker BR. Is 11beta-hydroxysteroid dehydrogenase type 1 a therapeutic target? Effects of carbenoxolone in lean and obese Zucker rats. J Pharmacol Exp Ther. 2003;305(1):167–172. doi: 10.1124/jpet.102.044842. [DOI] [PubMed] [Google Scholar]
- 16.Walker BR, Connacher AA, Lindsay RM, Webb DJ, Edwards CR. Carbenoxolone increases hepatic insulin sensitivity in man: A novel role for 11-oxosteroid reductase in enhancing glucocorticoid receptor activation. J Clin Endocrinol Metab. 1995;80(11):3155–3159. doi: 10.1210/jcem.80.11.7593419. [DOI] [PubMed] [Google Scholar]
- 17.Andrews RC, Rooyackers O, Walker BR. Effects of the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab. 2003;88(1):285–291. doi: 10.1210/jc.2002-021194. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, et al. Reduction of hepatic glucocorticoid receptor and hexose-6-phosphate dehydrogenase expression ameliorates diet-induced obesity and insulin resistance in mice. J Mol Endocrinol. 2008;41(2):53–64. doi: 10.1677/JME-08-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whorwood CB, Sheppard MC, Stewart PM. Licorice inhibits 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid levels and potentiates glucocorticoid hormone action. Endocrinology. 1993;132(6):2287–2292. doi: 10.1210/endo.132.6.8504732. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson JW, et al. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 21.Ignatova ID, et al. Tumor necrosis factor-alpha upregulates 11beta-hydroxysteroid dehydrogenase type 1 expression by CCAAT/enhancer binding protein-beta in HepG2 cells. Am J Physiol Endocrinol Metab. 2009;296(2):E367–E377. doi: 10.1152/ajpendo.90531.2008. [DOI] [PubMed] [Google Scholar]
- 22.Escher G, Galli I, Vishwanath BS, Frey BM, Frey FJ. Tumor necrosis factor alpha and interleukin 1beta enhance the cortisone/cortisol shuttle. J Exp Med. 1997;186(2):189–198. doi: 10.1084/jem.186.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, et al. Enhancement of cortisol-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by interleukin 1beta in cultured human chorionic trophoblast cells. Endocrinology. 2006;147(5):2490–2495. doi: 10.1210/en.2005-1626. [DOI] [PubMed] [Google Scholar]
- 24.D’Apolito M, et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest. 2010;120(1):203–213. doi: 10.1172/JCI37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16(4):400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: Mutational analysis of a membrane protein. Annu Rev Genet. 1990;24:133–170. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- 28.Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimomura I, et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96(24):13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44(3):230–234. doi: 10.1159/000183992. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol. 2009;296(6):F1297–F1306. doi: 10.1152/ajprenal.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobice DF, et al. Mass spectrometry imaging for dissecting steroid intracrinology within target tissues. Anal Chem. 2013;85(23):11576–11584. doi: 10.1021/ac402777k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotelevtsev Y, et al. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA. 1997;94(26):14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenfeld S, Garber AJ. Abnormal carbohydrate metabolism in chronic renal failure. The potential role of accelerated glucose production, increased gluconeogenesis, and impaired glucose disposal. J Clin Invest. 1978;62(1):20–28. doi: 10.1172/JCI109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaefer RM, et al. Normalization of enhanced hepatic gluconeogenesis by the antiglucocorticoid RU 38486 in acutely uraemic rats. Eur J Clin Invest. 1990;20(1):35–40. doi: 10.1111/j.1365-2362.1990.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Valadés AG, et al. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 2008;57(8):2199–2210. doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez-Valadés AG, et al. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol Ther. 2006;13(2):401–410. doi: 10.1016/j.ymthe.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 2007;104(31):12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA. 2010;107(7):3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y, Peake PW, Kelly JJ. Should we quantify insulin resistance in patients with renal disease? Nephrology (Carlton) 2005;10(6):599–605. doi: 10.1111/j.1440-1797.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 41.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9(5):255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 42.Esteves CL, et al. Regulation of adipocyte 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) by CCAAT/enhancer-binding protein (C/EBP) β isoforms, LIP and LAP. PLoS ONE. 2012;7(5):e37953. doi: 10.1371/journal.pone.0037953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harwood SM, et al. Calpain is activated in experimental uremia: Is calpain a mediator of uremia-induced myocardial injury? Kidney Int. 2003;63(3):866–877. doi: 10.1046/j.1523-1755.2003.00823.x. [DOI] [PubMed] [Google Scholar]
- 44. Home Office (2013) Draft Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 (as amended). Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/116843/aspa-draft-guidance.pdf. Accessed July 18, 2013.
- 45.Caton PW, Nayuni NK, Murch O, Corder R. Endotoxin induced hyperlactatemia and hypoglycemia is linked to decreased mitochondrial phosphoenolpyruvate carboxykinase. Life Sci. 2009;84(21-22):738–744. doi: 10.1016/j.lfs.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Douthwaite JA, Lees DM, Corder R. A role for increased mRNA stability in the induction of endothelin-1 synthesis by lipopolysaccharide. Biochem Pharmacol. 2003;66(4):589–594. doi: 10.1016/s0006-2952(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 47.Caton PW, Nayuni NK, Khan NQ, Wood EG, Corder R. Fructose induces gluconeogenesis and lipogenesis through a SIRT1-dependent mechanism. J Endoncrinol. 2011;208(3):273–283. doi: 10.1530/JOE-10-0190. [DOI] [PubMed] [Google Scholar]
- 48.Hu A, et al. Th2 cytokine-induced upregulation of 11beta-hydroxysteroid dehydrogenase-1 facilitates glucocorticoid suppression of proasthmatic airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol. 2009;296(5):L790–L803. doi: 10.1152/ajplung.90572.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.