Abstract
Candida albicans, an opportunistic fungal pathogen, can cause severe systemic infections in susceptible patient groups. Systemic candidiasis is mainly studied in the mouse intravenous challenge model, where progressive infection correlates with increased early renal chemokine levels.
To develop a new in vitro assay to assess C. albicans virulence, which reflects the events occurring in the murine infection model, renal M-1 cortical collecting duct epithelial cells were evaluated as the early producers of cytokines in response to C. albicans. We show that renal epithelial cells respond only to live C. albicans cells capable of forming hyphae, producing chemokines KC and MIP-2, with levels correlating with epithelial cell damage. By assaying epithelial cell responses to strains of known virulence in the murine intravenous challenge model we demonstrate that renal epithelial cells can discriminate between virulent and attenuated strains. This simple, novel assay is a useful initial screen for altered virulence of C. albicans mutants or clinical isolates in vitro and provides an alternative to the mouse systemic infection model.
Keywords: Candida albicans, virulence, innate immunity, renal epithelium, infection model
Introduction
Candida albicans and other Candida species, such as C. parapsilosis, C. glabrata, C. tropicalis, C. dubliniensis, and C. krusei, are common commensal fungal species found in the gastrointestinal and reproductive tracts of healthy individuals.1 However, Candida species can also cause mucosal (superficial) and systemic infections.2 Systemic candidiasis is one of the most important nosocomial infections in Europe and the US and is associated with high mortality (40%) rates among hospitalized patients, particularly those in intensive care units (ICU), people undergoing major surgery and in immunocompromised individuals.2-6
Mucosal and systemic candidiasis is mainly studied in animal models.7-10 However, there are limitations to these models; C. albicans is not a natural colonizer of small mammals10 and there are ethical and cost implications.11,12 These disadvantages, as well as Society’s wish to reduce the numbers of animals used in research, have encouraged scientists to explore in vitro models to refine, reduce, or replace (3Rs) animals in research.13
For mucosal infections, in vitro models include mucosal explants,14 monolayer cell cultures,15-17 multiple layer cell cultures, and reconstituted human epithelium.18-20 A good correlation has been found between immune responses measured in in vitro models and fungal virulence assayed in animal models.20-23
Although numerous models have been developed to investigate superficial candidiasis,24-27 in vitro models to study systemic infection are currently limited to interactions with immune cells or with blood vessel endothelial cells.28-33 Systemic infection is primarily studied in model hosts, such as invertebrate mini-hosts,34-39 the chick chorioallantoic membrane model,40,41 and small mammals.42-46 However, the mouse intravenous (IV) challenge model remains the most commonly used model to investigate C. albicans virulence.47-50 During infection the bloodstream and the majority of organs are cleared of the pathogen, but fungal burdens increase in the kidneys and brain, accompanied by increasing levels of renal cytokines and chemokines.51,52 Increased renal cytokine levels correlate with lesion severity and eventual infection outcome,49,52 with high levels of pro-inflammatory cytokines eventually causing sepsis and death of infected animals.
The escape of fungi from the bloodstream during systemic candidiasis has been modeled in vitro using endothelial cells.28-30,53 Endothelial cell damage and cytokine production was induced only by live, germinated fungal cells,53 and those C. albicans strains unable to damage endothelial cells were found to be less virulent in the mouse model of systemic candidiasis.29 However, as it is early cytokine and chemokine levels in the kidneys which correlate with C. albicans murine systemic infection outcome,52 we hypothesized that the host innate immune response is initiated in the kidneys. Epithelial cells, including renal cortical epithelial cells, are known to be capable of initiating an innate immune response through proinflammatory cytokine production, e.g., IL-8, IL-6, and IL-1.22,54-61 We, therefore, chose to evaluate murine renal epithelial cells as the basis for the development of a new assay to allow in vitro assessment of C. albicans virulence.
Results
Candida albicans–renal cells interactions
Based upon previous work,52 it was hypothesized that the interaction between fungal and renal epithelial cells initiates the innate immune response in the kidneys during systemic C. albicans infection. To examine how C. albicans physically interacts with a renal epithelial monolayer, murine M-1 cortical collection epithelial cells were co-incubated with C. albicans SC5314, a virulent strain in the mouse systemic infection model, at a co-incubation ratio of 1:1 C. albicans:M-1 cells. Within 3 h hyphae were evident (Fig. 1A) and had penetrated the epithelial monolayer (Fig. 1D). By 6 h some elongated hyphae were observed (Fig. 1B), with further evidence of penetration into the epithelial monolayer (Fig. 1E), while masses of hyphae were seen at 24 h post-infection (Fig. 1C).
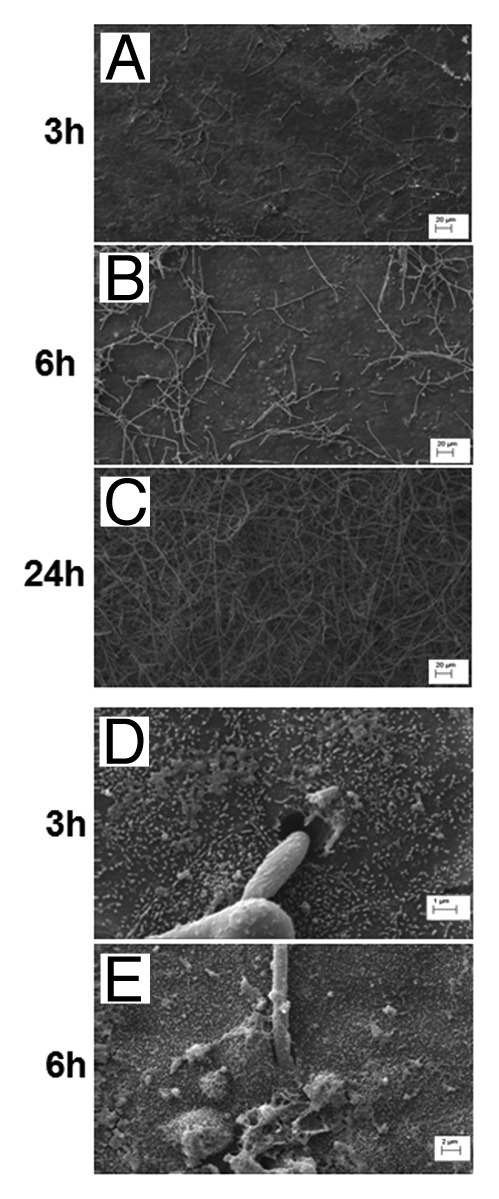
Figure 1. Scanning electron microscopy (SEM) images of C. albicans-renal epithelial cell co-incubation. M-1 renal epithelial cells and C. albicans SC5314 at 1:1 (A–C) C. albicans:renal cell ratio were co-incubated and visualized by SEM at 3 h (A), 6 h (B), and 24 h (C). Hyphal cell adherence to and penetration into renal cell monolayer is evident at 3 h and 6 h (D and E). Scale bars represent 20 μm (A–C), 1 μm (D), and 2 μm (E).
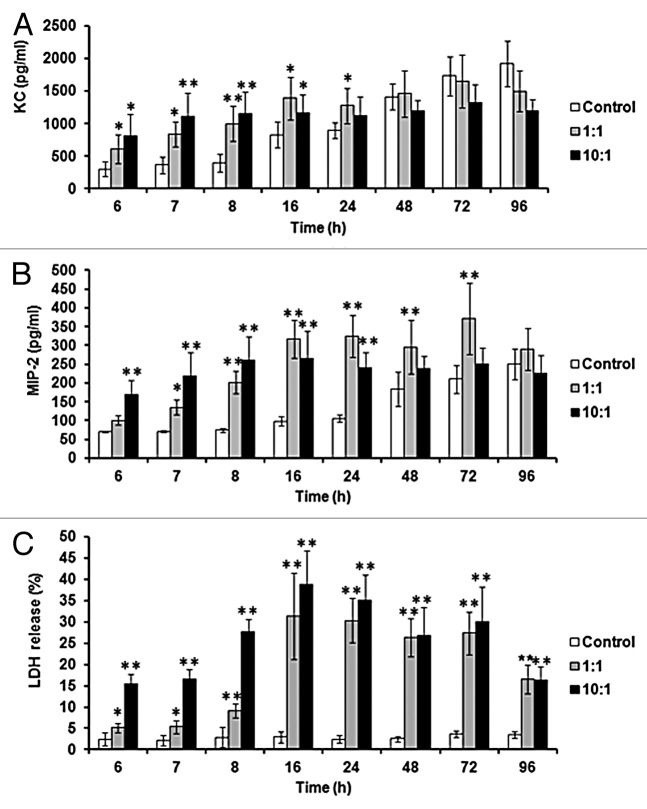
In order to further characterize the response of murine renal epithelial cells to C. albicans, chemokines and cytokines produced in response to C. albicans SC5314 were measured from 6 to 96 h post-infection. The majority of cytokines assayed (IL-6, TNF-α, IFN-γ, IL-12, IL-17, IL-10, and IL-1β)51,52,62 were undetectable over 96 h (data not shown), whereas KC and MIP-2 (equivalent to human IL-8) chemokine levels increased (Fig. 2A and B). Both KC and MIP-2 levels were significantly higher than uninfected controls at 6 h, increasing further by 8 h. However, later in infection control KC and MIP-2 levels also increased but remained significantly lower than co-incubations at 24 h, and even at 48 h in the case of MIP-2 (Fig. 2B). By 48 h control KC levels were similar to the 1:1 co-incubation (Fig. 2A) and by 96 h control MIP-2 had increased to levels similar to the 1:1 co-incubation (Fig. 2B).
Figure 2. Renal epithelial chemokine production and damage increases during incubation with C. albicans. KC (A) and MIP-2 (B) levels were measured over 96 h of co-incubation. Chemokine levels and LDH release (C) were measured for control, 1:1 (C. albicans:renal cells) and 10:1 co-incubations. Results represent the means ± standard deviation of three separate experiments using triplicate samples. Significant differences relative to uninfected control were determined by ANOVA and the Tukey post-hoc test (*P ≤ 0.05, **P < 0.001).
To assay whether C. albicans damaged renal cells during their interaction lactate dehydrogenase (LDH) release was measured (Fig. 2C). LDH levels reflected chemokine production by epithelial cells, where significantly higher damage occurred in the co-incubations compared with uninfected epithelial cells at 6 h post-infection. Incubation of 10 times more C. albicans cells with renal cells resulted in enhanced epithelial cell damage at earlier time points, although there was little difference later in infection (Fig. 2C).
Based upon the greatest significant differences for all parameters between uninfected and infected cells (Fig. 2), and in attempts to reflect localized fungal:epithelial cell ratios in the kidney, an 8 h time point and a 1:1 co-incubation was chosen as the basis for an in vitro assay, with KC, MIP-2, and LDH levels assayed.
Renal cells respond only to live C. albicans capable of switching morphology
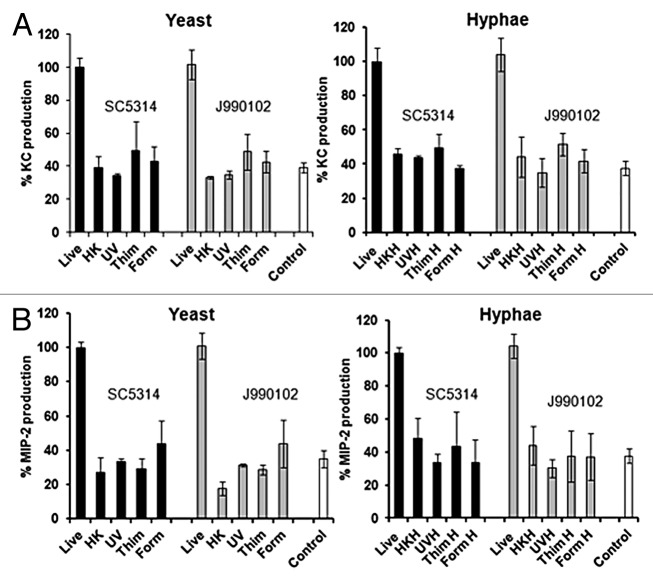
Previous studies with peripheral blood mononuclear cells (PBMC) have shown that heat-killed (HK) C. albicans yeast cells stimulate higher cytokine production than live cells.32,33,62 Endothelial cells have been shown to respond only to live C. albcians cells53 and oral epithelial cells produce significantly lower levels of cytokines in response to killed C. albicans cells.58 To investigate whether renal epithelial cell respond to killed cells, C. albicans yeast cells were killed either by heat-killing, by chemical killing, or by exposure to UV.63,64 Dead cells were then used in the assay and the responses compared with those induced by live cells. Using two different C. albicans strains known to be virulent in the mouse model, it was shown that only live cells induce significant KC and MIP-2 levels (Fig. 3A and B). Similar results were found when hyphal cells were assayed, with only live cells capable of inducing chemokine responses (Fig. 3A and B).
Figure 3. Renal epithelial cells respond only to live C. albicans cells. KC (A) and MIP-2 (B) measured at 8 h for a 1:1 (C. albicans-epithelial cell) co-incubation with live or killed C. albicans virulent strains, SC5314 or J990102. Live, heat killed (HK), UV killed (UV), formaldehyde (Form), and thimerosal (Thim) killed yeast and hyphal cells (A and B) were used. All values are expressed relative to SC5314 and results represent the means ± SEM of three separate experiments using triplicate samples. Significant differences relative to uninfected control were determined by ANOVA and the Tukey post-hoc test (*P < 0.05, **P < 0.001).
In our assay yeast cells rapidly form hyphae during co-incubation with renal epithelial cells. To investigate the importance of hyphal formation in the induction of innate immune responses by renal cells, morphological mutants were tested in our assay. The hgc1Δ and egf1Δ/cph1Δ (Table 1) null mutants, which are unable to form true hyphae, stimulated significantly lower KC and MIP-2 production (Fig. 4A and B), and caused less damage to the epithelial cells (Fig. 4C). Controls for these mutants, i.e., DAY185 and CAF2, did not differ in either chemokine production or epithelial cell damage relative to SC5314 (data not shown).
Table 1.C. albicans isolates and strains.
| Isolate/ mutant | Virulence (mouse model) | Description | References |
|---|---|---|---|
| SC5314 | Virulent | Clinical isolate | 66 |
| J951361 | Virulent | Clinical isolate | 52 |
| J990102 | Virulent | Clinical isolate | 52 |
| AM2003-020 | Virulent | Clinical isolate | 52 |
| AM2003/0100 | Attenuated | Clinical isolate | 52 |
| HUN96 | Attenuated | Clinical isolate | 52 |
| AM2003/0069 | Attenuated | Clinical isolate | 52 |
| FC28 | Attenuated | Clinical isolate | 52 |
| RV4688 | Attenuated | Clinical isolate | 52 |
| AM2003/0191 | Virulent | Clinical isolate | 52 |
| s20122.073 | Virulent | Clinical isolate | 52 |
| b30708/5 | Attenuated/intermediate | Clinical isolate | 52 |
| AM2005/0377 | Attenuated | Clinical isolate | 52 |
| AM2003/0074 | Attenuated | Clinical isolate | 52 |
| 78/028 | Attenuated | Clinical isolate | 52 |
| CAI4 + CIp10 (NYG152) | Virulent | Wild-type control strain | 68 |
| pmr1Δ + CIp10 (NGY355) | Attenuated | N- and O-glycosylation mutant (pmr1 null) | 65 |
| pmr1Δ + PMR1–CIp10(NGY356) | Virulent | PMR1 reintegrant | 65 |
| mnt1/2Δ + CIp10 (NGY337) | Attenuated/intermediate | O-glycosylation mutant (mnt1 and mnt2 null) | 67 |
| mnt1/2Δ + MNT1–CIp10 (NGY335) | Virulent | MNT1 reintegrant | 67 |
| hgc1Δ (WYZ12.1) | Attenuated | G1 cyclin mutant (hgc1Δ) | 76 |
| egf1Δ/cph1Δ (HLC54) | Attenuated | Transcriptional regulator and transcription factor double mutant (egf1Δ/cph1Δ) | 75 |
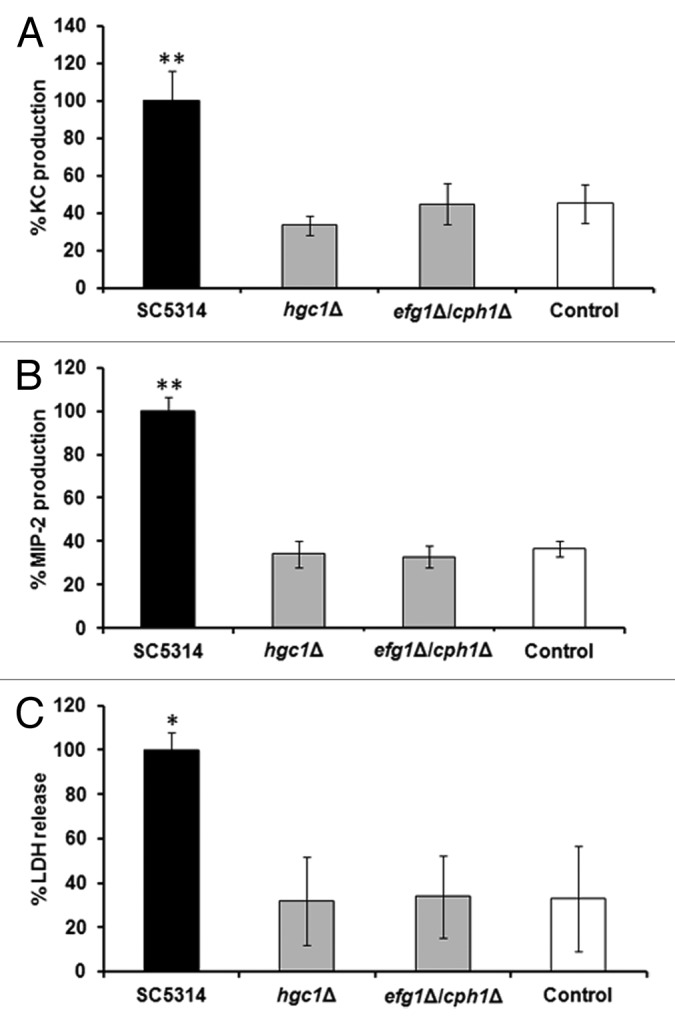
Figure 4. Switching from yeast to hyphal form is required to induce chemokine production and LDH release from renal cells. KC (A), MIP-2 (B), and LDH (C) levels at 8 h post-infection with 1:1 C. albicans:renal cells co-incubation with yeast-locked mutants and SC5314. All values are expressed relative to SC5314. Results represent the means ± SD of three separate experiments using triplicate samples. Significant differences relative to uninfected control were determined by ANOVA and the Tukey post-hoc test (*P < 0.05, **P ≤ 0.001).
Renal epithelial cells discriminate between virulent and attenuated C. albicans isolates and mutants
To explore whether renal epithelial cells can differentiate between attenuated and virulent C. albicans strains, the assay was performed using C. albicans clinical isolates of known virulence in the mouse intravenous challenge model of systemic candidiasis (Table 1).52 Only virulent isolates induced significant levels of KC, with the exception of isolate AM2003-020, relative to uninfected controls (Fig. 5A). MIP-2 production showed a similar pattern but significant increases in chemokine levels were also found for two attenuated isolates, AM2003/0100 and AM2003-0069 (Fig. 5B). Again, LDH release was highest for virulent strains, with little damage seen when known attenuated strains were tested (Fig. 5C).
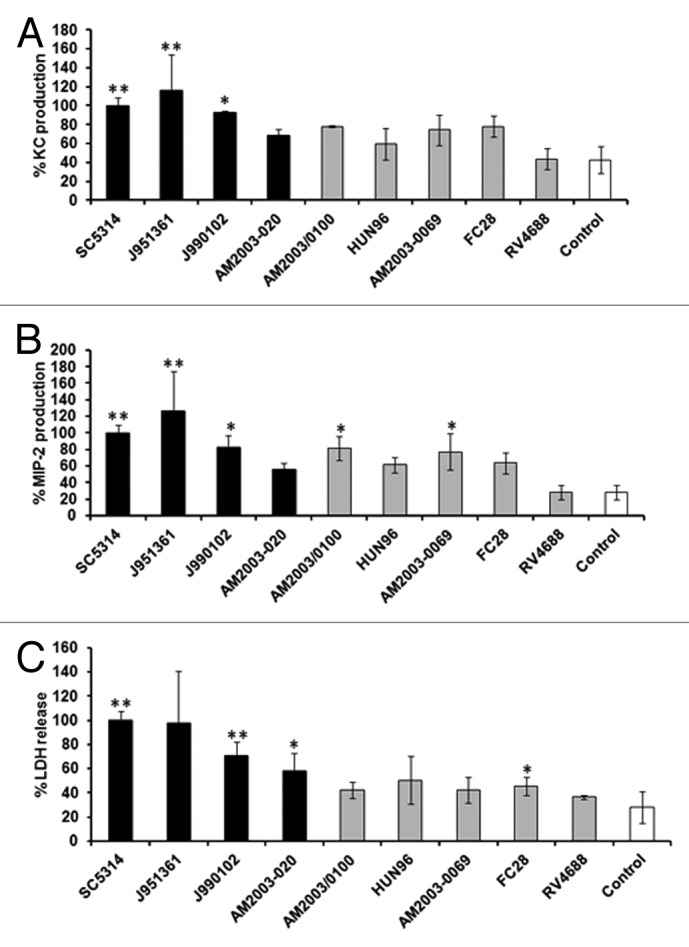
Figure 5. Renal epithelial cells discriminate between virulent and attenuated C. albicans isolates. KC (A), MIP-2 (B), and LDH (C) levels at 8 h post-infection for 1:1 C. albicans:renal cell co-incubation with different C. albicans isolates of known virulence in the mouse model.82 All values are expressed relative to SC5314. Results represent the means ± SD of three separate experiments using triplicate samples. Significant differences relative to the uninfected control were determined by ANOVA and the Tukey post-hoc test (**P < 0.001, *P < 0.05). Virulent strains are shown as black columns, attenuated strains as gray and control as white columns.
C. albicans null mutant strains known to be affected in virulence (Table 1)65-68 were also tested in the model, and were compared with the control strain CAI4 + CIp1068 (Fig. 6). pmr1Δ + CIp10, an attenuated strain in the mouse systemic model,65 showed reduced KC levels (Fig. 6A), MIP-2 levels (Fig. 6B), and epithelial cell damage (Fig. 6C) when compared with the control. This defect was restored in the reintegrant strain. However, a strain of intermediate virulence, mnt1/mnt2 + CIp10 mutant,67 stimulated similar levels of KC, MIP-2, and LDH release as the parental and reintegrant strains (Fig. 6).
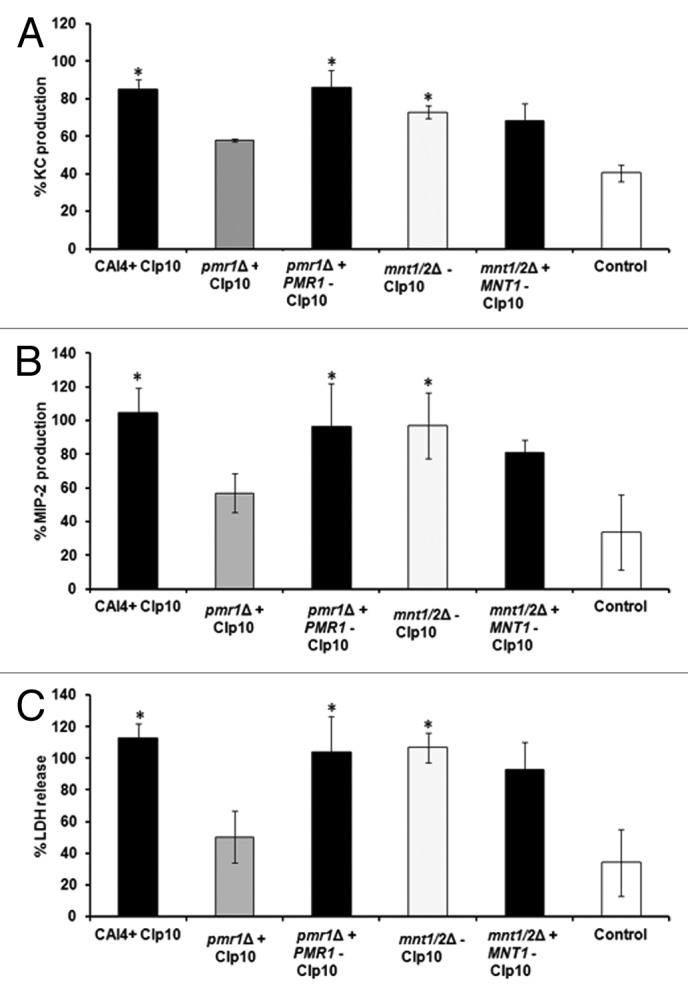
Figure 6. Attenuated C. albicans mutant strains fail to induce high levels of chemokine production by renal epithelial cells. KC (A), MIP-2 (B), and LDH (C) levels at 8 h post-infection for a 1:1 C. albicans:renal cell co-incubation with C. albicans null mutant strains with virulence defects in the mouse intravenous challenge model.65,67,68 All values are expressed relative to SC5314. Results represent the means ± SD of three separate experiments using triplicate samples. Significant differences relative to uninfected control were determined by ANOVA and the Tukey post-hoc test (**P < 0.001, *P < 0.05). Virulent strains are shown as black columns, attenuated as gray, intermediate strain as light gray, and control as white columns.
Further clinical isolates of known virulence were tested in blinded assays during evaluation of the assay to prevent unconscious bias. Virulence of the isolates in the mouse model was revealed after the assays had been completed. Similar to previous assays with clinical isolates (Fig. 5), virulent C. albicans isolates stimulated high KC and MIP-2 production, with the exception of a single strain, AM2003/0191, while intermediate and attenuated isolates induced similar chemokine levels (Fig. 7A and B) and LDH release (Fig. 7C) as uninfected controls. Clinical isolate AM2003/0191 formed a mix of yeast and hyphae in co-culture, rather than true hyphae as seen for other virulent strains. The majority of attenuated strains (Figs. 5 and 7), with the exception of 78/028 and AM2003/0074, formed long pseudohyphae in co-culture. However, the correlation is not complete as strains b30708/5 and AM2003/0100 formed true hyphae in co-culture, but did not cause significant damage to the epithelial cells or stimulate high levels of chemokines (data not shown).
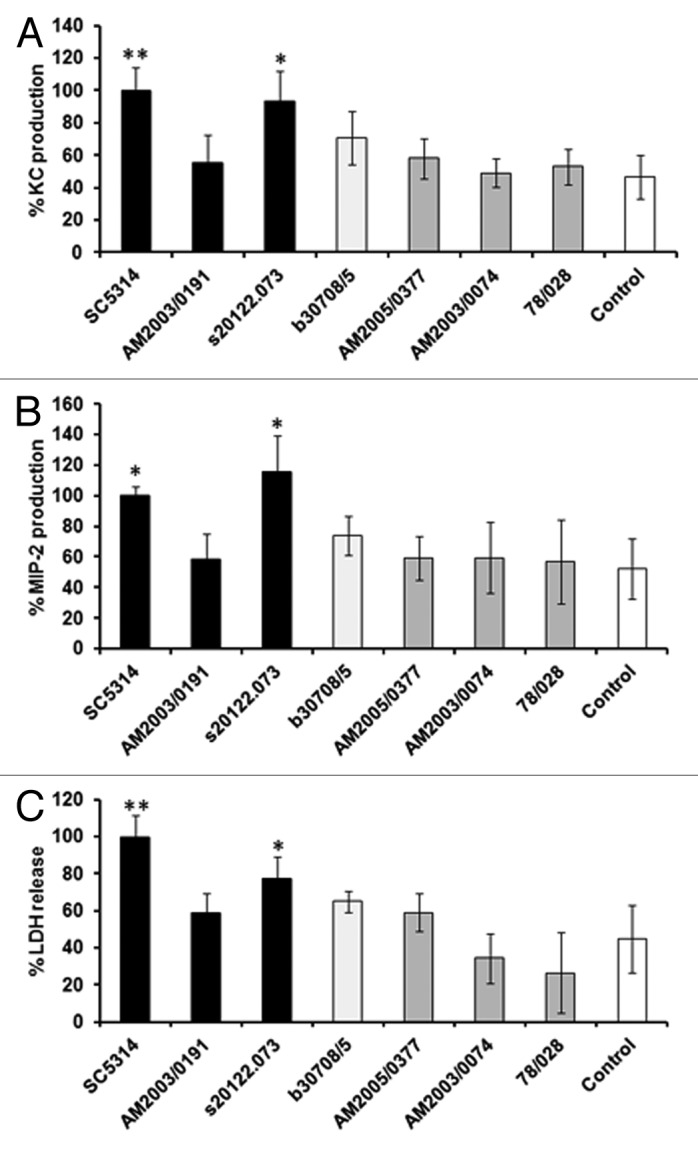
Figure 7. Identification of virulent C. albicans strains in a blinded assay. Six C. albicans clinical isolates were assayed in a blinded manner, with the experimentalist provided with the mouse virulence data after analysis of the results. KC (A), MIP-2 (B), and LDH (C) levels were determined at 8 h post-infection. All values are expressed relative to SC5314. Results represent the mean ± SD of three separate experiments using triplicate samples. Significant differences relative to the uninfected control were determined by ANOVA and the Tukey post-hoc test (**P < 0.001, *P < 0.05). Virulent strains (as determined in the mouse model) are shown as black columns, attenuated as gray, intermediate strain as light gray and control as white columns.
Chemokine levels and epithelial cell damage
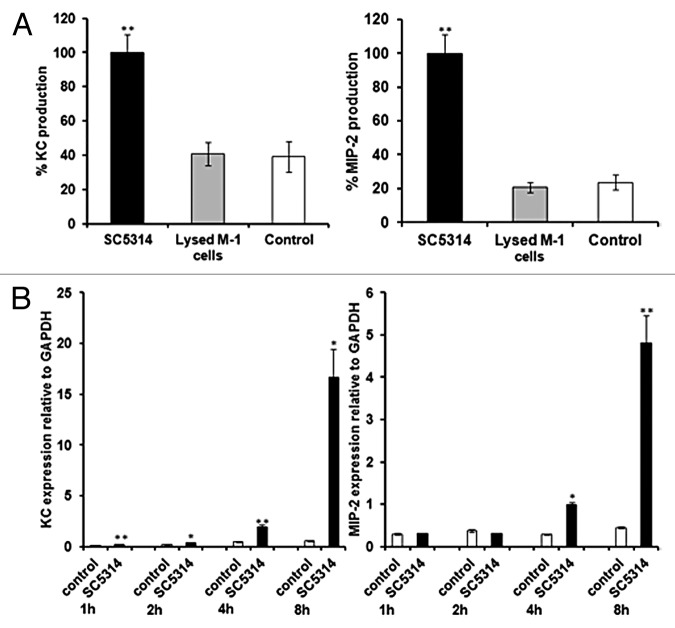
Generally, in our assay, trends for KC, MIP-2, and LDH levels were similar, with low levels for attenuated strains and higher levels for virulent strains. Correlation analyses of damage and chemokine production suggests that the two are related, with R2 = 0.778 for KC and LDH and R2 = 0.6918 for MIP-2 and LDH (Fig. 8). In order to establish whether chemokine levels merely reflect release of intracellular chemokine stores when epithelial cells are damaged chemokine levels from lysed epithelial cells after 8 h incubation were measured. Both KC and MIP-2 levels released from lysed cells were similar to those found for uninfected, intact M-1 epithelial cells, while chemokine production was significantly higher for epithelial cells co-incubated with C. albicans (Fig. 9A). Active production of both KC and MIP-2 was further confirmed by analysis of KC and MIP-2 gene expression during co-incubation with C. albicans, with KC levels increased from 1 h post-infection and MIP-2 significantly increased from 4 h post-infection (Fig. 9B). This suggests that chemokine production is an active process in response to damage caused by fungal cells.
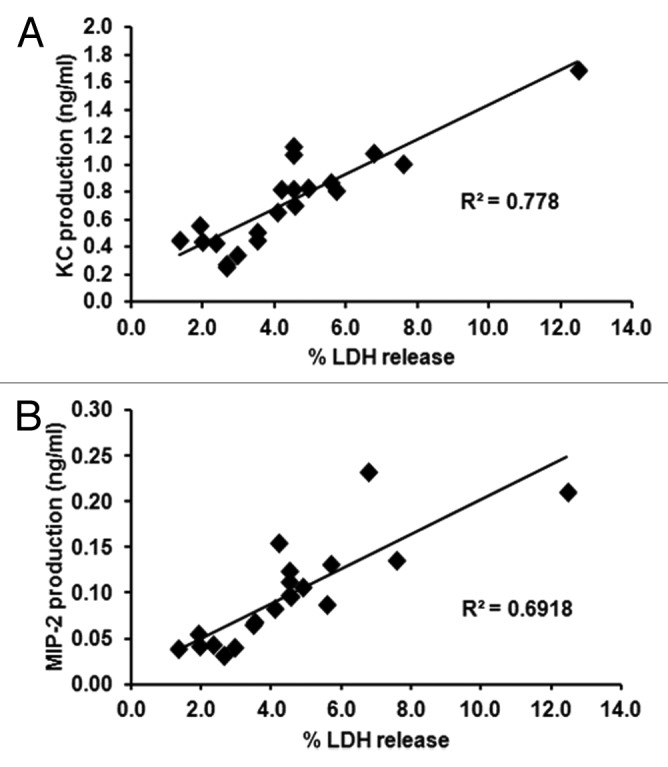
Figure 8. Chemokine production correlates with LDH release from renal epithelial cells. KC (A) and MIP-2 (B) levels relative to LDH levels for all experiments analyzed. Each data point represents the mean assay values for a C. albicans strain or mutant. Correlations were determined by the Spearman correlation test.
Figure 9. Chemokines are actively produced by renal epithelial cells. (A) KC and MIP-2 production by renal cells co-incubated with SC5314 (virulent strain), control uninfected epithelial cells and epithelial cells lysed using Triton-X-100. (B) KC and MIP-2 gene expression in renal cells co-incubated with SC5314 (virulent strain) and control cells at 1, 2, 4, and 8 h. Results in (A) represent the mean ± SD, and in (B) represent the mean ± SEM, of three separate experiments using triplicate samples. Black bars represent the co-incubation, gray bars are lysed epithelial cells and white bars represent uninfected controls. Significant differences relative to the uninfected control were determined by ANOVA and the Tukey post-hoc test (*P ≤ 0.05, **P ≤ 0.001) (A) or by the Student t test (*P ≤ 0.05, **P ≤ 0.001) (B).
Discussion
Animal models are most commonly used to investigate pathogenesis, infection progression and virulence of C. albicans.42-45,47-52,65-71 However, these experiments require the use of many animals, particularly mice. The aim of the present study was to develop a new in vitro model to assay C. albicans virulence, allowing a reduction in the number of mice used in systemic candidiasis studies, thus addressing the 3Rs.13
Our results clearly demonstrate that renal epithelial cells are capable of initiating an immune response to fungal pathogens, similar to other epithelial cell types.18,54,58,60,72 Interactions between renal epithelial cells and fungal cells, using a cell culture monolayer, showed that C. albicans formed hyphae and penetrated into M-1 epithelial cells at 3 h post-infection. C. albicans had previously been shown to adhere to the HEK293 human kidney cell line, but chemokine and cytokine responses were not investigated.73 Physical interaction between C. albicans and endothelial or oral and vaginal epithelial cells has previously been demonstrated to be required for initiation of a host response, with a switch to hyphal growth required.29,53,58,74 This ability to switch between yeast and hyphal forms is also required for full virulence of C. albicans in the mouse intravenous challenge model.34,75,76 However, results for endothelial and epithelial cells differ significantly from in vitro assays with human peripheral blood mononuclear cells (PBMCs), where the highest cytokine levels induced are in response to heat-killed, yeast form C. albicans.33,77,78
In our in vitro model early, active production of chemokines KC and MIP-2, important for the neutrophil recruitment,79 by renal epithelial cells was demonstrated in response to virulent C. albicans strains. These results reflect early events in the mouse model of systemic infection, where KC levels increase within the first 12 h of infection in the kidneys of mice infected with virulent isolates52 and virulent strains generally induce higher early levels of renal KC than attenuated strains.43,52 Other cytokines, e.g., IL-6, TNF-α, and IL-1β, also begin to increase in the kidneys after this time,52 but were undetectable in our in vitro assay, suggesting that these cytokines may be produced by infiltrating immune cells. However, it is also possible that the cell line used may be defective in pro-inflammatory cytokine production. This is in contrast to oral and vaginal epithelial cells which have been demonstrated to secrete IL-8, IL-1β, IL-6, and TNF-α in response to C. albicans.74,80
In general only C. albicans strains which are virulent in the mouse systemic model induced significant KC and MIP-2 production and caused damage to the renal epithelial cells. Damage to endothelial cells has been shown previously to induce production of immune modulators, and hence initiate an immune response.81 However not every virulent isolate behaved in the same way in our assay. Virulent isolates AM2003/0191 (clade 2) and AM2003-020 (clade 4)82 did not elicit significantly increased KC and MIP-2 production by epithelial cells, and two attenuated isolates, AM2003/0069 (clade 2) and AM2003/0100 (clade 2),82 stimulated significantly higher MIP-2 levels than uninfected controls. Although classed as a virulent isolate in the mouse model, AM2003-020 induces lower levels of KC in the kidneys of infected mice, relative to other virulent isolates,52 showing agreement with results obtained here. An imperfect correlation between murine virulence and endothelial cell damage has been observed previously, where some strains capable of causing endothelial cell damage were attenuated in a mouse infection model.29 Adaptation of the fungus in vivo may also play a role, as strain-specific differences in adaptation to the host environment, which could not be reproduced in vitro, were recently demonstrated.83 These alterations in in vivo adaptation could underlie results found for a minority of C. albicans strains where renal epithelial assay results did not correlate with virulence in the mouse model. However, one of the limitations of our model is that phagocytosis and blood clearance of fungi is not accounted for, which would affect the numbers of fungal cells reaching the kidneys in the mouse model, and thus infection outcome. Hence, mutants affected in susceptibility to neutrophils, e.g., the sod5 mutant, may not be detected as attenuated, even though this mutant is attenuated in the mouse.84,85
Our new model using renal epithelial cells is a useful, simple, and rapid assay of C. albicans virulence, which can be used as a screen prior to proceeding to animal infection models, thus reducing the numbers of mice used in virulence assays. In the future it would be of interest to extend this model to human kidney cell lines to determine whether C. albicans interacts in a similar way with human kidney cells.
Materials and Methods
Candida albicans culture and maintenance
C. albicans isolates and strains (Table 1) were routinely maintained on YPD agar at 4 °C (1% yeast extract, 2% mycological peptone, 2% glucose, 2% agar) or at –80 °C in glycerol for long-term storage. For co-incubation experiments C. albicans cells were grown in YPD at 30 °C 200 rpm overnight. Hyphae were induced by washing YPD overnight cultures three times with PBS, and then inoculating cells into DMEM:Ham’s F12 with 2 mM Glutamine (GIBCO) to a final concentration of ~5 × 106 cells/ml. Cultures were incubated for 4 h at 37 °C in 5% CO2. Cells (~1 × 107 cells/ml) were washed three times with PBS before being used in assays.
Preparation of killed C. albicans cells
Killed C. albicans cells were prepared either by heat-killing, by chemical killing or by UV killing.63,64 Heat-killed (HK) cells were obtained by incubating cells (~1 × 108/ml) at 70 °C for 2 h in a water bath. Chemically-killed cells were prepared by treating cells (~1 × 108/ml) overnight with 100 mM thimerosal or 1% formaldehyde, and then washing cells four times in PBS to remove residual chemicals. To UV kill cells, C. albicans (~1 × 108/ml) were exposed to 0.100 J/cm2 UV light 20 times on a UV-crosslinker (CL 508 S, Uvitec, Cambridge) with 254 nm UV emission tubes. All killed cells were stored at 4 °C before used for co-incubation.
For all killed cells, after re-suspension in PBS (~100 × 108 cells/ml), a 10 µl sample was plated onto YPD agar and incubated for 48 h at 30 °C to assay cell viability. The detection limit of the viability assay was 100 cells/ml. Viable cells were not detected in any of the cell suspensions used in these assays.
Renal epithelial cell cultures
The M-1 mouse kidney cortical collecting duct epithelial cell line (CRL-2038, ATCC) was used for co-incubation assays with C. albicans. Renal epithelial cells were grown in DMEM:Ham’s F12 with 2 mM glutamine (Gibco Life Technologies), 5 µM dexamethasone (Hspira UK Limited), 5% fetal bovine serum (FBS), and maintained in 5% CO2 at 37 °C. The growth medium was changed every second day. Only passage numbers 35–49 were used for assays.
C. albicans–renal epithelial cell co-incubation assays
For co-incubation of renal epithelial cells with C. albicans, 1 × 105 M-1 kidney cells were seeded into each well of a 24-well plate (Greiner Cellstar) and grown for 2 d to reach confluence. The growth medium was changed and fungal cells added at a ratio of 1:1 or 10:1 (C. albicans:M-1 cells). Plates were incubated for 6–96 h in 5% CO2 at 37 °C. Culture supernatants were collected at defined time points and stored at –20 °C for cytokine/chemokine measurements or at 4 °C for epithelial cell damage (LDH) assays. To determine intracellular chemokine levels, renal cells were lysed with Triton X-100 (1% final concentration) for 40 min and the supernatant stored at 4 °C. In some experiments C. albicans strains were provided to the experimenter in a blinded fashion, e.g., with a code rather than the strain name.
Cytokine and chemokine production
Cytokine production by epithelial cells during C. albicans co-incubation was measured by Duoset ELISA development systems or Quantikine colorimetric sandwich ELISAs (R&D Systems) (IL-12, TNF-α, IL-1β, IL-10, IL-17, and IFN-γ, KC, and MIP-2) (R&D Systems), according to the manufacturer’s instructions.17
RNA extraction and real-time PCR
RNA was extracted from cells using the RNeasy Mini Kit (QIAGEN) following the manufacturer’s instructions. cDNA was prepared using Superscript II (Invitrogen) as per manufacturer’s instructions. The real-time PCR assay was utilized the Roche universal probe library. Primers used were manufactured by Invitrogen: KC forward (5′-ACTCCAACAC AGCACCATGA-3′) and reverse (5′-TGGTCTGCAG GCACTGAC-3′) primers and MIP-2 forward (5′-CCCTGGTTCA GAAAATCATC C-3′) and reverse (5′-CTTCCGTTGA GGGACAGC-3′) primers. PCR was performed using the LC Dual Color Hydrolysis probe assay in a Roche Lightcycler 480. Roche universal probe 49 was used to detect amplification of KC and probe 63 for MIP-2, with the murine GAPDH Taqman assay (Applied Biosystems) included in the same RT-PCR reaction to detect GAPDH as a control, following the manufacturer’s instructions. Data was analyzed with the Lightcycler software by the Absolute Quantification Analysis using the Second Derivative maximum method.
Epithelial cell damage assay
Renal epithelial cell damage was assayed by lactate dehydrogenase (LDH) release into the culture supernatant.17 At each sampling time, 1 ml of supernatant was collected and LDH release determined using the cytotoxicity detection kit (Roche Applied Science), according to the manufacturer’s instructions. LDH levels were determined from an l-Lactate Dehydrogenase (Roche) 8 point standard curve (highest value of 0.125 µg/ml). To determine maximum LDH release with 100% cell death at each time point, Triton X-100 (1% final concentration) was added to the renal cell monolayer and incubated for 40 min to lyse the cells.17 Total cell lysis was confirmed by light microscopy. LDH release (%) for each time point was calculated relative to the 100% cell death value at the same time point.
Scanning electron microscopy
M-1 cells were grown on BD Falcon cell culture PET track-etched membrane inserts (Becton Dickinson Labware). Inserts had an effective growth area of 0.3 cm2 and 0.4 µm pore size. Membranes were carefully dissected and fixed in 2% glutaraldehyde in 0.1 M phosphate buffer overnight. Samples were washed in 0.1 M phosphate buffer and then incubated in 1% osmium teroxide for 1 h. Membranes were washed in 0.1 M phosphate buffer, and then dehydrated through a series of ethanol washes (70%, 80%, 90%, and 100%). Samples were placed in hexamethyldisilazane (HMDS) for 10 min and allowed to dry overnight before attaching to an aluminum stub and coating in gold for viewing.
Statistical analysis
Data were analyzed using IBM SPSS statistics version 20. Significant differences were determined by ANOVA and Tukey Post-hoc test. The correlation factors were calculated using the Spearman non-parametric correlation test, and significant differences in gene expression were determined using the Student t test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank to Mrs Irene Wood, University of Aberdeen, for technical assistance with tissue culture, Dr Debbie Wilkinson and Dr Kevin MacKenzie, Histology and EM Facility, University of Aberdeen, for SEM sample preparation, and the National Centre for Replacement, Refinement and Reduction of Animals in Research (NC3Rs) for funding the PhD studentship of E.K.S.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/27046
References
- 1.Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2008;2:1183–93. doi: 10.1038/ismej.2008.76. [DOI] [PubMed] [Google Scholar]
- 2.Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, Teutsch SM. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26:540–7. doi: 10.1086/502581. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers CM, Bal AM. Management of fungal infections in the intensive care unit: a survey of UK practice. Br J Anaesth. 2011;106:827–31. doi: 10.1093/bja/aer089. [DOI] [PubMed] [Google Scholar]
- 4.Das I, Nightingale P, Patel M, Jumaa P. Epidemiology, clinical characteristics, and outcome of candidemia: experience in a tertiary referral center in the UK. Int J Infect Dis. 2011;15:e759–63. doi: 10.1016/j.ijid.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Schelenz S, Barnes RA, Kibbler CC, Jones BL, Denning DW. Standards of care for patients with invasive fungal infections within the United Kingdom: a national audit. J Infect. 2009;58:145–53. doi: 10.1016/j.jinf.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.MacCallum DM. Candida infections and modelling disease. In: Ashbee R, Bignell EM, editors. The yeast handbook: pathogenic yeasts. Springer Berlin Heidelberg; 2010. p. 41-67 [Google Scholar]
- 7.McMillan MD, Cowell VM. Effects of chronic Candida albicans in the hamster cheek pouch. Oral Surg Oral Med Oral Pathol. 1992;74:492–8. doi: 10.1016/0030-4220(92)90302-7. [DOI] [PubMed] [Google Scholar]
- 8.de Bernardis F, Santoni G, Boccanera M, Spreghini E, Adriani D, Morelli L, Cassone A. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68:3297–304. doi: 10.1128/IAI.68.6.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balish E, Wagner RD, Vazquez-Torres A, Jones-Carson J, Pierson C, Warner T. Mucosal and systemic candidiasis in IL-8Rh-/- BALB/c mice. J Leukoc Biol. 1999;66:144–50. doi: 10.1002/jlb.66.1.144. [DOI] [PubMed] [Google Scholar]
- 10.Clemons KV, Gonzalez GM, Singh G, Imai J, Espiritu M, Parmar R, Stevens DA. Development of an orogastrointestinal mucosal model of candidiasis with dissemination to visceral organs. Antimicrob Agents Chemother. 2006;50:2650–7. doi: 10.1128/AAC.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage DC, Dubos RJ. Localization of indigenous yeast in the murine stomach. J Bacteriol. 1967;94:1811–6. doi: 10.1128/jb.94.6.1811-1816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell WMS, Burch RL. The Principles of Humane Experimental Technique. London: Methuen, 1959. [Google Scholar]
- 14.Ohnemus U, Willers C, Bubenheim M, Horstkotte MA, Houdek P, Fischer F, Schmage P, Moll I, Brandner JM. An ex-vivo oral mucosa infection model for the evaluation of the topical activity of antifungal agents. Mycoses. 2008;51:21–9. doi: 10.1111/j.1439-0507.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- 15.Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, Tappuni AR, Rodgers CA, Woodman AJ, Challacombe SJ, et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology. 2008;154:3266–80. doi: 10.1099/mic.0.2008/022293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahri R, Saidane-Mosbahi D, Rouabhia M. Candida famata modulates toll-like receptor, beta-defensin, and proinflammatory cytokine expression by normal human epithelial cells. J Cell Physiol. 2010;222:209–18. doi: 10.1002/jcp.21939. [DOI] [PubMed] [Google Scholar]
- 17.Dalle F, Wächtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, Labruère C, Bonnin A, Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–71. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 18.Semlin L, Schäfer-Korting M, Borelli C, Korting HC. In vitro models for human skin disease. Drug Discov Today. 2011;16:132–9. doi: 10.1016/j.drudis.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dent Res. 2010;89:666–75. doi: 10.1177/0022034510368784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaller M, Boeld U, Oberbauer S, Hamm G, Hube B, Korting HC. Polymorphonuclear leukocytes (PMNs) induce protective Th1-type cytokine epithelial responses in an in vitro model of oral candidosis. Microbiology. 2004;150:2807–13. doi: 10.1099/mic.0.27169-0. [DOI] [PubMed] [Google Scholar]
- 21.Schaller M, Schäfer W, Korting HC, Hube B. Differential expression of secreted aspartyl proteinases in a model of human oral candidosis and in patient samples from the oral cavity. Mol Microbiol. 1998;29:605–15. doi: 10.1046/j.1365-2958.1998.00957.x. [DOI] [PubMed] [Google Scholar]
- 22.Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118:652–7. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 23.Khodavandi A, Alizadeh F, Harmal NS, Sidik SM, Othman F, Sekawi Z, Jahromi MA, Ng KP, Chong PP. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. FEMS Microbiol Lett. 2011;315:87–93. doi: 10.1111/j.1574-6968.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- 24.Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, Wilson D, Hube B. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. 2012;7:e36952. doi: 10.1371/journal.pone.0036952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadev NP, Murdoch C, Saville SP, Thornhill MH. Evaluation of tissue engineered models of the oral mucosa to investigate oral candidiasis. Microb Pathog. 2011;50:278–85. doi: 10.1016/j.micpath.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Dongari-Bagtzoglou A, Kashleva H. Development of a novel three-dimensional in vitro model of oral Candida infection. Microb Pathog. 2006;40:271–8. doi: 10.1016/j.micpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Hollmer C, Essmann M, Ault K, Larsen B. Adherence and blocking of Candida albicans to cultured vaginal epithelial cells: treatments to decrease adherence. Infect Dis Obstet Gynecol. 2006;2006:98218. doi: 10.1155/IDOG/2006/98218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orozco AS, Zhou X, Filler SG. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect Immun. 2000;68:1134–41. doi: 10.1128/IAI.68.3.1134-1141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez AA, Johnston DA, Myers C, Edwards JE, Jr., Mitchell AP, Filler SG. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect Immun. 2004;72:598–601. doi: 10.1128/IAI.72.1.598-601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidl K, Solis NV, Bayer AS, Hady WA, Ellison S, Klashman MC, Xiong YQ, Filler SG. Divergent responses of different endothelial cell types to infection with Candida albicans and Staphylococcus aureus. PLoS One. 2012;7:e39633. doi: 10.1371/journal.pone.0039633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Veerdonk FL, Joosten LA, Devesa I, Mora-Montes HM, Kanneganti TD, Dinarello CA, van der Meer JW, Gow NA, Kullberg BJ, Netea MG. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1beta production by the fungal pathogen Candida albicans. J Infect Dis. 2009;199:1087–96. doi: 10.1086/597274. [DOI] [PubMed] [Google Scholar]
- 32.Mora-Montes HM, Bates S, Netea MG, Castillo L, Brand A, Buurman ET, Díaz-Jiménez DF, Jan Kullberg B, Brown AJ, Odds FC, et al. A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J Biol Chem. 2010;285:12087–95. doi: 10.1074/jbc.M109.081513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–71. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, Halder G, Kontoyiannis DP. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193:1014–22. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 35.Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–37. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell. 2009;8:1750–8. doi: 10.1128/EC.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall RA, Bates S, Lenardon MD, Maccallum DM, Wagener J, Lowman DW, Kruppa MD, Williams DL, Odds FC, Brown AJ, et al. The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans. PLoS Pathog. 2013;9:e1003276. doi: 10.1371/journal.ppat.1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesa-Arango AC, Forastiero A, Bernal-Martínez L, Cuenca-Estrella M, Mellado E, Zaragoza O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol. 2013;51:461–72. doi: 10.3109/13693786.2012.737031. [DOI] [PubMed] [Google Scholar]
- 39.Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006;8:2105–12. doi: 10.1016/j.micinf.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen ID, Grosse K, Berndt A, Hube B. Pathogenesis of Candida albicans infections in the alternative chorio-allantoic membrane chicken embryo model resembles systemic murine infections. PLoS One. 2011;6:e19741. doi: 10.1371/journal.pone.0019741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gow NA, Knox Y, Munro CA, Thompson WD. Infection of chick chorioallantoic membrane (CAM) as a model for invasive hyphal growth and pathogenesis of Candida albicans. Med Mycol. 2003;41:331–8. doi: 10.1080/13693780310001600859. [DOI] [PubMed] [Google Scholar]
- 42.Walker LA, Maccallum DM, Bertram G, Gow NA, Odds FC, Brown AJ. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet Biol. 2009;46:210–9. doi: 10.1016/j.fgb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castillo L, MacCallum DM, Brown AJ, Gow NA, Odds FC. Differential regulation of kidney and spleen cytokine responses in mice challenged with pathology-standardized doses of Candida albicans mannosylation mutants. Infect Immun. 2011;79:146–52. doi: 10.1128/IAI.01004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee KK, Maccallum DM, Jacobsen MD, Walker LA, Odds FC, Gow NAR, Munro CA. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother. 2012;56:208–17. doi: 10.1128/AAC.00683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tashiro M, Kimura S, Tateda K, Saga T, Ohno A, Ishii Y, Izumikawa K, Tashiro T, Kohno S, Yamaguchi K. Pravastatin inhibits farnesol production in Candida albicans and improves survival in a mouse model of systemic candidiasis. Med Mycol. 2012;50:353–60. doi: 10.3109/13693786.2011.610037. [DOI] [PubMed] [Google Scholar]
- 46.MacCallum DM. Mouse model of invasive fungal infection. Methods Mol Biol. 2013;1031:145–53. doi: 10.1007/978-1-62703-481-4_17. [DOI] [PubMed] [Google Scholar]
- 47.Szabo EK, MacCallum DM. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol Lett. 2011;320:1–8. doi: 10.1111/j.1574-6968.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 48.MacCallum DM, Odds FC. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses. 2005;48:151–61. doi: 10.1111/j.1439-0507.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 49.Spellberg B, Ibrahim AS, Edwards JE, Jr., Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336–43. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 50.Ashman RB, Papadimitriou JM. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol Cell Biol. 1987;65:163–71. doi: 10.1038/icb.1987.18. [DOI] [PubMed] [Google Scholar]
- 51.MacCallum DM. Massive induction of innate immune response to Candida albicans in the kidney in a murine intravenous challenge model. FEMS Yeast Res. 2009;9:1111–22. doi: 10.1111/j.1567-1364.2009.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacCallum DM, Castillo L, Brown AJ, Gow NA, Odds FC. Early-expressed chemokines predict kidney immunopathology in experimental disseminated Candida albicans infections. PLoS One. 2009;4:e6420. doi: 10.1371/journal.pone.0006420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filler SG, Pfunder AS, Spellberg BJ, Spellberg JP, Edwards JE., Jr. Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–17. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maza PK, Oliveira P, Toledo MS, Paula DMB, Takahashi HK, Straus AH, Suzuki E. Paracoccidioides brasiliensis induces secretion of IL-6 and IL-8 by lung epithelial cells. Modulation of host cytokine levels by fungal proteases. Microbes Infect. 2012;14:1077–85. doi: 10.1016/j.micinf.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 55.Whiley RA, Cruchley AT, Gore C, Hagi-Pavli E. Candida albicans strain-dependent modulation of pro-inflammatory cytokine release by in vitro oral and vaginal mucosal models. Cytokine. 2012;57:89–97. doi: 10.1016/j.cyto.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Gerritsma JS, Hiemstra PS, Gerritsen AF, Prodjosudjadi W, Verweij CL, Van Es LA, Daha MR. Regulation and production of IL-8 by human proximal tubular epithelial cells in vitro. Clin Exp Immunol. 1996;103:289–94. doi: 10.1046/j.1365-2249.1996.d01-617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmouder RL, Strieter RM, Wiggins RC, Chensue SW, Kunkel SL. In vitro and in vivo interleukin-8 production in human renal cortical epithelia. Kidney Int. 1992;41:191–8. doi: 10.1038/ki.1992.26. [DOI] [PubMed] [Google Scholar]
- 58.Dongari-Bagtzoglou A, Kashleva H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb Pathog. 2003;34:169–77. doi: 10.1016/S0882-4010(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 59.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP, 3rd, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–53. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steele C, Fidel PL., Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun. 2002;70:577–83. doi: 10.1128/IAI.70.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol. 2006;176:3717–24. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- 64.Seider K, Brunke S, Schild L, Jablonowski N, Wilson D, Majer O, Barz D, Haas A, Kuchler K, Schaller M, et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol. 2011;187:3072–86. doi: 10.4049/jimmunol.1003730. [DOI] [PubMed] [Google Scholar]
- 65.Bates S, MacCallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, Brown AJ, Odds FC, Gow NA. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J Biol Chem. 2005;280:23408–15. doi: 10.1074/jbc.M502162200. [DOI] [PubMed] [Google Scholar]
- 66.Gillum AM, Tsay EYH, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–82. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 67.Munro CA, Bates S, Buurman ET, Hughes HB, Maccallum DM, Bertram G, Atrih A, Ferguson MA, Bain JM, Brand A, et al. Mnt1p and Mnt2p of Candida albicans are partially redundant α-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J Biol Chem. 2005;280:1051–60. doi: 10.1074/jbc.M411413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brand A, MacCallum DM, Brown AJ, Gow NA, Odds FC. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell. 2004;3:900–9. doi: 10.1128/EC.3.4.900-909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badrane H, Cheng S, Nguyen MH, Jia HY, Zhang Z, Weisner N, Clancy CJ. Candida albicans IRS4 contributes to hyphal formation and virulence after the initial stages of disseminated candidiasis. Microbiology. 2005;151:2923–31. doi: 10.1099/mic.0.27998-0. [DOI] [PubMed] [Google Scholar]
- 70.Chiang LY, Sheppard DC, Bruno VM, Mitchell AP, Edwards JE, Jr., Filler SG. Candida albicans protein kinase CK2 governs virulence during oropharyngeal candidiasis. Cell Microbiol. 2007;9:233–45. doi: 10.1111/j.1462-5822.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 71.van de Veerdonk FL, Kullberg BJ, van der Meer JW, Gow NA, Netea MG. Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr Opin Microbiol. 2008;11:305–12. doi: 10.1016/j.mib.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Pathak E, Mayeux PR. In vitro model of sepsis-induced renal epithelial reactive nitrogen species generation. Toxicol Sci. 2010;115:475–81. doi: 10.1093/toxsci/kfq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li F, Palecek SP. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot Cell. 2003;2:1266–73. doi: 10.1128/EC.2.6.1266-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaller M, Korting HC, Borelli C, Hamm G, Hube B. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect Immun. 2005;73:2758–65. doi: 10.1128/IAI.73.5.2758-2765.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 76.Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–56. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–50. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–86. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–35. [PubMed] [Google Scholar]
- 80.Naglik JR, Moyes D. Epithelial cell innate response to Candida albicans. Adv Dent Res. 2011;23:50–5. doi: 10.1177/0022034511399285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filler SG, Ibe BO, Luckett PM, Raj JU, Edwards JE., Jr. Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis. 1991;164:928–35. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- 82.MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJ, Gow NA, Odds FC. Property differences among the four major Candida albicans strain clades. Eukaryot Cell. 2009;8:373–87. doi: 10.1128/EC.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013;9:e1003315. doi: 10.1371/journal.ppat.1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell. 2004;15:456–67. doi: 10.1091/mbc.E03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. 2005;56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x. [DOI] [PubMed] [Google Scholar]