Abstract
Pseudomonas aeruginosa infections represent one of the major threats for injured or transplanted lungs and for their healing. Considering that the mesenchymal stem cells (MSCs) are a major tool for the regenerative medicine, including therapy of lung damaging diseases, the aim of this paper was to investigate the effects of P. aeruginosa quorum sensing signaling molecules (QSSMs) on human MSCs death signaling pathways and cytokine profile. Our data revealed that N-(3-oxododecanoyl)-L-homoserine lactone (OdDHL), N-butanoyl-L-homoserine lactone (C4-HSL), 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS), and its precursor, 2-heptyl-4-quinolone (HHQ), significantly impact on several core signaling mechanisms of MSCs in a specific and time-dependent manner. Even if all tested autoinducers interfered with the MSCs apoptotic genes expression, only OdDHL and HHQ significantly promoted MSCs apoptosis, by 14- and 23-fold respectively, this aspect being confirmed by the flow cytometry assay. The tested QSSMs induced a heterogeneous cytokine profile of the treated MSCs. The level of IL-1β was increased by OdDHL, IL-8 production was stimulated by all tested autoinducers, IL-6 was modulated mostly by PQS and IL-10 by HHQ. The significant influence of the purified bacterial autoinducers on the MSCs signaling pathways may suggest that the accumulation of these mediators could interfere with the normal function of these cells in the human body, and eventually, impair or abolish the success of the stem cells therapy during P. aeruginosa infections.
Keywords: P. aeruginosa, autoinducers, mesenchymal stem cells, viability, apoptosis, cytokine profile
Introduction
Mesenchymal stem cells (MSCs) represent a group of adult stem cells recently considered a symbol of regenerative medicine, especially as a potential therapy for lung injuries. MSCs possess unique characteristics as low immunogenicity, immunomodulatory properties,1,2 ability to secrete endothelial and epithelial growth factors,3,4 and, more recently, to exhibit antimicrobial properties.5,6 Lungs healing process, including the replacement of normal/damaged epithelium by fibroblastic scar tissue is still poorly understood. Recent findings suggest that epithelial cells can become fibroblasts through epithelial-to-mesenchymal transition (EMT). It is also hypothesized that EMT frequently occurs in damaged lungs and plays a potential role in airway remodeling.7 During acute lung injury, some of the paracrine soluble factors produced by the mesenchymal stem cells, such as keratinocyte growth factor, angiopoietin-1, interleukin-1 receptor antagonist, interleukin-10, prostaglandin E2, and LL-37 may have a potential role in the acute lung injury by restoring alveolar fluid clearance, lung permeability, and inhibiting bacterial growth, while MSCs-derived immunomodulation of innate and adaptive immune cells may reduce alveolar inflammation.6 For these reasons, MSCs are considered a promising therapeutic alternative for patients underlying severe acute and chronic lung injuries (e.g., obliterative bronchiolitis [OB] associated with chronic rejection of lung allografts, irreversibly damaged lungs of cystic fibrosis [CF] patients, etc.).7 Pseudomonas aeruginosa is a versatile opportunistic pathogen that causes resistant and difficult to treat infections especially within the respiratory airways and lungs.8,9 Acquisition of P. aeruginosa infections in the transplanted airway has been shown to be a risk factor for the development of OB,10 this opportunistic pathogen representing the main cause of morbidity and mortality of CF patients.11 Recent in vitro studies demonstrate that P. aeruginosa drives or increases the EMT in the airway, by activating monocytic cells and subsequently, generating a pro-inflammatory microenvironment triggered by the pro-inflammatory cytokine IL-1β.10 Even though the molecular mechanisms of this interaction are not known, the acquisition of P. aeruginosa infection and the increased risk of developing OB- and CF-related lung damage are closely linked. In P. aeruginosa, the virulence, behavior, population fitness, and bacteria–host interactions are strictly controlled by cell-to-cell density-dependent signaling molecules, named quorum sensing (QS) signaling molecules (QSSMs) or autoinducers.12,13 QSSMs are also involved in pathogen–host crosstalk, being able to modulate host epithelial or phagocyte cells signaling pathways involved in apoptosis and immune response regulation.14 P. aeruginosa produces mainly two types of autoinducers (AIs): acyl homoserin lactones (AHLs) and 4-quinolones (4Qs). The most investigated AHLs are N-(3-oxododecanoyl)-L-homoserine lactone (OdDHL) and N-butanoyl-L-homoserine lactone (C4-HSL), while the most important quinolones are 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS) and its precursor, 2-heptyl-4-quinolone (HHQ).15 Despite their proven role on other eukaryotic cell types, there is no report regarding the impact of P. aeruginosa AIs on stem cells, including MSCs.
The aim of this paper was to investigate the influence of P. aeruginosa main QS autoinducers OdDHL, C4-HSL, PQS, and HHQ on MSCs cell death signaling pathways and cytokine profile, which represent the most investigated parameters of these cells for therapeutic approaches.
Results
In a preliminary stage of our experiment, we have investigated the effect of different P. aeruginosa QSSMs on the cell viability of MSCs using the Trypan Blue assay (TBA). Among the four tested QSSMs, the OdDHL in a concentration of 50 μM exhibited the greatest effect on the MSCs viability according to Tripan Blue test. The MSCs viability gradually decreased during the incubation time, from 81%, as observed after a short exposure time of 2 h, to 37% after 18 h (Table 1). PQS exhibited a moderate effect on the MSCs viability, the percentage of viable cells remaining greater than 90% after 12 h of incubation in the presence of 50 μM PQS and decreasing to 71% after 18 h incubation (Table 1). The negative impact of HHQ on MSCs viability was lower than the one exhibited by PQS, the viability rates after up to 12 h of incubation being higher than 93%, and slightly decreasing to 86% after 18 h (Table 1). On the other hand, C4-HSL, the second most important AHL signaling molecule in P. aeruginosa seems to have no significant influence on MSCs viability for at least 18 h, since viability rates exceeded 90% in all tested conditions.
Table 1. The percentage of viable MSCs (counted after Tripan Blue staining) after 2, 6, 12, and 18 h of treatment using P. aeruginosa QSSMs.
| Viability during time | ||||
|---|---|---|---|---|
| Experimental condition | 2 h | 6 h | 12 h | 18 h |
| MSC controla | 99% | 99% | 100% | 99% |
| 50 μM OdDHL | 81%* | 63%* | 55%** | 37%*** |
| 50 μM C4-HSL | 99% | 99% | 93% | 90% |
| 50 μM PQS | 99% | 94% | 91% | 71%* |
| 50 μM HHQ | 99% | 99% | 93% | 86%* |
Note: aMSC control contain the same amount of HPLC grade MeOH used for test samples. *P < 0.05, **P < 0.01, ***P < 0.001, based on ANOVA and Bonferroni post test of medians of 3 independent experiments performed in triplicate (n = 3).
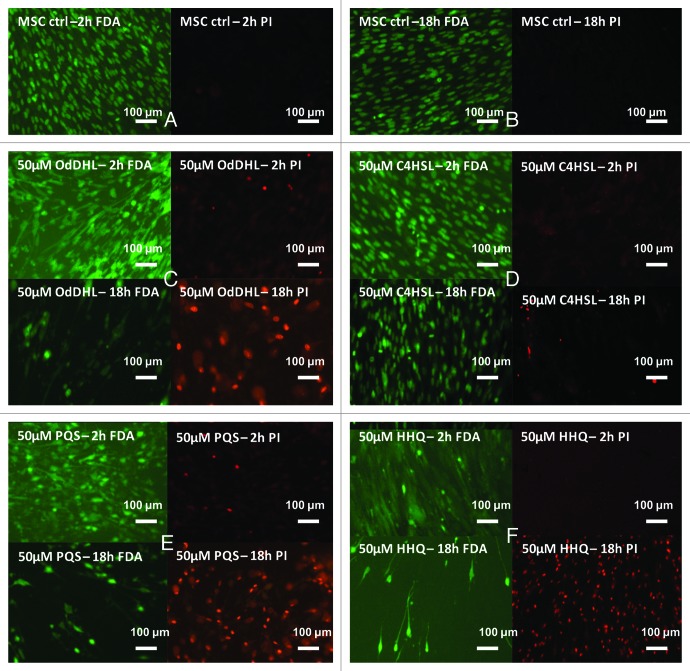
In the fluorescence microscopy examination, MSCs grown in the presence of 50 μM OdDHL for 2 h revealed irregular structures, developing lamellar pseudopode-like appendixes, which may indirectly indicate cytoskeleton reorganization induced by OdDHL.16 Furthermore, microscopy examination also revealed morphological changes typical for apoptotic cells, as nuclear fragmentation and cytoplasmic blebbing. This effect is strongly enhanced during time driving to loss-of-contact phenotypes and low viability, the PI/FDA staining revealing a high percentage of non-viable MSCs grown in the presence of OdDHL for up to 18 h (Fig. 1C).
Figure 1. Fluorescence micrographs of MSCs monolayer grown in the presence of 50 μM purified QSSMs, for 2 and 18 h. (A and B) MSCs untreated control (containing an equivalent amount of HPLC grade EtOH), (C) MSCs treated with OdDHL, (D) MSCs treated with C4HSL, (E) MSCs treated with PQS, (F) MSCs treated with HHQ. FDA, fluorescein diacetate staining (green, visualization at 488 nm); PI, propidium iodide staining (red, visualization at 546 nm). Immersion oil, 1000× magnification.
A concentration of 50 μM C4-HSL also induced moderate morphological changes of MSCs, revealing lamellar appendixes and cytoplasmic blebbing, but the viability was not significantly affected (Fig. 1D), as compared with the untreated control (Fig. 1A and B).
The 50 μM PQS affected the morphology of MSCs after 2 h of treatment, this early effect being enhanced during time and favoring loss-of-cell contact and subsequently cell death. After 18 h of incubation, the percentage of live MSCs was significantly lower than the red dyed, non-viable cells (Fig. 1E), as compared with control MSCs (Fig. 1A and B).
Microscopy results demonstrate that 50 μM HHQ does not reveal immediate detrimental effects against MSCs, but this autoinducer significantly decrease MSCs viability after 18 h incubation. HHQ treatment induced cell detachment and also cell membrane permeabilization, MSCs being able to assimilate the PI dye, similar to necrotic cells (Fig. 1F). Typical apoptotic changes, as membrane blebbing and nuclear fragmentation were also observed in specimens treated with HHQ.
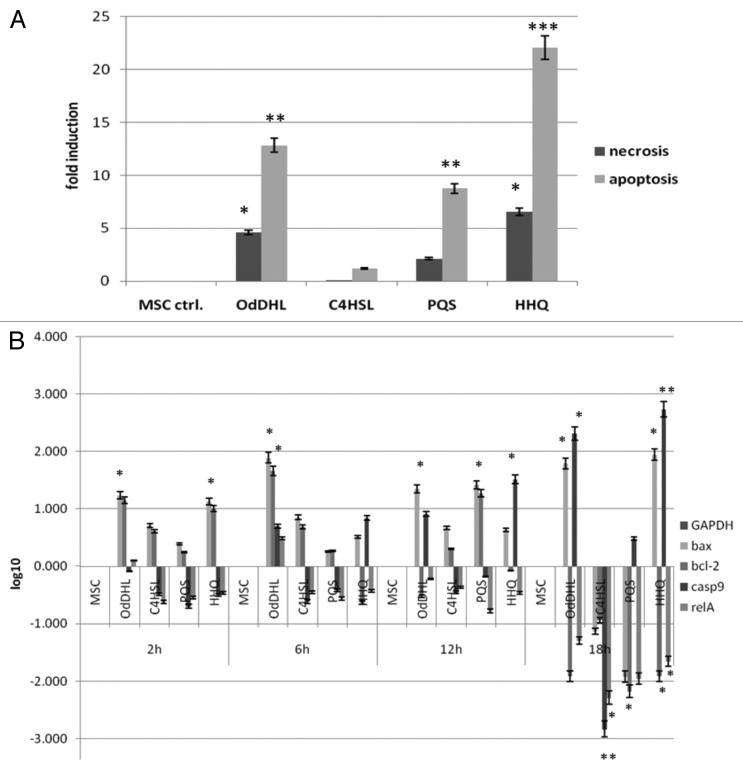
Flow cytometry assay was used to better discriminate between viable, apoptotic, and necrotic cells after the treatment. This assay revealed that OdDHL significantly impacts on MSCs viability, increasing both necrosis and apoptosis processes in the analyzed cells. On the other hand, MSCs grown in the presence of 50 μM C4-HSL exhibited a high percentage of viable cells, of about 94.6%. PQS produced a moderate effect against MSCs viability, as revealed by flow cytometry assay, this result being in a good accordance to microscopy data. Of MSCs grown in the presence of 50 μM PQS for 18 h, 14.8% proved to be necrotic, while only 1.2% of MSCs were apoptotic. In the presence of HHQ, the percentage of viable MSCs was about 52% after 18 h of incubation, much lower than that obtained for 50 μM OdDHL. Flow cytometry quantification of MSCs viability after incubated in the presence of 50 μM HHQ revealed that 45.9% of the analyzed MSCs undergone the necrotic and only 2.2% the apoptotic death pathway (Table 2).
Table 2. Percentages of viable, apoptotic, and necrotic MSCs, grown in the presence of 50 μM purified QSSMs for 18 h at 37 °C, 5%CO2, moist atmosphere.
| Staining, channel | MSC | OdDHL | C4HSL | PQS | HHQ |
|---|---|---|---|---|---|
| Q1: FITC−, FL3_PI+ | 7.01 | 32.2 | 5.2 | 14.8 | 45.9 |
| Q2: FITC+, FL3_PI+ | 0.007 | 0.508 | 0.022 | 0.337 | 0.389 |
| Q3: FITC+, FL3_PI− | 0.081 | 1.04 | 0.178 | 0.709 | 1.79 |
| Q4: FITC−, FL3_PI− | 92.9 | 66.2 | 94.6 | 84.1 | 51.9 |
MSC, untreated control (containing an equivalent amount of HPLC grade EtOH); Q1, gate comprising PI stained (necrotic) cells; Q2, gate comprising PI and annexin V-FITC stained (late apoptotic) cells; Q3, gate comprising annexin V-FITC stained (typical apoptotic) cells; Q4, gate comprising viable MSCs. Analysis was performed using CellQuest™ Pro software.
The analysis of MSCs gene expression profiles induced in the presence of the four different molecules revealed that OdDHL and HHQ activated the apoptosis signaling pathways (Fig. 2). The 50 µM OdDHL upregulated the expression of the pro-apoptotic bax and caspase9 genes and downregulated the expression of the anti-apoptotic bcl-2C and relA genes after 12–18 h of treatment. HHQ also promoted gene expression patterns indicating the occurrence of an apoptotic process, by upregulating pro-apoptotic and downregulating anti-apoptotic tested genes after only 6 h of treatment. Gene expression results strongly support the data provided by flow cytometry (Fig. 2).
Figure 2. Graphic representation of MSCs apoptosis and necrosis rates at phenotypic (A) and gene expression level (B) after grown in the presence of 50 µM purified QSSMs. (A) Apoptosis and necrosis calculated induction rates obtained after analyzing flow cytometry results of MSCs treated with 50 µM of OdDHL, C4-HSL, PQS, and HHQ for 18 h. MSC ctrl, untreated control; *P < 0.05, **P < 0.01, ***P < 0.001 (sample vs. untreated control, based on ANOVA and Bonferroni post test of medians of 3 independent experiments performed in triplicate [n = 3]). (B) Graphic representation of gene expression patterns for bax, bcl-2, casp9, and relA from MSCs grown in the presence of 50 µM OdDHL, C4-HSL, PQS, and HHQ for 2, 6, 12, and 18 h, incubation at 37 °C, 5% CO2.
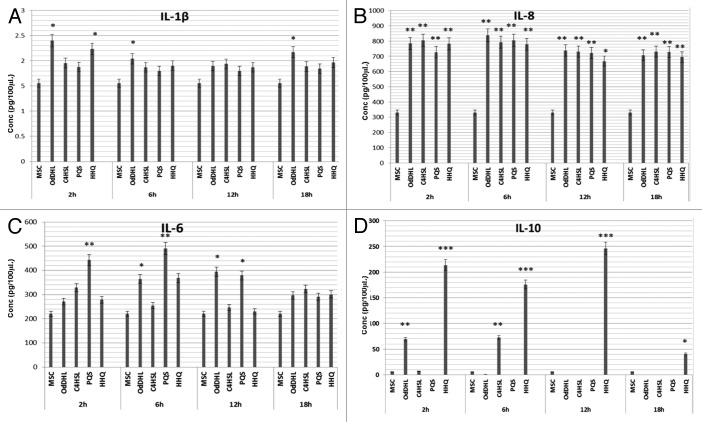
TNFα was not detected by ELISA in any of the tested samples, at any of the time points used for screening, even though a perfect test standard curve was obtained (data not shown). In exchange, 50 µM OdDHL slightly stimulated the IL-1β secretion after only 2 h of incubation, this low stimulatory effect remaining constant during time. All other tested QSSMs did not exhibit any effect on IL-1β levels for up to 18 h of experimentation (Fig. 3A).
Figure 3. Comparative evaluation of IL-1β (A), IL-8 (B), IL-6 (C), IL-10 (D) secretion profiles in MSCs supernatants after treatment with 50 μM of OdDHL, C4HSL, PQS, and HHQ for 2, 6, 12, and 18 h at 37 °C, 5% CO2. *P < 0.05, **P < 0.01, ***P < 0.001 (sample vs. untreated control, based on ANOVA and Bonferroni post test of medians of 3 independent experiments performed in duplicate).
The tested autoinducers significantly stimulated IL-8 accumulation in the MSCs supernatants, the intensity of this effect being stable for up to 18 h (Fig. 3B).
PQS greatly increased the IL-6 level for more than 2-fold. This effect occurred after 2–6 h of treatment and was slightly attenuated during time. The PQS molecular precursor, HHQ and the HSL autoinducer, OdDHL, also stimulated the IL-6 secretion, but at lower levels comparing with PQS. This stimulatory phenotype was significantly reduced after 6 h of treatment. C4-HSL exhibited no significant effect on IL-6 production at any of the tested time points (Fig. 3C).
HHQ has significantly stimulated IL-10 secretion by more than 20-fold, this strong effect appearing after 2 h of treatment and being maintained for at least 12 h. PQS proved no significant effect on the MSCs IL-10 secretion (Fig. 3D).
Discussion
The molecular interactions mediated by chemical signals occurring between pathogens and host during the infectious process are still poorly elucidated. Even if many recent studies are focused on the impact of bacteria QSSMs on host cells, findings state mainly the effect of AHL autoinducers, the interactions between 4Qs autoinducers and host cells remaining widely unknown. To our knowledge, this is the first report revealing the influence of P. aeruginosa-derived AHL and 4Q autoinducers on different morphological and biochemical parameters of MSCs. The Tripan Blue exclusion assay revealed that P. aeruginosa derived AHL and 4Q autoinducers impact differently on MSCs viability in a time-dependent manner, the most effective being OdDHL. This autoinducer induced MSCs cell death immediately after treatment, while C4-HSL, another investigated AHL signaling molecule had no effect on this phenotype at the tested concentration of 50 µM. This concentration was chosen according to previously reported literature data demonstrating that microbial QSSMs are rapidly degraded within host cells by the host defense mechanisms. Therefore, it is necessary to use higher amounts of purified QSSMs, of at least 10–50 μM17 or even 100 μM,18 comparing with the active concentrations of the autoinducers accumulated in a planktonic bacterial culture (of about 1–5 μM).17 These results confirm previous literature reports, performed on other cell types, which demonstrate that 50–100 μM OdDHL promotes cell death in macrophages and neutrophils, while most of short chain AHLs, including C4HSL have no impact on cell viability.19 The microscopic examination revealed that treated MSCs developed altered morphology and adherence, accompanied by modified staining properties. The observed morphological changes were different among the tested QSSMs, and they seemed to be enhanced during the incubation time. The OdDHL strongly altered the MSCs morphology after 2 h of treatment. The second HSL tested autoinducer, C4-HSL, proved a lower effect on MSCs morphology and staining properties. C4-HSL did not exhibit membrane permeability defects, since only few cells incorporated the PI dye, by comparison with MSCs undergoing HHQ treatment, which revealed increased membrane permeabilization for the PI dye. Annexin V–PI staining was used in order to efficiently discriminate between viable and non-viable cells and also between different cell deaths (i.e., apoptosis vs. necrosis). PI stains only DNA from cells with altered membrane (dead, necrotic), while annexin V can bind phosphatidylserine, exposed on the external side of apoptotic cells membrane. Flow cytometry assay revealed that the tested QSSMs exhibited different effects on MSCs viability after 18 h incubation. HHQ followed by OdDHL proved an enhanced detrimental impact on MSCs, supporting the results obtained by microscopy, inducing the highest percentages of necrotic and apoptotic cells. Even if flow cytometry results seemed to indicate that tested QSSMs have mainly a cytotoxic effect, promoting MSCs necrosis, detailed analysis revealed that apoptosis was also significantly affected. After the absolute quantification of obtained flow cytometry results it was observed that apoptosis was significantly promoted by tested QSSMs, many of the observed changes being statistically significant. The most significant calculated induction rate fold was observed when MSC cultures were grown in the presence of HHQ, the apoptosis rate exceeding 22-folds. Our results are contradictory to those obtained by Kim et al.20 on macrophages, showing that HHQ inhibited macrophage activation, but did not affect apoptosis, suggesting that their effects on immune system are not resulting from general alteration of cell functions.20 OdDHL and PQS induced a moderate apoptosis rate, comparing with HHQ, while C4-HSL failed to induce significant apoptosis or necrosis in MSCs, this result suporting the fact that bacterial QSSMs are able to diferentially impact on host cells signaling pathways.14 In order to demonstrate that some of tested QSSMs are able to regulate apoptosis in MSCs and also to interfere with host signaling pathways we performed qRT-PCR gene expression assay, targeting several genes involved in the control of apoptosis and secretory immune response, i.e., bax and caspase 9 pro-apoptotic genes and bcl-2C anti-apoptotic gene, as well as relA, the major subunit of NFκB, a nuclear factor involved both in apoptosis and immune response signaling pathways. It has been previously shown that P. aeruginosa induces host cell apoptosis upregulating the expression of bax and downregulating expression of bcl-2, resulting in increased levels of cytochrome c release and increased caspase 3 and caspase 9 in human U937 monocyte cells.21 Also, human mast cells undergo P. aeruginosa-mediated apoptosis through a mechanism involving both the Bcl family protein and mitochondrial-dependent pathway.22 A total of 30 pathways were found to be significantly modulated by OdDHL in chistic fibrosis airway epithelial cells, of which a substantial number (21/30) related to the activation of cellular innate immune and inflammatory responses, including TNF-α pathway, NFκB pathway, and cytokine production.23 Our results revealed that tested QSSMs differentially regulate MSCs gene expression in a time-dependent manner. Even if all tested molecules are able to drive changes within MSCs gene expression, only the expression profiles obtained for OdDHL and HHQ indicated the occurrence of an apoptotic process, by downregulating the expression of anti-apoptotic genes and upregulating pro-apoptotic genes. The ability of OdDHL and C4-HSL to induce apoptotic death in other epithelial and phagocyte cells was reported,19 but no data are available regarding the impact of AHLs and quinolone-derived autoinducers on the MSCs apoptosis. The finding that HHQ signaling molecule is able to induce the MSCs apoptosis by modulating the expression of apoptosis-regulatory genes, while PQS has a significantly diminished impact, represents the first observation demonstrating the effect of 4Qs on MSCs apoptosis.
There is substantial redundancy in the signaling pathways activated in response to pathogen-associated molecular patterns (PAMPs) released by gram-negative rods in the airway lumen. Host cells respond to these ligands via the apical display of TLRs and induction of NFκB-mediated proinflammatory gene expression that includes IL-6 and IL-8.24,25 OdDHL was extensively investigated for host immune response modulation, being shown that OdDHL has the ability to stimulate respiratory epithelial cells to produce IL-8 in a dose-dependent manner, and also to induce lymphocyte B IgE secretion. This effect is also correlated with peritoneal macrophage-derived IL-12 and TNF-α inhibition. Furthermore, OdDHL can also exhibit a mitogenic activity.26,27 Nevertheless, the impact of OdDHL and other QSSMs on mesenchymal stem cells cytokine profile and the temporal dynamics of the triggered effects were not previously reported to our knowledge. Considering their essential role in bacteria-derived immune response, we screened for the production of IL-1β, IL-6, IL-8, IL-10, and TNFα cytokines in the MSCs cultures treated with various QSSMs. ELISA assay revealed that P. aeruginosa-derived autoinducers have heterogeneous effects on the MSCs cytokines production profile depending on the exposure time. The level of IL-1β was increased in OdDHL treated MSCs supernatants. The most significantly stimulated cytokines were IL-8 (stimulated by all tested autoinducers), IL-6 (stimulated mostly by PQS, followed by HHQ and OdDHL), and IL-10 (significantly stimulated by HHQ and less by OdDHL and C4HSL). The concomitant activation of IL-1β and IL-8, both exhibiting an important role in neutrophils chemotaxis, may be responsible for the severe lung damage that accompanies P. aeruginosa infections.26 IL-8 increases the density of adhesins on phagocytes membrane, stimulating neutrophils activations, migration and lysosomal content release, next to the inflammatory center, explaining chronic inflammation and pulmonary injuries in infected patients.28 The fact that purified bacterial autoinducers are able to interfere with MSCs signaling pathways, modulating cellular death and their secretory profile may suggest that the accumulation of these mediators could interfere with their normal functions within the host body. The current enthusiasm surrounding the potential use of MSCs for therapeutic purposes is mainly based on their immunomodulatory and antimicrobial properties.9 If this balance is disturbed, their positive specific properties can be removed or even hijacked for pathogens own benefit, knowing their great versatility.
Conclusions
This paper is the first report on the effects of P. aeruginosa derived QSSMs on human stem mesenchymal cells viability, death signaling pathways, and cytokine profile. Our results demonstrate that the main pseudomonadal AHL and 4Q autoinducers differentially modulate MSCs death signaling pathways and secretory profiles. These observations may impact on the regenerative medicine field, since microbial infections represent one of the major treats for stem cells-based therapies and provide new insights in the mechanisms by which different QSSMs activate the host cells and promote tissue damages during P. aeruginosa infections.
Materials and Methods
Quorum sensing signaling molecules
N-(3-Oxododecanoyl)-L-homoserine lactone (OdDHL), N-([RS]-3-hydroxybutyryl)-L-homoserine lactone (C4-HSL), and 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS) were purchased from Sigma Aldrich, while HHQ (2-heptyl-4[1H]-quinolinone) was obtained from Chem Spider. All compounds were diluted with HLPC grade Methanol (Sigma Aldrich) and 10 mM stock solutions were stored at −20 °C.
Mesenchymal stem cells isolation
MSCs were obtained from human bone marrow, following a protocol adapted after Quiroz and collaborators,29 and respecting all required ethical issues. The protocol was accepted by the Faculty of Biology IRB committee, University of Bucharest (206:2004).30 Briefly, the bone marrow was harvested on EDTA in sterile flasks, less than 4 h before separation. Marrow was diluted using 2–4 volumes of phosphate saline buffer (PBS) and 35 mL diluted marrow was mixed with 15 mL separation medium Biocoll (Biochroma AG), warmed at 37 °C. The mixture was centrifuged for separation and the superior layer was removed. The cell ring containing MSCs, obtained on the separation medium interface was collected and washed two times by centrifugation in order to remove platelets and other debris. Approximately 40% MSCs were isolated with the current protocol. The cells were diluted to 3 × 106/flask and maintained at 37 °C, 5% CO2, moist atmosphere in αMEM medium (GIBCO), supplemented with 10% human serum (Gibco). After reaching ~80% confluence MSCs monolayer was treated with 50 µM of P. aeruginosa QS molecules and flasks were incubated at 37 °C, 5% CO2, in moist atmosphere. This concentration of QSSMs was selected based on previous literature reports and since our dose response curves data demonstrated that this is the minimum concentration required to insure the best significance and reproducibility of the results (data not shown). Furthermore, this dose is more likely to be found in vivo. Even though 10 µM methanol (MeOH) had no effect on the tested phenotypes the same amount used for the dilution of QSSMs was added for all controls.
The treated cells were harvested after 2, 6, 12, and 18 h of incubation. Each experiment was performed in triplicate and repeated on at least three separate occasions.
Morphology, viability, and cytotoxicity assay
The cell morphology was analyzed using a Nikon Eclipse TS100 (Nikon) fluorescence microscope. For microscopic examination, the treated MSCs were washed with PBS, fixed with cold Methanol and stained with propidium iodide (PI) and fluorescein diacetate (FDA) (Sigma Aldrich), following a protocol adapted from.31 PI/FDA double staining allows efficient discrimination between live (green) and dead (red) cells. At least 3 microscopic fields were analyzed for each sample.
Viability assessments were performed using (0.4%) Trypan blue (Invitrogen) staining using an automated cell counter, Countess (Invitrogen), according to the manufacturer instructions.
Flow cytometry was used for quantifying the number of apoptotic/necrotic cells after treatment. Harvested MSCs were stained with annexin V-FITC and PI (Sigma Aldrich), following manufacturer’s recommendations. Samples were analyzed using a FACS Calibur (BD) cytometer and results were quantified using CellQuest™ Pro software. Absolute apoptosis and necrosis fold induction rates were calculated by subtracting the average value obtained for each QSSM from untreated control, which appears as 0 on the graph.
Gene expression assay
Gene expression of pro-apoptotic (bax, caspase 9) and anti-apoptotic (relA, bcl-2) selected genes was quantified using real-time quantitative reverse transcription PCR (qRT-PCR). These genes were chosen according to previous published reports.19,32 Treated MSCs were harvested, adhered cells being detached after adding one volume of 0.25% preheated trypsin and mechanical pipetting. The obtained cell suspension was centrifuged and MSCs sediments were used for Trizol (Ambion®) RNA purification. Reverse transcription was performed using Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Scientific), following manufacturer’s recommendations. Real Time PCR was performed using an ABI 7300 Real Time PCR System using Taqman Universal PCR Master Mix (Applied Biosystems) and pre-validated Taqman Gene Expression Assay kits (Applied Biosystems). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control (Assay ID: Hs99999905_m1, Applied Biosystems). Each sample was performed in triplicate and repeated on at least three separate occasions. Results were analyzed using RQ study software (Applied Biosystems). The ΔΔCT method was used to compare the relative expression levels of pro-/anti-apoptotic genes mRNAs. Gene expression was normalized based on the levels of mRNA for GAPDH. The normalized gene expression level of untreated MSCs was set as 1, to which the expression levels of samples were compared and then presented as fold changes. The gene expression levels were plotted as log10 values, therefore, the expression level of the calibrator samples appear as 0 in the graphs.
Enzyme linked immunosorbent assay (ELISA)
The IL-1β, IL-6, IL-8, IL-10, and TNFα levels in MSCs supernatants were determined by ELISA commercial kits (Pierce, Thermo Scientific), according to the manufacturer’s instructions. Absorbance of the samples was assessed at 450 nm and 550 nm, using a combined spectrophotometer (GeniosPro, Tecan). All tests were performed in duplicate and repeated on at least three separate occasions.
Statistical analysis
One way analysis of variance (ANOVA) was used to analyze the data (GraphPad In Stat software). Bonferroni post test was used when appropriate. P values < 0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was possible with the financial support of the Grant Ideas 154/2011.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/27571
References
- 1.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–22. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–38. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–9. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward C, Forrest IA, Murphy DM, Johnson GE, Robertson H, Cawston TE, Fisher AJ, Dark JH, Lordan JL, Kirby JA, et al. Phenotype of airway epithelial cells suggests epithelial to mesenchymal cell transition in clinically stable lung transplant recipients. Thorax. 2005;60:865–71. doi: 10.1136/thx.2005.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saviuc C, Marinas I, Grumezescu AM, Bleotu C, Chifiriuc C, Mihaiescu D, Lazar V. Phytochemical composition of the fennel fruits essential oil and its influnence on prokariotic cells growth and pathogenic features. Biointerf Res in Appl Chem. 2012;l:300–5. [Google Scholar]
- 9.Grumezescu AM, Holban AM, Andronescu E, Tomoiaga M, Ficai A, Bleotu C, Chifiriuc MC. Microbiological applications of a new water dispersible magnetic nanobiocomposite. Lett. Appl. NanoBioSci. 2012;1:83–90. [Google Scholar]
- 10.Borthwick LA, Sunny SS, Oliphant V, Perry J, Brodlie M, Johnson GE, Ward C, Gould K, Corris PA, De Soyza A, et al. Pseudomonas aeruginosa accentuates epithelial-to-mesenchymal transition in the airway. Eur Respir J. 2011;37:1237–47. doi: 10.1183/09031936.00088410. [DOI] [PubMed] [Google Scholar]
- 11.Smith L, Rose B, Tingpej P, Zhu H, Conibear T, Manos J, Bye P, Elkins M, Willcox M, Bell S, et al. Protease IV production in Pseudomonas aeruginosa from the lungs of adults with cystic fibrosis. J Med Microbiol. 2006;55:1641–4. doi: 10.1099/jmm.0.46845-0. [DOI] [PubMed] [Google Scholar]
- 12.Holban AM, Lazar V. Inter-kingdom cross-talk: the example of prokaryotes - eukaryotes communication. Biointerf Res Appl Chem. 2011;1:95–110. [Google Scholar]
- 13.Saviuc C, Cotar AI, Holban AM, Banu O, Grumezescu AM, Chifiriuc MC. Phenotypic and molecular evaluation of Pseudomonas aeruginosa and Staphylococcus aureus virulence patterns in the presence of some essential oils and their major compounds. Lett in Appl NanoBioSci. 2013;2:91–6. [Google Scholar]
- 14.Holban AM, Chifiriuc MC, Lazǎr V. Host cells response in Pseudomonas aeruginosa infections - role of quorum sensing molecules. Afr J Microbiol Res. 2013;7:2140–9. [Google Scholar]
- 15.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Cámara M, et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol. 2007;14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Chifiriuc MC, Demetra S, Veaceslav M, Lazar V, Grigore M, Bleotu C. Host immune response to Chlamydia infection. In Chlamydia, Edited by Mihai Mares, Published by InTech, 1012, ISBN 978-953-51-0470-49. [Google Scholar]
- 17.Kravchenko VV, Ulevitch RJ, Kaufmann GF. Modulation of mammalian cell processes by bacterial quorum sensing molecules. Methods Mol Biol. 2011;692:133–45. doi: 10.1007/978-1-60761-971-0_10. [DOI] [PubMed] [Google Scholar]
- 18.Vikström E, Magnusson KE, Pivoriūnas A. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone stimulates phagocytic activity in human macrophages through the p38 MAPK pathway. Microbes Infect. 2005;7:1512–8. doi: 10.1016/j.micinf.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;71:5785–93. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Kim SH, Lépine F, Cho YH, Lee GR. Global gene expression analysis on the target genes of PQS and HHQ in J774A.1 monocyte/macrophage cells. Microb Pathog. 2010;49:174–80. doi: 10.1016/j.micpath.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Chai WS, Zhu XM, Li SH, Fan JX, Chen BY. Role of Bcl-2 family members in caspase-3/9-dependent apoptosis during Pseudomonas aeruginosa infection in U937 cells. Apoptosis. 2008;13:833–43. doi: 10.1007/s10495-008-0197-6. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins CE, Swiatoniowski A, Power MR, Lin TJ. Pseudomonas aeruginosa-induced human mast cell apoptosis is associated with up-regulation of endogenous Bcl-xS and down-regulation of Bcl-xL. J Immunol. 2006;177:8000–7. doi: 10.4049/jimmunol.177.11.8000. [DOI] [PubMed] [Google Scholar]
- 23.Mayer ML, Sheridan JA, Blohmke CJ, Turvey SE, Hancock RE. The Pseudomonas aeruginosa autoinducer 3O-C12 homoserine lactone provokes hyperinflammatory responses from cystic fibrosis airway epithelial cells. PLoS One. 2011;6:e16246. doi: 10.1371/journal.pone.0016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng J, Do J, Widdicombe JH, Machen TE. Innate immune responses of human tracheal epithelium to Pseudomonas aeruginosa flagellin, TNF-alpha, and IL-1beta. Am J Physiol Cell Physiol. 2006;290:C678–90. doi: 10.1152/ajpcell.00166.2005. [DOI] [PubMed] [Google Scholar]
- 25.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123:1630–7. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J Immunol. 2001;167:366–74. doi: 10.4049/jimmunol.167.1.366. [DOI] [PubMed] [Google Scholar]
- 27.Smith RS, Kelly R, Iglewski BH, Phipps RP. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J Immunol. 2002;169:2636–42. doi: 10.4049/jimmunol.169.5.2636. [DOI] [PubMed] [Google Scholar]
- 28.Belay T, Sonnenfeld G. Differential effects of catecholamines on in vitro growth of pathogenic bacteria. Life Sci. 2002;71:447–56. doi: 10.1016/S0024-3205(02)01683-1. [DOI] [PubMed] [Google Scholar]
- 29.Quiroz FG, Posada Estefan OM, Gallego Pérez D, Higuita Castro N, Sarassa Velásquez CA, Hansford DJ, Florez PA, López Rojas LE. Isolation of human bone marrow mesenchymal stem cells and evaluation of their osteogenic potential. Rev Ingen Bioméd. 2008;2:48–55. [Google Scholar]
- 30. http://www.bio.unibuc.ro/pdf/cod_etica/cod_animale_om.pdf
- 31.Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985;33:77–9. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- 32.Iordache C, Bleotu C, Holban A, Lixandru M, Cotar A, Lazar V, Antohe F, Chifiriuc MC. Differential effects on caspase mediated apoptosis of HeLa cells induced by different Pseudomonas aeruginosa culture fractions. IJABPT. 2011;2:132–8. [Google Scholar]