Abstract
Quantitative reductions in T-cell receptor (TCR) signalling are associated with severe immunodeficiency, yet in certain cases can lead to autoimmunity. Mutation of the tyrosine kinase ZAP-70 can cause either of these outcomes, yet the limits of its signal transducing capacity are not well defined. To investigate these limits we have made use of mrtless: a chemically induced mutation of Zap70 associated with T-cell deficiency. Unlike cells devoid of ZAP-70, mrtless thymocytes showed partial induction of CD5 and CD69, and were sensitive to TCR stimulation with a dose–response shifted approximately 10-fold. However, essentially no T cells were able to compensate for the mrtless mutation and mature beyond the CD4+ CD8+ stage. This outcome contrasts with a ZAP-70 Src Homology 2 domain mutant strain, where high-affinity self-reactive TCR are positively selected rather than deleted. We discuss these data with respect to current models of TCR signalling in thymocyte selection.
Keywords: genetics, T cells, thymic selection
Introduction
Tyrosine kinases are essential for T-cell antigen recognition, and propagate signals from the T-cell receptor (TCR) by phosphorylating and activating downstream substrates. The two most critical tyrosine kinases involved in TCR signalling are ZAP-70 and Lck. Lck is recruited to the TCR by CD4 and CD8 co-receptors, activating ZAP-70 by direct phosphorylation and by creating docking sites on CD3 subunits to which the tandem Src Homology 2 (SH2) domains of ZAP-70 bind. Mice lacking ZAP-70 exhibit a complete arrest of αβ T-cell development at the CD4+ CD8+ stage: a point at which cells are selected for TCRs with low avidity recognition of self-peptide/MHC complexes.1–3 Residual development of CD4+ CD8− T cells occurs in ZAP-70-deficient humans as a result of the compensatory action of the related kinase SYK,4 although these cells are unresponsive to TCR stimulation.4–7 Mice deficient for both ZAP-70 and Syk have an arrest of T-cell development at the double-negative 3 (DN3, CD4− CD8− CD44− CD25+) stage, where thymocytes are selected for productive rearrangement of the Tcrb locus.8
Null alleles of ZAP-70 and other TCR signalling molecules prevent T-cell maturation, but much less is known about how inherited variants causing quantitative changes in TCR signalling affect T-cell function. This is significant from both a mechanistic and a clinical perspective, because subtle single amino acid substitutions represent the most common functional genetic variation in humans. In the case of human ZAP70, 0·338% of alleles in a cohort of 6503 exomes were found to be missense variants (http://evs.gs.washington.edu/EVS/), and more than a quarter of these are predicted to be damaging (14/44 annotated as probably damaging by polyphen-2). At these frequencies, more than one person in every 10 000 could be expected to inherit a combination of deleterious mutations in ZAP70, although which of these combinations will confer a clinical phenotype is more difficult to predict.9
It is clear from studies of null mutations in mouse and man that 50% of normal ZAP-70 activity is well tolerated, whereas an absence of activity leads to severe immunodeficiency. Activity between 0% and 50%, on the other hand, is often associated with unusual combinations of immunodeficiency and autoimmunity.10–15 Quantitative differences in TCR signalling are also known to play a central role in establishing the correct repertoire of circulating T cells:16 TCRs with high avidity for self-peptide/MHC trigger thymocyte negative selection, TCRs with intermediate avidity trigger positive selection, and TCRs with avidity that is too low fail to support thymocyte survival and maturation. Consistent with a quantitative model for signalling negative versus positive selection, strongly self-reactive TCRs have been shown to escape negative selection and instead undergo positive selection in mice with an amino acid substitution in the SH2 domain of ZAP-70.11 By contrast, the catalytic domain mutant described here can still partially transduce TCR signals, yet does not allow appreciable numbers of T cells to mature.
Materials and methods
Mice
Zap70mrt (MGI:3614790) was derived from a C57BL/6 male mouse treated intraperitoneally with 100 mg/kg N-ethyl-N-nitrosourea at 3-weekly intervals. Mutation mapping and DNA sequencing were performed as previously described.17 Zap70tm1Weis (Zap70−/−, MGI:2176252), NOD.H2k and 3A9 TCR transgenic (MGI:3512685) mice have been described previously,3,18,19 and B10.Br/SgSnJ were obtained from The Jackson Laboratory. Mice were housed in a specific pathogen-free facility and all studies were conducted in compliance with protocols approved by the Animal Ethics and Experimentation Committee of The Australian National University.
Genotyping
The mrtless strain was maintained by breeding heterozygous carriers. Heterozygotes and homozygotes were identified by mutagenically separated PCR.20 Primers used were 5′-CCGCAATGTTCTACTGGTCA-3′ (sense), 5′-GAAAGTTGATGCACTCTGGCGCGTATCA-3′ (antisense wild-type) and 5′-CACTGCGGCTGGAGAACTTCCTCAAGTTGATGCACTCTGGCGCGTGCCG-3′ (antisense mutant). Amplification consisted of an initial 2 min at 94°; followed by 35 cycles of 20 seconds at 94°, 20 seconds at 62° and 30 seconds at 72°. The final cycle included an additional 10 min at 72°.
Flow cytometry
Blood was collected from the retro-orbital plexus in 10 μl of 500 mm EDTA. Red blood cells were lysed by addition of Tris–ammonium chloride, and the remaining cells were washed in PBS supplemented with 2% fetal calf serum. Suspensions of blood, thymus or spleen were stained with FITC-conjugated αCD4 (GK1.5), αCD45.1 (A20), αCD8 (53-6.7), αIgM (II/41) or αThy1.2 (53-2.1); phycoerythrin-conjugated αCD4 (GK1.5), αCD8 (53-6.7) or αIgD (IA6-2) (BD), or biotinylated αCD21 (C. Goodnow), αCD45.1 (A20), αCD45.2 (104), αCD5 (53-7.3), αCD69 (H1.2F3) or αTCR-β (H57-597) (Becton Dickinson, Franklin Lakes, NJ) followed by streptavidin Cy-chrome. Data were acquired on a FACSCalibur (Becton Dickinson) and analysed with flowjo software (Treestar, Ashland, OR).
Western blotting
Thymocytes were sorted using a FACSVantage SE with DiVa Option (Becton Dickinson). Cell lysates were prepared in nonidet P-40 lysis buffer with sodium fluoride, PMSF, leupeptin hemisulphate, phenylarsine oxide, aprotinin and sodium orthovanadate. Samples were separated by SDS–PAGE using standard protocols and Western blotting was performed using a purified mouse monoclonal antibody against ZAP-70 (provided by A. Weiss), followed by goat anti-mouse horseradish peroxidase (ICN Biomedicals, Costa Mesa, CA). Blots were visualized by chemiluminescence (Amersham, Little Chalfont, UK).
Proliferation assay
Ninety-six-well flat-bottom tissue culture plates (Greiner Bio-One, Frickenhausen, Germany) were coated with a solution of PBS + 5 μg/ml αCD3 (2C11) for 75 min at 37°. Then, 5 × 105 CFSE-labelled splenocytes in a solution of complete RPMI-1640 + 1 μg/ml αCD28 (37.51) were added to each well, and incubated for 72 hr at 37° in 5% CO2. After stimulation, cells were stained with CD4-peridinin chlorophyll protein-Cy5.5, CD8-phycoerythrin, CD69-phycoerythrin-Cy7 and Annexin V-allophycocyanin, and analysed by flow cytometry.
In vitro stimulation
The 96-well flat-bottom tissue culture plates (Corning Life Sciences, Corning, NY) were coated overnight at 4° with αCD3 (2C11) or αTCR (H57-597) in PBS, then washed and coated overnight with αCD28 (37.51). Thymocytes were resuspended in complete RPMI-1640, plated at 5 × 105 cells/well and incubated for 20 hr at 37° 5% CO2. After stimulation, cells were stained for CD4, CD8 and CD69 and analysed by flow cytometry.
In vitro stimulation with hen egg lysozyme
Splenocytes were collected from a B10.Br mouse and irradiated (20 000 rads). Cells were plated on 96-well flat-bottom tissue culture plates and incubated for 6 hr at 37° 5% CO2 in the presence of hen egg lysozyme (HEL) 46-61 peptide (1 μm). Thymocyte suspensions were then added to the plates and incubated for 15 hr at 37°, stained for CD4, CD8 and CD69, and analysed by flow cytometry.
In vivo stimulation with HEL
Mice on an H2k/b background expressing the 3A9 TCR transgene were injected intravenously with 1 mg HEL (Sigma, St Louis, MO) in PBS, or with PBS alone. Thymi were collected the following day, stained for CD4, CD8 and CD69, and analysed by flow cytometry. Where appropriate, B10.Br mice (H2k/k) were used as wild-type controls.
Bone marrow chimeras
C57BL/6 CD45.1+ recipient mice were given two doses of irradiation (500 rads each) separated by a 3-hr interval, then injected with 2 × 106 bone marrow cells through the tail vein. Recipient mice were killed for analysis 10 weeks later.
Results
Identification of a Zap70 missense mutation
In an investigation of genes required for lymphocyte development, blood samples from third-generation (G3) offspring of N-ethyl-N-nitrosourea-treated C57BL/6J sires were analysed by flow cytometry.21 Within one pedigree, multiple individuals were found to lack CD4+ and CD8+ cells (Fig. 1a). Further breeding established that the phenotype, named mrtless (mrt), was inherited in an autosomal recessive manner.
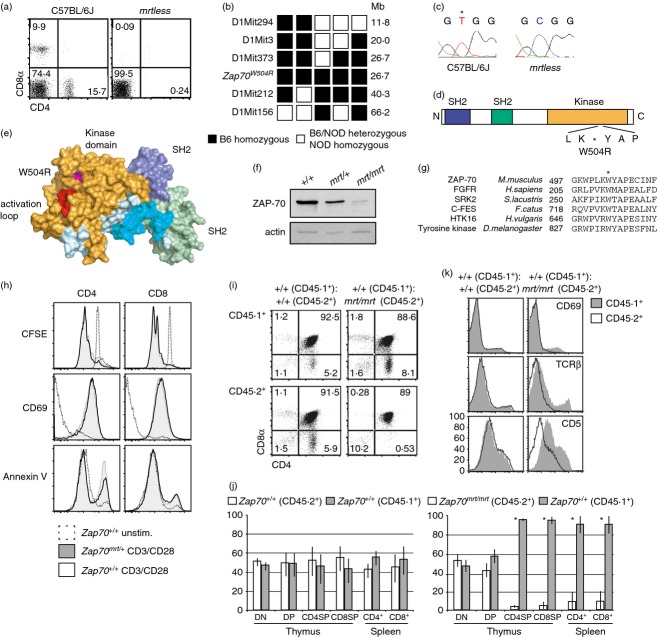
Figure 1.
A loss-of-function missense variant of Zap70. (a) Flow cytometric analysis of peripheral blood lymphocytes from wild-type C57BL/6J and mrtless mice. (b) Individual F2 intercross (F2IC) mice with the mrtless phenotype were typed for the markers shown. Haplotypes of key recombinant F2IC mice are displayed in columns. Black squares represent C57BL/6 homozygosity, while white squares represent C57BL/6-NOD heterozygosity or homozygosity. (c) Sequencing the cDNA of mrtless thymocytes identified a T to C transition at nucleotide 1618 of Zap70. (d) Schematic showing the mrtless mutation within the ZAP-70 protein. (e) Structural representation of ZAP-70 (PDB:2OZO), with W504 highlighted in magenta, and activation loop (CGNFGSVR) in red. (f) ZAP-70 Western blot on FACS-purified CD4+ CD8+ thymocyte lysates from mice of the indicated genotypes. (g) Cross-species kinase domain sequence alignment. Asterisk indicates the tryptophan mutated in the mrtless pedigree. (h) CFSE-labelled splenocytes from Zap70+/+ and Zap70mrt/+ mice were cultured in the presence or absence of anti-CD3 and anti-CD28 antibodies for 72 hr. Representative histograms (n = 4) show CFSE dilution and surface marker expression on CD4+ and CD8+ cells. (i,j) Sub-lethally irradiated CD45.1+ mice were transplanted with a 1 : 1 mixture of either Zap70+/+ and Zap70+/+ bone marrow, or Zap70+/+ and Zap70mrt/mrt bone marrow, and percentages of cells derived from each donor were established by flow cytometry. Bars in (j) indicate mean ± standard deviation, n = 3. (k) Histograms comparing CD69, T-cell receptor-β and CD5 expression upon CD45.1+ cells (solid histogram) and CD45.2+ cells (open histograms). Asterisk indicates statistical significance as determined by two-tailed Student's t-test (P < 0·05). Data are representative of one (i–k), two (f, h) or more than two (a–c) independent experiments.
A mapping cross was performed between C57BL/6J mrtless and NOD.H2k mice to determine the chromosomal location of the mrtless mutation. Of the 213 F2 intercross (F2IC) mice generated, 55 (25·8%) exhibited the mrtless phenotype, consistent with a fully penetrant recessive trait. Pooled DNA from the F2IC was tested for linkage using a genome-wide panel of microsatellite markers, linking the T-cell-deficient phenotype to a marker on chromosome 1. DNA samples from individual F2IC mice were then typed at additional microsatellite markers within the linked region, narrowing the mutation-containing interval to 13·6 Mb between D1Mit373 and D1Mit212 (Fig. 1b). Within this interval lay Zap70: a strong candidate given its known role in TCR signalling. Zap70 cDNA from mrtless and C57BL/6 thymocytes was sequenced, and comparison revealed a T to C transition at nucleotide 1618 of Zap70 (Fig. 1c) corresponding to a W504R amino acid substitution (Fig. 1d). W504 is located within the kinase domain of ZAP-70, adjacent to the catalytic activation loop (Fig. 1e), and is highly conserved among tyrosine kinases (Fig. 1g). Western blotting of sorted CD4+ CD8+ thymocyte lysates demonstrated that Zap70mrt/mrt mice did indeed express ZAP-70 protein, although at greatly diminished quantities compared with Zap70+/+ controls. This reduction may be due to the structural instability of ZAP-70 introduced by the W504R substitution, which may also affect the catalytic activity of the residual protein. Zap70mrt/+ CD4+ CD8+ thymocytes had a ˜ 50% reduction in expression of ZAP-70 compared with wild-type (Fig. 1f), despite having no obvious impairment in T-cell proliferation, CD69 induction, or stimulation-induced phosphatidylserine expression (Fig. 1h). Nevertheless, heterozygosity for the Zap70mrt allele was sufficient to partially disrupt intracellular calcium flux in splenic T cells.12,15
To confirm that the block in T-cell development originated from T-cell-intrinsic defects in ZAP-70 function, mixed bone marrow chimeras were established to allow a direct comparison of CD45.2+ cells (from Zap70mrt/mrt or Zap70+/+ donors), and control CD45.1+ cells developing within the same animal (Fig. 1i). Equal proportions of CD45.1+ and CD45.2+ CD4− CD8− thymocytes confirmed the establishment of balanced haematopoietic chimerism (Fig. 1j). In Zap70mrt/mrt mixed chimeras, however, there was a near complete absence of CD45.2+ CD4+ CD8−, CD4− CD8+ and peripheral CD4+ or CD8+ cells (Fig. 1i,j). This demonstrated that the mrtless phenotype originated from a T-cell-intrinsic developmental arrest at the CD4++ CD8+ stage, because very few Zap70mrt/mrt T cells were able to develop even under conditions where wild-type T cells could mature fully.
Expression of CD69, TCR-β and CD5 was also reduced on Zap70mrt/mrt thymocytes in mixed bone marrow chimeras (Fig. 1k). Few Zap70mrt/mrt-derived thymocytes expressed CD69, whereas wild-type cells developing in the same thymus contained a normal subset of CD69+ cells. Expression of TCR-β and CD5 was also diminished on Zap70mrt/mrt cells compared with wild-type cells in the same thymus, indicating that reduced induction of CD69, CD5 and TCR-β were all phenotypes intrinsic to Zap70mrt/mrt T cells.
Zap70mrt/mrt mice are phenotypically distinct to Zap70−/−
Zap70mrt/mrt mice had normal numbers of CD4− CD8− and CD4+ CD8+ thymocytes, but an almost complete lack of CD4+ CD8− or CD4− CD8+ cells in the thymus and spleen: a phenotype equivalent to that observed in Zap70−/− mice (Fig. 2a,b). In response to pre-TCR and TCR signalling, CD4+ CD8+ thymocytes increase expression of CD3, CD5, CD69 and TCR-β before becoming a single-positive CD4+ or CD8+ cell.22 Zap70mrt/mrt CD4+ CD8+ thymocytes showed weak expression of CD69, were devoid of CD3εhi and TCR-βhi cells, and CD5 expression was markedly lower than Zap70+/+ controls (Fig. 2c,d). Compared with Zap70−/− CD4+ CD8+ cells, however, CD5 and CD69 were still partially induced on Zap70mrt/mrt CD4+ CD8+ cells (Fig. 2c,d), confirming that these responses were ZAP-70-dependent and that the ZAP-70W504R mutant kinase was partially capable of supporting this response.
Figure 2.
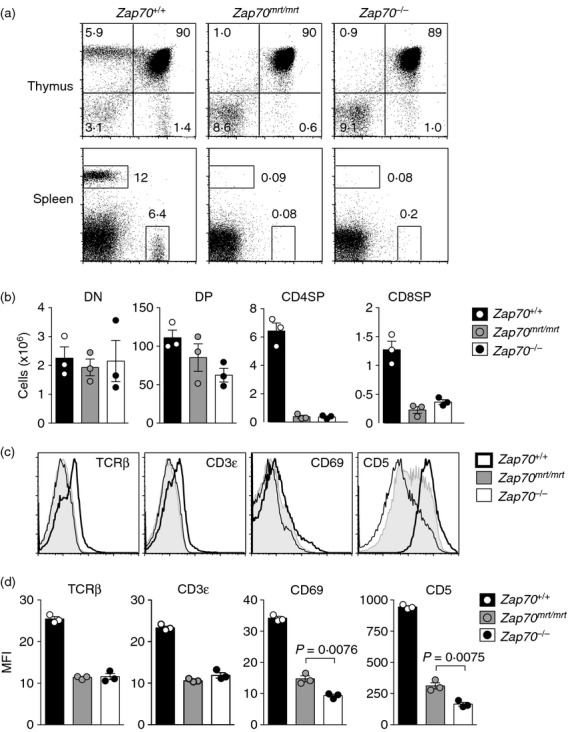
Evidence for partial ZAP-70 function in Zap70mrt/mrt mice. Flow cytometric analysis of thymic and splenic lymphocytes, with numbers indicating the percentages of total lymphocytes (a) or absolute numbers (b). (c, d) Comparison of surface marker expression upon CD4+ CD8+ thymocytes from Zap70+/+ (thick black line), Zap70mrt/mrt (solid grey histogram) and Zap70−/− (thin black line) mice. Bars in (b) and (d) represent standard error of the mean, and P-values were calculated by two-tailed Student's t-test. Data are from one independent experiment (n = 3).
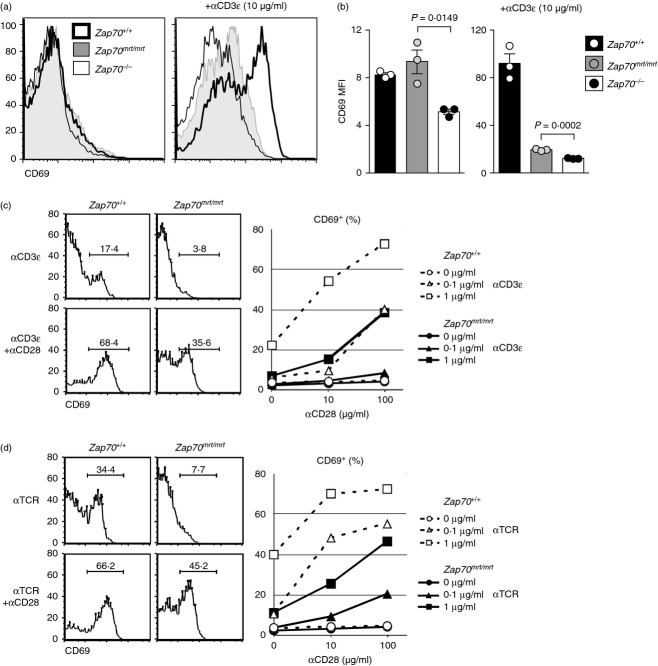
Zap70mrt reduces, but does not abolish, responsiveness to TCR-derived signals
As T-cell development was intrinsically dysfunctional in Zap70mrt/mrt mice, it was of interest to assess the consequences of TCR/CD28 stimulation. Surprisingly, Zap70mrt/mrt CD4+ CD8+ thymocytes increased surface expression of CD69 in response to stimulation by αCD3 and αTCR antibodies in vitro, and did so to a greater extent than Zap70−/− cells (Fig. 3a,b). The response of Zap70mrt/mrt CD4+ CD8+ thymocytes was nevertheless lower than Zap70+/+ controls at any given dose and combination of αCD3/αTCR and αCD28 (Fig. 3c,d). For instance, the CD28 dose–response of Zap70mrt/mrt cells to 1 μg/ml αCD3 resembled that of Zap70+/+ cells to 0·1 μg/ml αCD3 (Fig. 3c).
Figure 3.
Shifted dose–response kinetics following T-cell receptor (TCR) stimulation in vitro. Thymocytes were stimulated for 20 hr in culture wells coated with anti-CD3 (a–c) or anti-TCR (d) in the presence or absence of anti-CD28, and the CD4+ CD8+ thymocyte response was examined by median CD69 expression (a) or by the percentage of cells that were CD69 positive (c,d). (a) Representative histogram of CD69 expression on Zap70+/+ (filled), Zap70mrt/mrt (open, solid line) and Zap70−/− (open, broken line) after stimulation with 1 μg/ml anti-CD3 + 1 μg/ml anti-CD28. Bars in (b) represent standard error of the mean, and P-values were calculated by two-tailed Student's t-test. Data are representative of one (a, b) or two (c) independent experiments.
To determine the effects of the mrtless mutation upon antigen-specific responses, Zap70mrt/mrt mice were bred with mice transgenic for the α-and β-chains of the 3A9 TCR, which recognizes a HEL-derived peptide in the presence of H2k.19 3A9 transgenic Zap70mrt/mrt;H2k/b mice maintained the blockage in T-cell development at the CD4+ CD8+ stage, with very few CD4+ CD8− or CD4− CD8+ thymocytes present (Fig. 4a). Expression of TCR-β was higher and more uniform on CD4+ CD8+ thymocytes from TCR transgenic mice, and was reduced by approximately 50% in Zap70mrt/mrt thymocytes. CD5 expression was 80% lower on transgenic Zap70mrt/mrt CD4+ CD8+ thymocytes compared with controls (Fig. 4b). After a 15-hr stimulation with irradiated B10.Br (H2k) splenocytes loaded with HEL antigen, CD69 was induced on both Zap70mrt/mrt and Zap70mrt/+ thymocytes, although Zap70mrt/mrt cells had a mildly reduced response compared with Zap70mrt/+ thymocytes (Fig. 4c).
Figure 4.
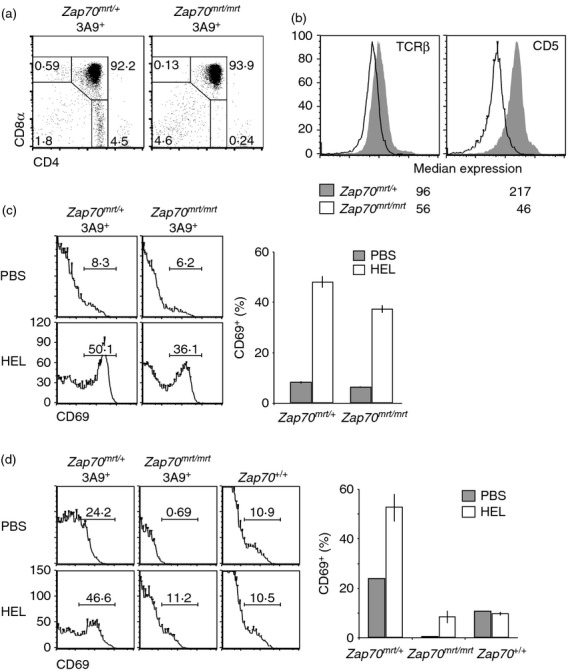
Effects of the Zap70mrt/mrt mutation on T cells with a defined T-cell receptor (TCR). (a) Flow cytometric analysis of thymocytes from 3A9 TCR transgenic mice (H2k/b) carrying the Zap70mrt mutation. (b) Representative histograms of TCR-β and CD5 expression on CD4+ CD8+ thymocytes from Zap70mrt/+ (solid grey histograms) and Zap70mrt/mrt transgenic mice (unfilled histograms) (n = 2). (c) Thymocytes were stimulated in vitro for 15 hr with irradiated B10.Br (H2k/k) splenocytes in the presence or absence of hen egg lysozyme (HEL) peptide, and flow cytometry was performed to measure CD69 induction on CD4+ CD8+ thymocytes. Representative histograms (left) and graphs (right) indicate mean ± standard deviation of CD69+ cells after no stimulation (grey bars, n = 2) or HEL stimulation (white bars, n = 2). (d) Mice were injected intraperitoneally with PBS, or 1 mg HEL in PBS, and thymi were collected 15 hr later. Flow cytometry was performed as in (c). Grey bars represent PBS control stimulation (n = 1), white bars represent HEL stimulation (n = 2). Data are from one independent experiment.
Finally, to test antigen-specific responses in vivo, H2k/k TCR transgenic Zap70mrt/mrt or Zap70mrt/+ mice and Zap70+/+ controls were injected intraperitoneally with HEL in PBS, or with PBS alone. While HEL treatment had no measurable effect in non-transgenic Zap70+/+ controls, it led to a strong induction of CD69 expression on Zap70mrt/+ CD4+ CD8+ thymocytes. Consistent with the polyclonal dose–response shift, CD69 induction was 80% lower in Zap70mrt/mrt transgenic mice (Fig. 4d).
Discussion
Mutation of the human ZAP70 gene (including those within the conserved catalytic domain), can abolish ZAP-70 activity and severely impair T-cell development.4,6,7,23 Comparison with the published structure of the ZAP-70 kinase domain indicated that the mutated residue described here (ZAP-70W504R) lies adjacent to the active site, and as such is likely to influence catalytic function.24 A > 90% decrease in steady-state ZAP-70 protein in Zap70mrt/mrt mice was similar to that observed in mice homozygous for the Zap70st (R464C) allele,2 suggesting that catalytic site mutations can also destabilize ZAP-70 protein.
The fact that TCR-β, CD3ε and CD5 levels are diminished on Zap70mrt/mrt CD4+ CD8+ cells, even in the presence of TCR-αβ transgenes, confirms that signalling through ZAP-70 is required to induce CD5 and maintain surface levels of TCR.2 Reduced TCR expression on CD4+ CD8+ cells may indicate either a failure to increase translation of CD3 subunits, which normally occurs as thymocytes mature, or increased activity of the Lck/Cbl/SLAP pathway that down-regulates surface TCR. Indeed, over-expression of ZAP-70 in Jurkat cells competitively antagonizes Lck/Cbl/SLAP-mediated TCR down-regulation,25 such that the reduction of ZAP-70 in Zap70mrt/mrt thymocytes may allow enhanced activity of the Cbl/SLAP pathway.
It was clear from comparison with Zap70−/− mice, however, that the Zap70mrt allele retained a limited degree of function. CD5 and CD69 expression were both higher on ex vivo CD4+ CD8+ thymocytes, as was induction of CD69 expression in response to TCR stimulation. Differences such as these were reminiscent of alleles encoding phosphoinositide-3-kinase γ, where homozygous knockout animals show immunodeficiency and severe cardiac pathology, while kinase-dead mutants are only immunodeficient.26 This difference has been ascribed to the ability of phosphoinositide-3-kinase γ to act as a macromolecular scaffold independently of its kinase activity. Similarly, while ZAP-70 kinase function and thymocyte maturation are presumably lost in both Zap70−/− and Zap70mrt/mrt animals, the retention of < 10% ZAP-70 protein with functional SH2-domains appears to be sufficient for partial induction of CD5 and CD69. Consistent with this, catalytic inactivation of ZAP-70 by a small molecule has revealed ZAP-70-dependent pathways that are entirely independent of its kinase activity, including regulatory T-cell suppression, and TCR-induced activation of the Rap1 GTPase.27
Given the ability of strong TCR agonists to induce CD69, and the evidence that a large proportion of newly rearranged TCRs have high avidity for self peptides,28 it is surprising that very few CD4+ CD8+ cells in Zap70mrt/mrt mice express CD69 at levels associated with positive selection, and that almost no TCRs can compensate for the signalling defect to mature into single-positive T cells and accumulate in the periphery. This failure of TCR selection to compensate for the Zap70mrt/mrt mutation is all the more surprising when compared with mice homozygous for the Zap70skg allele (W163C, in the proximal SH2 domain).11 Zap70skg homozygotes have a 50% decrease in the number of single positive thymocytes or peripheral cells, and animals develop lymphadenopathy, hypergammaglobulinemia and autoimmune arthritis.11 When crossed with TCR transgenic strains, the Zap70skg/skg mutation decreases the efficiency of positive selection of the DO11 and HY TCRs approximately 90% and 96%, respectively, allowing male-specific TCRs with high self-avidity to escape negative selection and instead be positively selected.11
It is informative to consider how the disparate consequences of Zap70mrt and Zap70skg mutations fit with current models for TCR-induced positive versus negative selection. A simple TCR/ZAP-70 threshold model, where weak signals induce positive selection and strong signals induce negative selection, would predict that strongly self-reactive TCRs would compensate for the diminished ZAP-70 in Zap70mrt/mrt thymocytes. To fit the different effects of the Zap70mrt and Zap70skg mutations with this model, one might postulate that the Zap70mrt mutation causes a quantitatively greater decrease in TCR signalling to all pathways. Hence, while many TCRs are able to compensate for the Zap70skg mutation and support positive selection, presumably by recognizing self-peptides with higher avidity, TCRs with sufficiently high self-avidity to compensate for the Zap70mrt mutation are rarely generated. In this view, it is surprising that CD69 and CD5 are still induced in Zap70mrt/mrt thymocytes, and that rare high-avidity cells do not homeostatically expand in the periphery of Zap70mrt/mrt mice.
Rather than quantitative thresholds for TCR/ZAP-70 signalling, an alternative explanation is that the Zap70mrt/mrt mutation causes qualitative and temporal changes in TCR signalling. These allow partial signalling to peptide exposure, which is sufficient for limited CD5 and CD69 induction in CD4+ CD8+ cells but insufficient to support the sustained signalling required for differentiation of CD4 and CD8 lineages.29,30 Qualitative differences could involve changes in the substrate specificity of ZAP-70W504R that prevent activation of particular signalling pathways. Alternatively, the temporal activity of the kinase may be altered such that it is unable to support positive selection due to increased turnover of the mutant protein, or because of saturating negative regulation by CD5, but can nevertheless deliver an acute burst of signalling for partial CD69 induction. One could also speculate that diminished amounts of ZAP-70 in Zap70mrt but not Zap70skg thymocytes allows enhanced inhibitory signalling through Lck-Cbl-SLAP. Since the Cbl-SLAP pathway inhibits positive selection through ZAP-70-dependent and-independent pathways,25 enhancement of this pathway may compound diminished signalling through ZAP-70W504R to bring about the failure of positive selection in Zap70mrt/mrt mice. A temporal sequence of TCR signalling in CD4+ CD8+ thymocytes has also been put forward as a mechanism for setting the bandwidth for TCR-positive selection, whereby TCR/ZAP-70 signals through the calcium/calcineurin/nuclear factor of activated T cells pathway are needed to enhance sensitivity of the CD4+ CD8+ cells to TCR/ZAP-70/extracellular signal-regulated kinase signalling, with both pathways necessary for positive selection.31 Failure to sustain TCR signalling through this sequence could explain the low induction of CD69 observed on Zap70mrt/mrt CD4+ CD8+ thymocytes compared with Zap70−/− thymocytes, yet the failure to induce CD69 to high levels. Which of these alternatives (if any) can explain the Zap70mrt phenotype remains to be seen.
In conclusion, our data reveal a lower limit for ZAP-70 activity in thymic positive selection. This limit is clearly above zero, as emphasized by the different outcomes of TCR stimulation in Zap70−/− and Zap70mrt/mrt thymocytes. Although it is not yet clear if residual ZAP-70W504R protein is catalytically active, or simply fills a cellular niche, it is nevertheless insufficient for the positive selection of thymocytes bearing low-and high-affinity TCRs alike. On the other hand, compound heterozygosity for ZAP-70W504R and ZAP-70I367F 12 or ZAP-70W504R and ZAP-70A243V15 is sufficient for positive selection, yet not for peripheral activation, whereas mutation of only one allele is sufficient for both. Given the diversity of rare variants in TCR signalling, and their association with unique physiological outcomes,13 the allelic series of Zap70 variants will help define the narrow boundaries between immunity, severe immunodeficiency and immune dysregulation.
Acknowledgments
CCG designed and supervised the study; OMS, ALY, SS and SL performed experiments; AL contributed reagents; and OMS and CCG wrote the paper. We thank A. Weiss and T. Kadlecek for Zap70−/− mice and anti-ZAP-70 monoclonal antibody, and the staff of the Australian Phenomics Facility for expert genotyping and animal husbandry. CCG is an Australian Research Council Federation Fellow, and was supported by grant R01 AI52127 from the National Institutes of Health-NIAID.
Glossary
- Lck
lymphocyte protein tyrosine kinase
- Syk
spleen tyrosine kinase
- SH2
Src Homology 2
- HEL
hen egg lysozyme
- Cbl
casitas B-lineage lymphoma
- SLAP
Src-like adaptor protein
- Rap1
RAS-related protein-1a
Disclosures
The authors declare that they have no competing financial interests.
References
- 1.Negishi I, Motoyama N, Nakayama K, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–8. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 2.Wiest DL, Ashe JM, Howcroft TK, et al. A spontaneously arising mutation in the DLAARN motif of murine ZAP-70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997;6:663–71. doi: 10.1016/s1074-7613(00)80442-2. [DOI] [PubMed] [Google Scholar]
- 3.Kadlecek TA, Oers van NS, Lefrancois L, et al. Differential requirements for ZAP-70 in TCR signaling and T cell development. J Immunol. 1998;161:4688–94. [PubMed] [Google Scholar]
- 4.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–58. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 5.Chu DH, Oers van NS, Malissen M, Harris J, Elder M, Weiss A. Pre-T cell receptor signals are responsible for the down-regulation of Syk protein tyrosine kinase expression. J Immunol. 1999;163:2610–20. [PubMed] [Google Scholar]
- 6.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–9. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 7.Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo WL, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AM, Negishi I, Anderson SJ, Chan AC, Bolen J, Loh DY, Pawson T. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc Natl Acad Sci USA. 1997;94:9797–801. doi: 10.1073/pnas.94.18.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews TD, Sjollema G, Goodnow CC. Understanding the immunological impact of the human mutation explosion. Trends Immunol. 2013;34:99–106. doi: 10.1016/j.it.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Jakob T, Köllisch GV, Howaldt M, et al. Novel mouse mutants with primary cellular immunodeficiencies generated by genome-wide mutagenesis. J Allergy Clin Immunol. 2008;121:179–184.e7. doi: 10.1016/j.jaci.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi N, Takahashi T, Hata H, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–60. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 12.Siggs OM, Miosge LA, Yates AL, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–26. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liston A, Enders A, Siggs OM. Unravelling the association of partial T-cell immunodeficiency and immune dysregulation. Nat Rev Immunol. 2008;8:545–58. doi: 10.1038/nri2336. [DOI] [PubMed] [Google Scholar]
- 14.Hsu L-Y, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009;206:2527–41. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauwe B, Tian L, Franckaert D, Pierson W, Staats KA, Schlenner SM, Liston A. A novel Zap70 mutation with reduced protein stability demonstrates the rate-limiting threshold for Zap70 in T-cell receptor signalling. Immunology. 2014;141:377–87. doi: 10.1111/imm.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–63. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 17.Miosge LA, Blasioli J, Blery M, Goodnow CC. Analysis of an ethylnitrosourea-generated mouse mutation defines a cell intrinsic role of nuclear factor κB2 in regulating circulating B cell numbers. J Exp Med. 2002;196:1113–9. doi: 10.1084/jem.20020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. I-E+ nonobese diabetic mice develop insulitis and diabetes. J Exp Med. 1993;178:793–803. doi: 10.1084/jem.178.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho WY, Cooke MP, Goodnow CC, Davis MM. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994;179:1539–49. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rust S, Funke H, Assmann G. Mutagenically separated PCR (MS-PCR): a highly specific one step procedure for easy mutation detection. Nucleic Acids Res. 1993;21:3623–9. doi: 10.1093/nar/21.16.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelms KA, Goodnow CC. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity. 2001;15:409–18. doi: 10.1016/s1074-7613(01)00199-6. [DOI] [PubMed] [Google Scholar]
- 22.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–11. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picard C, Dogniaux S, Chemin K, et al. Hypomorphic mutation of ZAP70 in human results in a late onset immunodeficiency and no autoimmunity. Eur J Immunol. 2009;39:1966–76. doi: 10.1002/eji.200939385. [DOI] [PubMed] [Google Scholar]
- 24.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–46. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Sosinowski T, Killeen N, Weiss A. The Src-like adaptor protein downregulates the T cell receptor on CD4+ CD8+ thymocytes and regulates positive selection. Immunity. 2001;15:457–66. doi: 10.1016/s1074-7613(01)00195-9. [DOI] [PubMed] [Google Scholar]
- 26.Patrucco E, Notte A, Barberis L, et al. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and-independent effects. Cell. 2004;118:375–87. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Au-Yeung BB, Levin SE, Zhang C, Hsu L-Y, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11:1085–92. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–60. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 29.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci USA. 2005;102:13574–9. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Adams A, Wildt KF, Aronow B, Feigenbaum L, Bosselut R. Restricting Zap70 expression to CD4+ CD8+ thymocytes reveals a T cell receptor-dependent proofreading mechanism controlling the completion of positive selection. J Exp Med. 2003;197:363–73. doi: 10.1084/jem.20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallo EM, Winslow MM, Canté-Barrett K, et al. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–5. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]