Abstract
NrdH redoxins are small protein disulfide oxidoreductases behaving like thioredoxins but sharing a high amino acid sequence similarity to glutaredoxins. Although NrdH redoxins are supposed to be another candidate in the antioxidant system, their physiological roles in oxidative stress remain unclear. In this study, we confirmed that the Corynebacterium glutamicum NrdH redoxin catalytically reduces the disulfides in the class Ib ribonucleotide reductases (RNR), insulin and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), by exclusively receiving electrons from thioredoxin reductase. Overexpression of NrdH increased the resistance of C. glutamicum to multiple oxidative stresses by reducing ROS accumulation. Accordingly, elevated expression of the nrdH gene was observed when the C. glutamicum wild-type strain was exposed to oxidative stress conditions. It was discovered that the NrdH-mediated resistance to oxidative stresses was largely dependent on the presence of the thiol peroxidase Prx, as the increased resistance to oxidative stresses mediated by overexpression of NrdH was largely abrogated in the prx mutant. Furthermore, we showed that NrdH facilitated the hydroperoxide reduction activity of Prx by directly targeting and serving as its electron donor. Thus, we present evidence that the NrdH redoxin can protect against the damaging effects of reactive oxygen species (ROS) induced by various exogenous oxidative stresses by acting as a peroxidase cofactor.
INTRODUCTION
Reactive oxygen species (ROS) are harmful by-products formed during aerobic metabolism, and the excess production of ROS results in oxidative stress and cellular damage. To protect against the adverse effects of ROS, bacteria have evolved an elaborate antioxidant system, including antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), glutaredoxin (Grx), and thioredoxin (Trx), and low-molecular-weight antioxidants, such as the tripeptide glutathione (GSH), β-carotene, and vitamins (vitamins C and E) (1). The major antioxidant enzymes, thioredoxin and glutaredoxin, are small thiol-disulfide oxidoreductases containing a conserved catalytic site (CXXC) with a redox-active disulfide, which is essential for reducing disulfide bonds of target proteins and maintaining intracellular redox homoeostasis (2, 3). Trx and Grx can reduce disulfides of metabolic enzymes such as ribonucleotide reductase (4, 5), phosphoadenosine-phosphosulfate reductase (6, 7), methionine sulfoxide reductase (8, 9), and arsenate reductase (10) by using their active-site cysteine residues in the CXXC motif. Trx and Grx can also provide electrons to peroxiredoxins and nonheme peroxidases to protect against oxidative stress via removing hydroperoxides (11). On the other hand, the most abundant low-molecular-weight thiol, GSH, constitutes a redox buffer in the cytoplasm and is considered to be the main nonenzymatic antioxidant in Gram-negative bacteria and eukaryotes (12, 13). The cysteine thiol of GSH can protect cells against ROS by directly clearing free radicals and also by cooperating with Grx and functioning to reduce disulfide bonds for antioxidant enzymes such as glutathione peroxidase and methionine sulfoxide reductase (9, 11, 14).
Similar to GSH from eukaryotes and Gram-negative bacteria, mycothiol (1-d-myo-inosityl-2-[N-acetyl-l-cysteinyl] amido-2-deoxy-α-d-glucopyranoside; MSH) is the major nonenzymatic antioxidant in the high-G+C-content Gram-positive Actinobacteria, including a group of bacteria of medical, industrial, and environmental significance, such as members of Corynebacterium, Mycobacterium, Rhodococcus, and Streptomyces (15, 16). MSH has been reported to play important roles in tolerance to oxidative and acid stresses, antibiotics, aromatic compounds, and heavy metal ions (15–18). After substantial progress was made in MSH research in the past decade, a lot of efforts have been put on the identification and characterization of novel antioxidant enzymes in Actinobacteria recently. Ordóñez et al. (10) located two hypothetical mycoredoxins, Mrx1 (NCgl0808) and Mrx2 (NCgl2445), in the Corynebacterium glutamicum genome based on homology to the Escherichia coli glutaredoxin genes. Mrx1 shares 34% sequence identity with E. coli Grx1 and is characterized by a thioredoxin structural fold with a putative MSH binding site. It was further confirmed to be a mycothiol-dependent thiol-disulfide reductase that reduces MSH mixed disulfide bonds using electrons from the MSH/mycothione reductase (Mtr)/NADPH pathway and can be considered the glutaredoxin (Grx) analog of the Actinomycetes (10). Using an mrx1 deletion mutant, Van Laer et al. (19) showed that Mrx1 is involved in the protection against oxidative stress in Mycobacterium smegmatis. However, Mrx2 was recently proposed to be an NrdH redoxin but not a true mycoredoxin, as it showed an amino acid sequence identity of 75% with NrdH redoxin from Corynebacterium ammoniagenes and was specifically reduced by TrxR but not MSH (20).
NrdH redoxins are small protein disulfide oxidoreductases functioning as hydrogen donors for the class Ib ribonucleotide reductases (RNR) (21–23). Although NrdH redoxins share a high level of sequence similarity to that of glutaredoxins, they behave like thioredoxins and contain a typical thioredoxin fold with the active-site motif CXXC (24, 25). NrdH redoxins have been investigated mainly in GSH-lacking bacteria, such as Staphylococcus aureus (23), Lactococcus lactis (26), and Bacillus anthracis (27), and in GSH-containing E. coli (21). In these bacteria, they function as efficient general disulfide oxidoreductase that reduces insulin, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and the manganese-containing class Ib RNR in the presence of NADPH and thioredoxin reductase (21, 23, 28). Recently, Van Laer et al. (20) solved the crystal structure of NrdH from C. glutamicum and revealed the presence of a typical thioredoxin fold with the active-site CXXC motif and a TrxR binding hydrophobic pocket located near its CXXC motif at the surface. Both C. glutamicum and Mycobacterium tuberculosis NrdH redoxins catalytically reduce the disulfide in DTNB, by exclusively receiving electrons from thioredoxin reductase (TrxR) but not MSH (20).
Although NrdH redoxins were supposed to be another candidate in the antioxidant system of bacteria lacking GSH, their physiological functions have not been experimentally confirmed. The nrdH gene in S. aureus appears to have no role in coping with oxidative stress, as the nrdH mutant grew as well as the wild type even under severe oxidative stress (23). In contrast, the NrdH-encoding gene seems to be essential for C. glutamicum, as mutants lacking nrdH are not viable (20). In this study, in addition to biochemical characterization, we also examined the physiological roles of NrdH in response to oxidative stress by overexpressing this gene in C. glutamicum. We present evidence that the NrdH redoxin can protect against the damaging effects of ROS induced by various exogenous oxidative stresses by acting as a peroxidase cofactor.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. C. glutamicum and E. coli strains were cultured in Luria-Bertani (LB) broth aerobically on a rotary shaker (220 rpm) or on LB plates at 30°C and 37°C, respectively. To construct the C. glutamicum ΔnrdH and Δprx in-frame deletion mutants, plasmid pK18mobsacB-ΔnrdH and pK18mobsacB-Δprx were transformed into C. glutamicum RES167 by electroporation, and chromosomal integration was selected by plating on LB agar plates supplemented with kanamycin and nalidixic acid. The deletion mutants were subsequently screened on LB agar plates containing 20% sucrose and confirmed by PCR and DNA sequencing as previously described (29). When needed, antibiotics were used at the following concentrations: chloramphenicol, 20 μg ml−1 for E. coli and 10 μg ml−1 for C. glutamicum; kanamycin, 50 μg ml−1 for E. coli and 25 μg ml−1 for C. glutamicum; nalidixic acid, 40 μg ml−1 for C. glutamicum; ampicillin, 100 μg ml−1 for E. coli.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype description | Reference or source |
|---|---|---|
| Strains | ||
| Corynebacterium glutamicum | ||
| RES167 | Restriction-deficient mutant of ATCC 13032, Δ(cglIM-cglIR-cglIIR) | 61 |
| Δprx mutant | Fragment of DNA encoding amino acids 10 to 164 of Prx was deleted in RES167 | This study |
| WT(pXMJ19) | RES167 containing pXMJ19 vector | This study |
| WT(pXMJ19-nrdH) | Overexpression of nrdH in RES167 | This study |
| WT(pXMJ19-nrdH::SXXS) | Overexpression of nrdH::SXXS in RES167 | This study |
| Δprx(pXMJ19) | Δprx mutant containing pXMJ19 vector | This study |
| Δprx(pXMJ19-nrdH) | Overexpression of nrdH in Δprx mutant | This study |
| Escherichia coli | ||
| BL21(DE3) | Host for expression vector pET28a | Novagen |
| XL1-Blue | Host for expression vector pGEX6p-1 | Stratagene |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′(traD36proAB lacIq lacZΔM15) | Stratagene |
| BTH101 | Host for the bacterial two-hybrid system | 31 |
| Plasmids | ||
| pK18mobsacB | Suicide plasmid carrying sacB for selecting double crossover in C. glutamicum, Kmr | 62 |
| pK18mobsacB-Δprx | Construct used for in-frame deletion of prx | This study |
| pK18mobsacB-ΔnrdH | Construct used for in-frame deletion of nrdH | This study |
| pK18mobsacB-PnrdH::lacZ | PnrdH::lacZ promoter fusion in pK18mobsacB | This study |
| pXMJ19 | Shuttle vector (Ptac lacIq pBL1 oriVC. glutamicum pK18 oriVE. coli), Cmr | 63 |
| pXMJ19-nrdH | nrdH cloned into pXMJ19 for complementation | This study |
| pXMJ19-nrdH::SXXS | nrdH::SXXS cloned into pXMJ19 for complementation | This study |
| pKT25 | p15A origin of replication encoding CyaA1–224; Kmr | 31 |
| pUT18C | ColE1 origin of replication encoding CyaA225–399; Ampr | 31 |
| pKT25-zip | BTH-positive control | 31 |
| pUT18C-zip | BTH-positive control | 31 |
| pKT25-prx | prx in pKT25 | This study |
| pUT18C-nrdH | nrdH in pUT18C | This study |
| pUT18C-nrdH::CXXS | nrdH::CXXS in pUT18C | This study |
| pUT18C-nrdH::SXXC | nrdH::SXXC in pUT18C | This study |
| pUT18C-nrdH::SXXS | nrdH::SXXS in pUT18C | This study |
| pGEX6P-1 | Expression vector with N-terminal GST tag, Ampr | GE Healthcare |
| pGEX6P-1-prx | pGEX6P-1 derivative for expression of prx | This study |
| pET28a | Expression vector with N-terminal His6 tag, Kmr | Novagen |
| pET28a-nrdH | pET28a derivative for expression of nrdH | This study |
| pET28a-nrdH::SXXC | pET28a derivative for expression of nrdH::SXXC | This study |
| pET28a-nrdH::CXXS | pET28a derivative for expression of nrdH::CXXS | This study |
| pET28a-nrdH::SXXS | pET28a derivative for expression of nrdH::SXXS | This study |
| pET28a-mtr | pET28a derivative for expression of mtr | This study |
| pET28a-trxR | pET28a derivative for expression of trxR | This study |
| pET28a-trx | pET28a derivative for expression of trx | This study |
| pET28a-mrx1 | pET28a derivative for expression of mrx1 | This study |
| pET28a-prx | pET28a derivative for expression of prx | This study |
| pET28a-nrdE | pET28a derivative for expression of nrdE | This study |
| pET28a-nrdF | pET28a derivative for expression of nrdF | This study |
Plasmid construction.
All primers used in this study are listed in Table S1 in the supplemental material. The genes encoding C. glutamicum thioredoxin (Trx; NCgl2985 gene), thioredoxin reductase (TrxR; NCgl2984 gene), mycoredoxin 1 (Mrx1; NCgl0808 gene), NrdH (Mrx2; NCgl2445 gene), mycothione reductase (Mtr; NCgl1928 gene), thiol peroxidase (Prx; NCgl1041 gene), ribonucleotide-diphosphate reductase subunit beta (NrdF; NCgl2438 gene), and ribonucleotide-diphosphate reductase subunit alpha (NrdE; NCgl2443 gene) were amplified by PCR using genomic DNA of C. glutamicum RES167 as the template, with the primers indicated in Table S1. These DNA fragments were digested and afterward subcloned into appropriately digested pET28a, pGEX6p-1, pKT25, or pUT18C vectors, obtaining plasmids pET28a-trx, pET28a-trxR, pET28a-prx, pET28a-mrx1, pET28a-nrdH, pET28a-mtr, pET28a-nrdE, pET28a-nrdF, pGEX6p-1-prx, pKT25-prx, and pUT18C-nrdH. The plasmid pK18mobsacB-ΔnrdH was used to construct the C. glutamicum nrdH deletion mutant. The 900-bp upstream PCR product and 862-bp downstream PCR product of nrdH were amplified using primer pairs DNrdH-F1/DNrdH-R1 and DNrdH-F2/DNrdH-R2, respectively. In the next step, the upstream and downstream PCR fragments were fused together with primer pairs DNrdH-F1/DNrdH-R2 by overlap PCR (30), and the resulting DNA fragments were digested with BamHI/SalI and inserted into similarly digested suicide plasmid pK18mobsacB to create pK18mobsacB-ΔnrdH. The plasmid pK18mobsacB-Δprx used to construct the prx mutant was constructed similarly by using primers DPrx-F1/DPrx-R1 and DPrx-F2/DPrx-R2. Site-directed mutagenesis was carried out by overlap PCR (30) to mutate the active-site cysteine residue at position 11 of NrdH into a serine residue (NrdH::SXXC). In brief, the mutant nrdH::SXXC DNA segment was amplified by two rounds of PCR. Primer pairs DNrdH-F1/NrdH-C11S-R and NrdH-C11S-F/DNrdH-R2 were used to amplify segments 1 and 2, respectively. The second round of PCR was carried out by using NrdH-F/NrdH-R as the primer pair, while fragment 1 and fragment 2 were used as the templates to get the nrdH::SXXC segment, which contained a mutation in the 11C site of NrdH. The nrdH::SXXC DNA fragment was digested and cloned into similar digested pET28a or pUT18C plasmid, obtaining plasmid pET28a-nrdH::SXXC or pUT18C-nrdH::SXXC, respectively. The nrdH::CXXS and nrdH::SXXS DNA fragments were obtained by using a procedure similar to that described above, with the primers indicated in Table S1 in the supplemental material. These DNA fragments were also cloned into plasmid pET28a and pUT18C, obtaining plasmid pET28a-nrdH::CXXS, pET28a-nrdH::SXXS, pUT18C-nrdH::CXXS, or pUT18C-nrdH::SXXS. For overexpression of nrdH in C. glutamicum RES167, nrdH and nrdH::SXXS DNA fragments were digested and cloned into similarly digested pXMJ19 vector to yield pXMJ19-nrdH and pXMJ19-nrdH::SXXS, respectively. The resulting plasmids, pXMJ19-nrdH and pXMJ19-nrdH::SXXS, were transformed into wild-type C. glutamicum RES167 and the prx mutant by electroporation, respectively. The transformants were selected on LB agar plates supplemented with nalidixic acid and chloramphenicol. Expression in C. glutamicum was induced by the addition of 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) into cultures. The lacZ fusion reporter vector pK18mobsacB-PnrdH::lacZ was made by fusion of the nrdH promoter to the lacZY reporter gene by overlap PCR (30). In the first round of PCR, the 1,000-bp NrdH promoter DNA fragment and the lacZY DNA fragment were amplified with the primer pair PnrdH-F/PnrdH-R and lacZY-F/lacZY-R, respectively. The first-round PCR products were used as the template in the second round of PCR with PnrdH-F and lacZY-R as primers, and the resulting PCR fragment was digested with SalI/PstI and inserted into the SalI/PstI site of the suicide vector pK18mobsacB to get the pK18mobsacB-PnrdH::lacZ fusion construct. The fidelity of all constructs was confirmed by DNA sequencing (Sangon Biotech, Shanghai, China).
Overexpression and purification of recombinant proteins.
To express and purify GST- and His6-tagged proteins, recombinant pGEX6p-1 and pET28a plasmids were transformed into E. coli XL1-Blue and BL21(DE3) host strains, respectively. For protein production, bacteria were grown at 37°C in LB medium to an optical density at 600 nm (OD600) of 0.5, shifted to 22°C, and then induced with 0.4 mM IPTG and cultivated for an additional 12 h at 22°C. Harvested cells were disrupted by sonication and purified with the His·Bind Ni-nitrilotriacetic acid (NTA) resin or the GST·Bind resin (Novagen, Wisconsin, USA) based on the manufacturer's instructions. Purified recombinant proteins were dialyzed against phosphate-buffered saline (PBS) overnight at 4°C and stored at −80°C until use. Protein concentrations were determined using the Bradford assay based on the manufacturer's instructions (Bio-Rad, California, USA), with bovine serum albumin as the standard.
Bacterial two-hybrid assay.
Bacterial two-hybrid complementation assays were carried out as previously described (31, 32). Efficiencies of interactions between different hybrid proteins were quantified by measurement of β-galactosidase activity of overnight cultures (OD600 = 1.5) grown in LB broth at 37°C. The overnight cultures (50 μl) were permeabilized with 420 μl PBS buffer containing 20 μl chloroform and 10 μl 0.1% SDS at 30°C for 1 h, and β-galactosidase activities were assayed with o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate as described previously (33). All the experiments were performed at least three times.
GST pulldown assay.
The GST pulldown assay was performed as previously described (34, 35) with the following modifications. Briefly, purified GST-Prx was mixed with His6-NrdH::CXXS or His6-NrdH::SXXS in PBS on a rotator for 2 h at 4°C, and GST was used as a negative control. After 40 μl of prewashed glutathione bead slurry was added, binding was allowed to proceed for another 2 h at 4°C. The beads were then washed 5 times with TEN buffer (100 mM Tris-Cl [pH 8.0], 10 mM EDTA, 500 mM NaCl). Retained proteins were detected by immunoblotting after SDS-PAGE by using the anti-His antibody (Millipore, Massachusetts, USA).
Western blot analysis.
For Western blot analysis, samples resolved by SDS-PAGE were transferred onto polyvinylidene difluoride (PVDF) or nitrocellulose membranes. After blocking with 4% milk for 2 h at room temperature, membranes were probed with the appropriate primary antibody overnight at 4°C: anti-His (Millipore, Massachusetts, USA), 1:1,000; anti-DNP (2,4-dinitrophenol) antibody (Millipore, Massachusetts, USA), 1:500. The blots were washed several times in PBST buffer (PBS buffer containing 0.2% Tween 20) and then incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated secondary antibody (Shanghai Genomics Inc., Shanghai, China) in PBST for 2 h. The proteins were visualized by using the ECL plus kit (GE Healthcare, New Jersey, USA) by following the manufacturer's specified protocol.
Construction of chromosomal fusion reporter strains and β-galactosidase assay.
The lacZ fusion reporter plasmid pK18mobsacB-PnrdH::lacZ was transformed into the wild-type C. glutamicum by electroporation, and the chromosomal pK18mobsacB-PnrdH::lacZ fusion reporter strain was selected by plating on LB agar plates supplemented with kanamycin. β-Galactosidase activities were assayed with ONPG as the substrate (33). The β-galactosidase data represent the means from one representative assay performed in triplicate, and the error bars represent the standard deviation (SD). Statistical analysis was carried out with Student's t test.
MSH purification.
MSH was purified from C. glutamicum RES167 with thiopropyl Sepharose 6B and successively with Sephadex LH-20 chromatography as described previously (36). Overnight-grown cells were harvested and lysed with equal volumes of 0.75 M perchloric acid at room temperature for 3 h. Acid-insoluble cellular debris was removed by centrifugation at 10,000 × g for 30 min. The supernatant was adjusted to pH 4.6 with 4 M KOH and then stored at 0°C for 2 h. Precipitated potassium perchlorate was removed by centrifugation at 10,000 × g for 30 min. The supernatant was mixed with buffer A (0.4 M Tris-HCl, 2 M NaCl, 4 mM EDTA, pH 7.5) and applied onto a thiopropyl Sepharose 6B column (Amersham Biosciences). The column was washed with buffer B (0.1 M Tris-HCl, 0.5 M NaCl, 1 mM EDTA, pH 7.5) and then eluted with buffer C (0.1 M Tris-HCl, 1 mM EDTA, 30 mM dithiothreitol [DTT], pH 7.5). The active eluant was applied onto a Sephadex LH20 column (Amersham Biosciences) and fractionated with 50% methanol in water, pH 4.5. The active fractions were concentrated with a rotary evaporator, lyophilized, and finally dissolved in water. The concentration of purified MSH was measured by the thiol-specific fluorescent-labeling high-performance liquid chromatography (HPLC) method (16) with commercial glutathione (GSH) as the thiol standard reference. The HPLC used in this study was equipped with an Extend-C18 column (Zorbax; 250 by 4.6 mm) and was operated with gradients of buffer D (0.25% acetic acid in distilled water titrated to pH 3.6 with NaOH) and buffer E (HPLC-grade methanol), with an eluant flow rate of 0.9 ml/min. The proportion of buffer E in continuous gradients was as follows: 12% at 0 to 5 min, 50% at 5 to 15 min, 100% at 15 to 30 min. The bimane derivative of MSH was eluted at about 15 min in this system.
Assays for ribonucleotide reductase.
Ribonucleotide reductase (RNR) activity assays were performed based on the method described by Ceylan et al. (37) with minor modifications, such as the following: RNR activity was measured from the conversion of GDP to dGDP by coupling the reaction to NADPH oxidation. With the TrxR-based regeneration system used in the assay, the reaction was determined in the presence of 50 mM HEPES, 100 mM KCl, 7 mM MgCl2 (pH 7.6), 200 μM NADPH, 500 μM dTTP, 1.6 mM ATP, 3 μM NrdE, 12 to 18 μM NrdF, 3 μM TrxR, and different NrdH variants (10 μM). In alternative assays with the Mtr/MSH-based regeneration system used, TrxR was replaced by 3 μM Mtr and 1 mM MSH. The reaction was started by adding 500 μM GDP, and the NADPH oxidation was monitored at 340 nm. The absorbance value (ΔA) was obtained by subtracting the background absorbance measurement in the absence of GDP from the value given by the reaction, and the reaction was carried out for 10 min. RNR activity was calculated from the ΔΔA/min value [ΔΔA/min = (ΔA10 − ΔA0)/10 min, where the subscript values indicate time in minutes]. As a positive control, Trx (10 μM) was used in place of NrdH in the same conditions.
Insulin disulfide reduction assay.
Insulin disulfide reduction was assayed as described previously (38). Briefly, insulin (0.32 mM final concentration) was added to a colorimetric tube containing either 0.5 ml of 1 mM MSH, 0.2 mM NADPH, 3 μM Mtr, 0.1 mg/ml bovine serum albumin, 50 mM Tris-Cl (pH 8.0), or 0.5 ml of 50 mM Tris-HCl (pH 7.5), as well as 1 mM EDTA, 200 μM NADPH, and 10 μM TrxR. The reaction was started by adding different NrdH variants (NrdH, NrdH::SXXC, NrdH::CXXS, or NrdH::SXXS; final concentration of 10 μM) and monitored by measuring the decrease in absorbance at 340 nm during the first hour of reaction every 1 min. As a positive control, Trx (10 μM) was used in place of NrdH in the same conditions.
DTNB assay.
DTNB [5,5′-dithiobis-(2-nitrobenzoic acid)] assays were performed upon the method described by Holmgren (5). Reduction of DTNB disulfides (final concentration of 1 mM) was measured in the presence of 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 200 μM NADPH, 10 μM TrxR, and either 10 μM NrdH or its variants (NrdH::SXXC, NrdH::CXXS, or NrdH::SXXS). The activity was monitored by following the increase at 412 nm during the first 10 min due to production of 3-carboxy-4-nitrobenzenethiol (NBT). Trx (10 μM) was used in place of NrdH as a positive control in the same conditions. The activity of NrdH and its variants (final concentration of 10 μM) as reductants of DTNB disulfides (final concentration of 1 mM) was also determined in the mixture containing 1 mM MSH, 200 μM NADPH, 3 μM Mtr, 0.1 mg/ml bovine serum albumin, and 50 mM Tris-Cl at pH 7.5. The activity was in this case monitored as the increase at 412 nm due to cleavage of the disulfide of DTNB.
HED assay.
The hydroxyethyl disulfide (HED) assay was performed based on Kim et al. (39) by measuring the NrdH-catalyzed reduction of the HED-SSM. The mixed disulfide (HED-SSM) was formed by incubating 0.7 mM HED with 1 mM MSH at 30°C for 5 min. The HED-SSM (final concentration of 0.25 mM) acting as the substrate of NrdH was added to the electron transfer medium in a final volume of 200 μl with 0.5 μM Mtr, 200 μM NADPH, and 10 μM NrdH or medium with 10 μM TrxR, 200 μM NADPH, and 10 μM NrdH. The reactions were carried out at 25°C for 10 min, and the absorption of NADPH at 340 nm was recorded. As a positive control, Mrx1 was used in place of NrdH in the reaction mixture.
Peroxidase activity assay.
Peroxidase activity of NrdH with H2O2 and cumene hydroperoxide (CHP) was determined as described previously (40) by monitoring the rate of NADPH oxidation. The reaction mixture (500 μl) comprised 50 mM Tris-HCl buffer (pH 8.0), 2 mM EDTA, 200 μM NADPH, 3 μM TrxR, 2 μM Prx, and 5 μM NrdH or its variants. The reaction was started by the addition of H2O2 or CHP at final concentrations of 100 μM following a 5-min preincubation, and the oxidation of NADPH at 340 nm was monitored. One unit of enzymatic activity is defined as the oxidation of 1 nmol of NADPH/min. Trx (5 μM) was used in place of NrdH as a positive control in the same conditions. Apparent kinetic parameters of Prx toward H2O2-CHP and NrdH were determined at 5 μM NrdH, 0 to 400 μM H2O2-CHP, or 200 μM H2O2-CHP, 0 to 30 μM NrdH. The consumption of NADPH was monitored for 15 min, with a step of 1 min.
Peroxidase activity was also detected by using the ferrous xylenol orange (FOX) assay (41). The consumption of peroxides, such as H2O2 and CHP, was measured kinetically at different time points during the initial linear rate of catalysis. The reaction mixture contained 50 mM HEPES-NaOH (pH 7.0), 200 μM NADPH, 2 μM Prx, 5 μM NrdH, 3 μM TrxR, and either 100 μM H2O2 or 100 μM CHP. After reactions were initiated by the addition of H2O2, or CHP, 16.7 μl of HCl (1 M) was added into 83.3 μl of reaction mixture to stop the reactions at different intervals. Then, the resulting mixture (100 μl) was mixed with 900 μl FOX reagent (88 mg butylated hydroxytoluene, 7.6 mg xylenol orange, and 9.8 mg (NH4)2Fe(SO4)2 · 6H2O added to 90 ml methanol and 10 ml 250 mM sulfuric acid) and incubated at 37°C for 30 min. The color developed was read colorimetrically at 560 nm. All the assays were performed three times, and error bars indicate the SD. The kinetic constants were calculated by a nonlinear regression using the program GraphPad Prism 5.
Sensitivity assays for antibiotics, oxidative agents, and heavy metals.
To measure the response to various environmental stress conditions, overnight cultures of C. glutamicum strains grown in LB medium at 30°C were diluted 10-fold with LB medium, and the diluted cells were exposed to various antibiotics (2 h) and oxidants and toxins (30 min) at 30°C with shaking. After treatment, the cultures were serially diluted and plated onto LB agar plates, and colonies were counted after 36 h of growth at 30°C. Percentage survival was calculated as follows: [(CFU ml−1 after challenge at different stresses)/(CFU ml−1 before stress challenge)] × 100. Heavy metal stress experiments were performed according to Helbig et al. (42) with minor modifications. Overnight cultures of C. glutamicum strains were diluted 1:100 in fresh LB medium with different concentrations of various heavy metals and cultivated for over 24 h with shaking at 30°C. Cellular growth was monitored by determining optical density at 600 nm. All these assays were performed in triplicate at least three times.
Measurement of intracellular ROS level.
To quantify in vivo levels of ROS, the 2′,7′-dichlorofluorescein diacetate (DCFH-DA)-based assay (43) was used, with the following modifications. Briefly, cells grown aerobically (OD600 = 1.6) were collected, washed, and resuspended in 50 mM PBS (pH 7.4) prior to preincubation with 2 μM DCFH-DA at 28°C for 20 min. Various stressors at indicated concentrations were added to these mixtures and incubated for another 30 min in the dark. After that, cells were washed twice with 50 mM PBS, centrifuged, and resuspended in 50 mM PBS. The fluorescence intensity was measured with a Spectromax spectrofluorimeter (excitation, 502 nm; emission, 521 nm).
Determination of the cellular level of protein carbonylation.
The protein carbonylation assays were performed based on the method described by Vinckx et al. (44), with minor modifications. Briefly, C. glutamicum strains were grown overnight in LB medium and treated with different oxidants for 30 min with shaking at 30°C. After that, cultures were collected by centrifugation, washed with 25 mM Tris-HCl (pH 8.0), and resuspended in 25 mM Tris-HCl (pH 8.0) containing protease inhibitor cocktails (Sigma), and sonication was performed to obtain a clear cell lysate. The soluble protein fraction was collected by centrifugation, and concentration was measured by using the Bradford assay according to the manufacturer's protocol (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard. Subsequently, protein carbonylation levels were detected, based upon the manufacturer's instructions, with an OxyBlot protein oxidation detection kit (Millipore, Massachusetts, USA), which measures carbonyl groups of proteins generated by oxidative reactions. Carbonyl groups in proteins were derivatized with 2,4-dinitrophenyl hydrazine (DNPH), 20 μg of each DNPH-derivatized protein was loaded, and electrophoresis was conducted on a 15% SDS-PAGE gel. After electrophoresis, derivatized proteins were electroblotted onto nitrocellulose membranes, and immunodetection of DNPH-derivatized proteins was done with a rabbit anti-DNP antibody.
RESULTS
Biochemical characterization of NrdH.
Although the putative C. glutamicum NrdH redoxin (NCgl2445, the hypothetical mycoredoxin Mrx2) was suspected to act as an electron donor for NrdEF, the experimental evidence was lacking. To explore this activity, we employed an in vitro assay system (see Materials and Methods) started by adding GDP as the substrate for RNR and monitored the coupled NADPH oxidation by TrxR at 340 nm. As shown in Fig. 1A, purified recombinant NrdH functions as a reductant for the class Ib RNR with NADPH and TrxR as the hydrogen donor system, suggesting that NrdH was capable of reducing class Ib RNR and was recycled by TrxR, which is similar to the previous findings in E. coli and S. aureus (21, 23). The reduction activity of NrdH was significantly more efficient than that of thioredoxin (NCgl2985) in transferring electrons from NADPH via the TrxR pathway, as the reduction rates measured by the ΔΔA (at 340 nm)/min were 0.062 for NrdH and only 0.024 for thioredoxin (Fig. 1A). To assess whether C. glutamicum NrdH is exclusively involved in reduction of class Ib RNRs or possesses general thiol-disulfide redox activity as thioredoxin, we further tested the capacity of recombinant NrdH to reduce protein disulfides and small-molecule artificial disulfide compounds. To determine the protein disulfide reduction activity of NrdH, we employed the classic insulin reduction assay that was typically used for thioredoxin determination. As shown in Fig. 1B, in the presence of TrxR/NADPH, the addition of NrdH to the reaction mixture caused a notable decrease in absorption at 340 nm. As expected, the addition of C. glutamicum thioredoxin to the reaction mixture also caused a similar decrease in absorption at 340 nm (Fig. 1B). These results demonstrate that the NrdH is almost as effective as thioredoxin in reducing insulin disulfides by using the TrxR/NADPH regeneration system. We further explored the capability of C. glutamicum NrdH to reduce exposed disulfide bonds by using DTNB as an artificial disulfide substrate. As shown in Fig. 1C, only the complete NrdH/TrxR pathway emerges as a catalytically relevant reducing agent of DTNB in the presence of TrxR and NADPH, and the activity is much less effective than that of the C. glutamicum thioredoxin control.
FIG 1.
NrdH reduces disulfide bonds by the TrxR/NADPH pathway but not the MSH/Mtr/NADPH pathway. (A to C) Reduction of RNR (A), insulin (B), and DTNB (C) by NrdH (10 μM) coupled to the TrxR/NADPH regeneration system. Trx (10 μM) was used as a positive control. Negative controls are the omission of TrxR in the presence of NrdH (−Control 1), omission of TrxR in the presence of Trx (−Control 2), and omission of both Trx and NrdH in the presence of TrxR (−Control 3). (D to F) Reduction of RNR (D), insulin (E), and DTNB (F) by NrdH (10 μM) coupled to the MSH/Mtr/NADPH regeneration system. Mrx1 (10 μM) was used as a positive control. Negative controls are the omission of Mtr in the presence of NrdH (−Control 1), omission of Mtr in the presence of Mrx1 (−Control 2), omission of both Mrx1 and NrdH in the presence of Mtr (−Control 3), and omission of Mtr in the presence of Mrx1 and NrdH (−Control 4). The reduction of RNR (A and D) and insulin (B and E) were recorded by measuring the decrease of NADPH oxidation at 340 nm. The reduction of DTNB was recorded as an increase in absorption at 412 nm (C and F).
To determine whether the conserved cysteines in the CXXC motif of NrdH (20) are essential for its function, we created both single and double substitution mutations of Cys to Ser in the CXXC motif (NrdH::CXXS, NrdH::SXXC, and NrdH::SXXS). We performed the aforementioned insulin, DTNB, and RNR reduction assays by using the TrxR/NADPH regeneration system to examine whether these mutations would alter NrdH function. As shown in Fig. 2A to C, the activities of the mutant NrdH::CXXS, NrdH::SXXC, and NrdH::SXXS proteins were significantly less efficient than observed for the wild-type NrdH. These results indicate that both conserved cysteines in the CXXC domain are important for NrdH function.
FIG 2.

The active-site cysteines of NrdH are essential for reduction of disulfide bonds. RNR (A), insulin (B), and DTNB (C) reduction were measured in the presence of 10 μM of each NrdH mutant by using the TrxR/NADPH regeneration system as described in Fig. 1.
We further asked whether NrdH was able to reduce NrdEF via the recently identified MSH/Mtr/NADPH pathway, a novel electron transfer pathway identified in MSH-producing high-G+C-content Gram-positive Actinobacteria that is functionally equivalent to the widely distributed GSH/Grx/NADPH pathway (10, 19). While the TrxR/NADPH pathway uses the flavoprotein TrxR to shuttle electrons from NADPH to disulfide bonds, the MSH/Mtr/NADPH pathway utilizes MSH, Mtr, and NADPH to reduce disulfide bonds. However, no activity was detected when thioredoxin reductase TrxR was replaced by mycothiol and mycothione reductase in the ribonucleotide reductase assay mixture (Fig. 1D). In the alternative experiments, we measured the capacity of NrdH to reduce insulin and DTNB by using the MSH/Mtr/NADPH regeneration system (Fig. 1E and F). No activities were observed in both cases. However, though no activity was observed for DTNB reduction, Mrx1 did show activity for insulin reduction by using the MSH/Mtr/NADPH regeneration system as anticipated (Fig. 1E).
As previously reported, Mrx1 could reduce MSH mixed disulfide bonds using electrons from the MSH/Mtr/NADPH pathway (19). Thus, it was of interest to examine whether NrdH, the protein sharing high similarity to Mrx1, also exhibited MSH-disulfide oxidoreductase activity. To detect this, we employed the standard mycothiol-coupled hydroxyethyl disulfide (HED) assay with Mrx1 as a positive control. As shown in Fig. S1A in the supplemental material, while Mrx1 displayed effective reduction of the mixed disulfide formed between HED and MSH, as judged by the consumption of NADPH monitored spectrophotometrically at OD340, no MSH-disulfide oxidoreductase activity could be detected for NrdH by the MSH/Mtr/NADPH pathway. Meanwhile, no reduction of the mixed disulfides by NrdH was observed by the TrxR/NADPH pathway in the standard HED assay mixture (see Fig. S1B in the supplemental material). These results agree with previous research, indicating that not all dithiol oxidoreductases containing the CXXC active site are able to catalyze the reduction of mixed disulfide bonds (21). Collectively, these data demonstrate that NrdH acts not only as an NrdEF reductant but also as a protein disulfide oxidoredoxin exclusively using the TrxR/NADPH but not the MSH/Mtr/NADPH electron transfer pathway.
Physiological roles of the C. glutamicum NrdH. (i) nrdH is an essential gene in C. glutamicum.
To assess the physiological roles of C. glutamicum NrdH, we tried to construct the nrdH-null mutant in C. glutamicum. Unfortunately, consistent with a recent report that the nrdH gene seems to be an essential gene (20), no viable colonies were obtained after multiple attempts to knock out this gene, even in media containing dithiothreitol (DTT) and cysteine. We therefore evaluated the physiological phenotype of nrdH by overexpressing this gene in the C. glutamicum RES167 wild-type strain.
(ii) Overexpression of nrdH promotes resistance to oxidative stress conditions.
We speculated that, as a small intracellular oxidoreductase of the thioredoxin superfamily, NrdH might be another candidate in the redox system in C. glutamicum that plays a role in protection against oxidative stress. To investigate the oxidative stress protection role of NrdH, we first compared the viability of the C. glutamicum strains transformed with either pXMJ19-nrdH or vector alone in the presence of CHP (11 mM), H2O2 (55 mM), CDNB (2,4-dinitrochlorobenzene; 70 mM), and iodoacetamide (IAM; 40 mM). As shown in Fig. 3A, overexpression of nrdH significantly increased the resistance of the wild-type C. glutamicum to these conditions compared with the vector-alone control. Interestingly, while overexpression of NrdH increased resistance to oxidative stress conditions, overexpression of the NrdH mutant (NrdH::SXXS) had no effect, indicating that the oxidoreductase activity of NrdH is critical for protection against oxidative stress.
FIG 3.
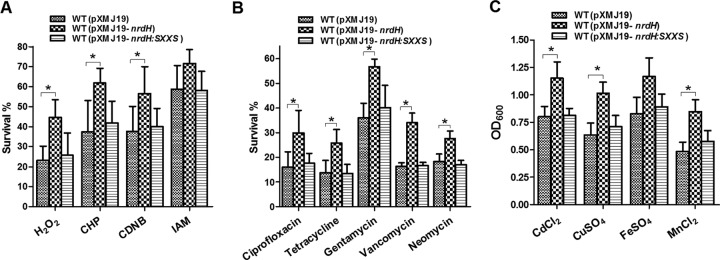
Effects of NrdH overexpression on multiple-stress resistance in C. glutamicum. (A) Survival for the C. glutamicum WT(pXMJ19), WT(pXMJ19-nrdH), and WT(pXMJ19-nrdH::SXXS) strains after challenging with H2O2 (55 mM), CHP (11 mM), IAM (40 mM), and CDNB (70 mM) for 30 min. (B) Survival for the C. glutamicum WT(pXMJ19), WT(pXMJ19-nrdH), and WT(pXMJ19-nrdH::SXXS) strains after challenging with ciprofloxacin (375 μg/ml), tetracycline (115 μg/ml), gentamicin (50 μg/ml), vancomycin (150 μg/ml), and neomycin (450 μg/ml) for 2 h. (C) The growth (OD600) of the C. glutamicum WT(pXMJ19), WT(pXMJ19-nrdH), and WT(pXMJ19-nrdH::SXXS) strains after 24 h at 30°C in LB medium containing CdCl2 (15 μM), CuSO4 (2,400 μM), FeSO4 (225 μM), and MnCl2 (800 μM) was recorded. Mean values with standard deviations (error bars) from at least three repeats are shown. *, P ≤ 0.05.
(iii) Overexpression of nrdH promotes resistance to bactericidal antibiotics.
It has been proposed that bactericidal antibiotics can induce cellular death through a common oxidative damage mechanism that relies on the production of ROS (45). To examine the impact of overexpression of nrdH on resistance to bactericidal antibiotics, cell viability of WT(pXMJ19), WT(pXMJ19-nrdH), and WT(pXMJ19-nrdH::SXXS) was tested in LB medium containing ciprofloxacin (375 μg/ml), tetracycline (115 μg/ml), gentamicin (50 μg/ml), vancomycin (150 μg/ml), and neomycin (450 μg/ml). As shown in Fig. 3B, the WT(pXMJ19-nrdH) strains exhibited higher cell viability (29.9%, 25.8%, 56.8%, 33.9%, and 27.6%, respectively) than the WT(pXMJ19) control strain (16.0%, 13.7%, 35.9%, 16.3%, and 18.3%, respectively). In addition, the difference of the survival rates between the WT(pXMJ19) strain and WT(pXMJ19-nrdH) strain showed a dose-dependent increase in response to antibiotic treatment (data not shown). As expected, overexpression of the NrdH::SXXS mutant in C. glutamicum had no effect in increasing the survival rate of the wild-type strain (Fig. 3B). However, no significant difference was found in the survival rate for bacteriostatic antibiotics, such as erythromycin, lincomycin, and spectinomycin, between the WT(pXMJ19) and WT(pXMJ19-nrdH) strains (data not shown).
(iv) Overexpression of nrdH promotes resistance to heavy metal stress.
In general, heavy metals can lead to ROS production and oxidative stress when taken up in excessive amounts, which subsequently results in protein peroxidation and other biomolecule damage (46). Redox-active metals such as iron and copper lead to hydroxyl radical generation and promote oxidative stress by their redox cycling activity (42, 47). Non-redox-active metals, such as cadmium, can indirectly induce oxidative stress by exhausting free radical scavengers such as GSH and protein-bound sulfhydryl groups (48–50). To determine whether NrdH also plays an important role in protecting C. glutamicum from heavy metal toxicity, WT(pXMJ19), WT(pXMJ19-nrdH), and WT(pXMJ19-nrdH::SXXS) were tested for growth in LB medium containing increased concentrations of heavy metals. Figure 3C showed that under conditions challenged with heavy metals CdCl2 (15 μM), CuSO4 (2,400 μM), FeSO4 (225 μM), and MnCl2 (800 μM), the WT(pXMJ19-nrdH) grew significantly better than WT(pXMJ19) and WT(pXMJ19-nrdH::SXXS). However, no effect was observed for ZnSO4 and CoCl2 in our experimental conditions (data not shown). These data suggest that overexpression of active NrdH in wild-type C. glutamicum enhances its resistance to heavy metal stress.
All together, these findings displayed that NrdH plays important roles for protection of C. glutamicum against ROS-inducing oxidants, alkylating agents, antibiotics, and heavy metal stress, which prompted us to further investigate whether stress tolerance in C. glutamicum conferred by NrdH overexpression was associated with the removal of ROS.
NrdH is able to reduce ROS levels under stress conditions.
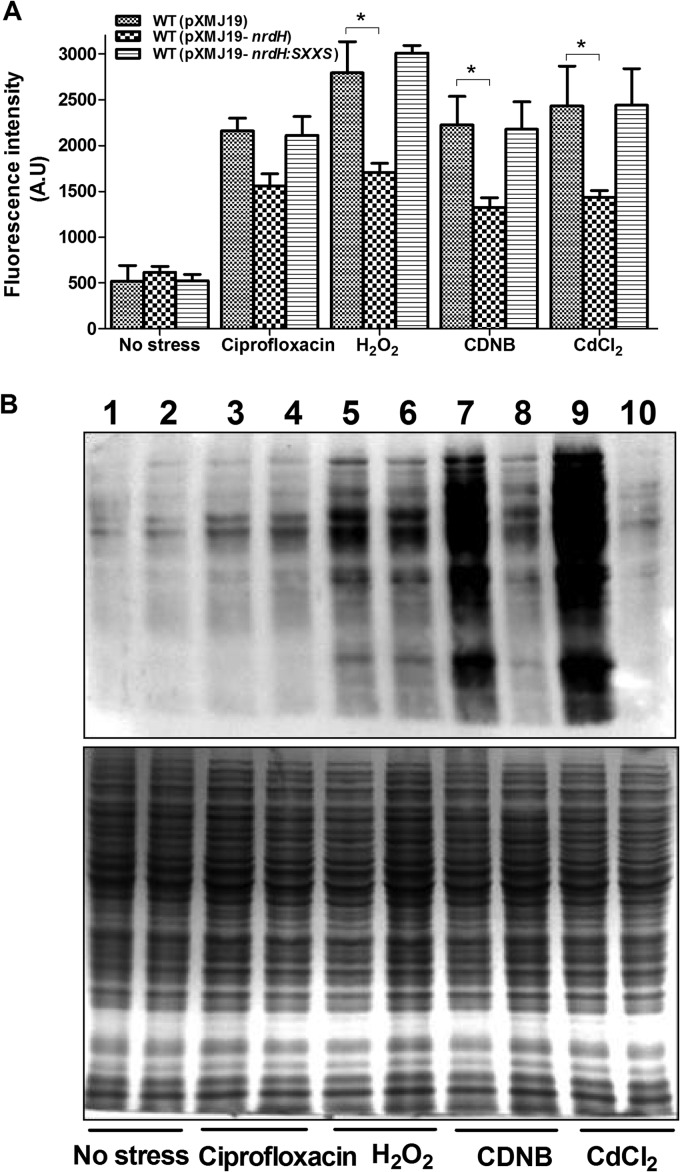
A great number of researches have presented that the exposure of microorganisms to various stresses, such as heavy metals, antibiotics, xenobiotics, and acid stress, can increase the production of ROS and then induce oxidative stress (45–47). Microorganisms employ a battery of enzymatic and nonenzymatic antioxidants to cope with continuous ROS production. As a previous study has shown that excessive generation of ROS triggers nrdHIEF expression in E. coli (51), we were prompted to examine whether elevated nrdH plays a role in removing free radicals under oxidative stress in C. glutamicum. The intracellular levels of ROS were assessed fluorometrically with the fluorogenic probe 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA; Sigma-Aldrich). As shown in Fig. 4A, the WT(pXMJ19-nrdH) strain has significantly lower levels of ROS content than the WT(pXMJ19) strain, after exposure to H2O2 (55 mM), ciprofloxacin (375 μg/ml), CDNB (70 mM), and CdCl2 (300 μM) for 0.5 h. For example, on exposure to 55 mM H2O2, 1.6-fold-higher levels of ROS were observed in the WT(pXMJ19) cells than in the WT(pXMJ19-nrdH) cells. For all these stress conditions tested, WT(pXMJ19) showed more increase in ROS accumulation compared to that in WT(pXMJ19-nrdH), indicating that overexpression of nrdH in C. glutamicum wild type reduced ROS levels induced by oxidative stresses. However, no reduction of ROS levels was observed in the nrdH mutant-overexpressed WT(pXMJ19-nrdH::SXXS) cells compared to in the vector-alone control (Fig. 4A). These findings suggest that NrdH is involved in ROS reduction induced by multiple oxidative stresses in C. glutamicum.
FIG 4.
Overexpressed NrdH reduces ROS production and protein oxidation under oxidative stress conditions. (A) The ROS levels in C. glutamicum strains expressing NrdH and NrdH::SXXS were measured by using the DCF fluorescence determination assay after exposure to the indicated oxidative reagents. The bars represent the fluorescence intensity in arbitrary units (A.U). *, P ≤ 0.05. (B) Protein carbonyl contents were analyzed by Western blotting with anti-DNP antibody after exposure to various oxidative reagents for 30 min at 30°C. A parallel run stained with Coomassie brilliant blue is shown in the bottom panel. Total proteins were extracted from vector-expressing (lanes 1, 3, 5, 7, and 9) and nrdH-expressing (lanes 2, 4, 6, 8, and 10) C. glutamicum cells.
ROS escaping from the antioxidant defense system are more apt to react with cysteine thiol groups of proteins which result in irreversible sulfoxidation products, inter- or intraprotein disulfides (PrSSPr, PrSSPr), and mixed disulfides with low-molecular-weight thiol and eventually lead to protein carbonylation (52, 53). Given that NrdH is able to reduce ROS in C. glutamicum, we hypothesized that NrdH may also function in protecting against protein carbonylation under oxidative stress conditions. To test this hypothesis, we isolated total proteins from strain WT(pXMJ19-nrdH) overexpressing nrdH and the vector-alone control strain WT(pXMJ19) grown under oxidative stresses. Carbonyl groups in the proteins were derivatized with 2,4-dinitrophenyl hydrazine (DNPH) and detected by Western blotting with the anti-DNP antibody. Interestingly, different stresses resulted in great difference in protein carbonylation formation in C. glutamicum. As shown in Fig. 4B, while CDNB and Cd(II) stress resulted in marked protein carbonylation in C. glutamicum, H2O2 stress resulted in moderate protein carbonylation, and ciprofloxacin stress had only marginal effects. It is noteworthy that the protein carbonylation levels for extracts from WT(pXMJ19-nrdH) were significantly lower than those from the WT(pXMJ19) control under CDNB and Cd(II) stresses. However, there is no significant difference in carbonyl content between the WT(pXMJ19) and WT(pXMJ19-nrdH) extracts under H2O2 and ciprofloxacin stress, correlating to the lower protein carbonylation levels induced by these stresses.
Induced expression of nrdH by multiple stressors.
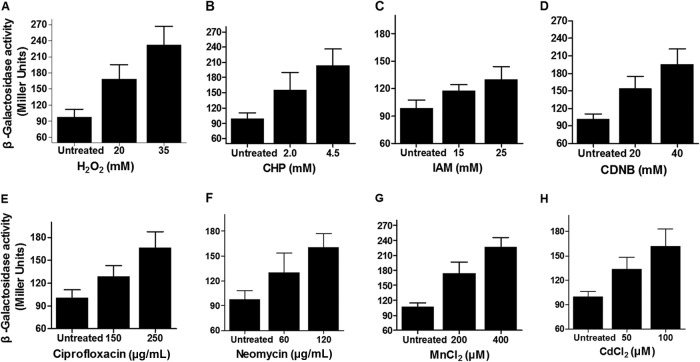
Since NrdH has been shown to promote survival of C. glutamicum in multiple stresses, these results prompted us to examine whether nrdH expression responded to these stress conditions. We thus introduced a single-copy PnrdH::lacZ fusion (the lacZ gene fused to the nrdH promoter) into the chromosome of the wild-type C. glutamicum. The LacZ activity of the resulting strain was quantitatively measured under a variety of stress conditions, including H2O2, CHP, IAM, CDNB, ciprofloxacin, neomycin, MnCl2, and CdCl2 at different concentrations (Fig. 5A to H). The expression levels of nrdH increased by at least 2.4-, 2.1-, 1.32-, 1.92-, 1.67-, 1.65-, 2.1-, and 1.62-fold in the H2O2 (35 mM)-, CHP (4.5 mM)-, IAM (25 mM)-, CDNB (40 mM)-, ciprofloxacin (250 μg/ml)-, neomycin (120 μg/ml)-, MnCl2 (400 μM)-, and CdCl2 (100 μM)-treated samples compared to those of untreated samples, respectively. Further, expression of the PnrdH::lacZ fusion exhibited a dose-dependent increase in response to these environmental stressors (Fig. 5). The induction effect by H2O2 is the strongest among all stressors tested. These data clearly demonstrate that multiple environmental stresses induce the expression of nrdH, which in turn directly contributes to tolerance to these adverse stresses. The increase in the expression levels of nrdH again correlated with a decrease in the levels of ROS formation, and an increase in cell viability collectively indicated a critical role of NrdH in protection of C. glutamicum against oxidative stress.
FIG 5.
Induction of the nrdH gene expression by multiple oxidative stresses. β-Galactosidase analyses of the nrdH gene expression in C. glutamicum strain containing the PnrdH::lacZ chromosomal fusion reporter after challenging with H2O2 (A), CHP (B), IAM (C), CDNB (D), ciprofloxacin (E), neomycin (F), MnCl2 (G), and CdCl2 (H) at the indicated concentrations. Error bars represent the standard deviations (SD) from three different determinations.
NrdH aids peroxidase to eliminate H2O2 and other alkyl hydroperoxides.
Previous studies have shown that yeast Grx1 and rice Grx have glutathione peroxidase activities toward H2O2 and other alkyl hydroperoxides, which directly remove H2O2 and other alkyl hydroperoxides (54, 55). As NrdH plays critical roles in reducing ROS levels and promoting the resistance of C. glutamicum to oxidative stresses, we examined the peroxidase activity of NrdH. As shown in Fig. 6A and Fig. S2 in the supplemental material, H2O2 or CHP was added to the TrxR and NADPH system with and without purified recombinant NrdH, and the activity of NrdH to reduce H2O2 and CHP was investigated by following the oxidation of NADPH. No peroxidase activity of NrdH was observed, as addition of NrdH to the reaction mixture did not increase the rate of NADPH consumption, thus indicating that the NrdH protein was not directly involved in reducing CHP or H2O2 via the TrxR and NADPH system without peroxidase (Fig. 6A; see also Fig. S2).
FIG 6.
NrdH acts as a Prx cofactor to facilitate its peroxidase activity. (A) NrdH-dependent peroxidase activity of Prx. The activity of Prx was monitored by recording NADPH oxidation at 340 nm in the presence of the TrxR/NADPH system and shown with the progress curves. Reaction mixtures contained 50 mM Tris-HCl buffer (pH 8.0), 2 mM EDTA, 200 μM NADPH, 3 μM TrxR, 2 μM Prx, and 5 μM NrdH in a final volume of 500 μl. Reactions were started by the addition of H2O2 (100 μM, left) or CHP (100 μM, right). Trx was used as a positive control, and reaction mixtures lacking NrdH, TrxR, or peroxidase in the assay system served as negative controls. (B) The active-site cysteines of NrdH are required for aiding the Prx peroxidase activity. The assay was carried out as described above. (C) Bacterial two-hybrid complementation assays were carried out to analyze interactions between Prx and NrdH. β-Galactosidase activities were assayed with ONPG as the substrate. Assays were performed in triplicate. (D) GST pulldown assay. His6-NrdH::CXXS or His6-NrdH::SXXS was incubated with GST-Prx or GST in PBS, and potential protein complexes captured with glutathione beads were detected with anti-His antibody. (E) NrdH-mediated protection against oxidative stress is dependent on Prx. C. glutamicum wild-type cells and prx mutant were transformed with pXMJ19 and pXMJ19-nrdH, respectively, and tested for sensitivity to H2O2 (55 mM), CHP (11 mM), CDNB (70 mM), and CdCl2 (300 μM) challenge.
However, other studies have also shown that Grx and Trx were able to aid peroxidases of the Prx family indirectly in the reduction of H2O2 and lipid peroxides (11, 56). To determine whether NrdH, the homolog of Grx and Trx, is able to aid peroxidase in the reduction of H2O2 and lipid peroxides, we detected the consumption of NADPH in the NrdH/TrxR/NADPH reaction system containing purified recombinant peroxidase (NCgl1041). In agreement with previous reports (56, 57), the addition of purified peroxidase to the Trx/TrxR/NADPH system with CHP or H2O2 as the terminal electron acceptor resulted in significant NADPH consumption (Fig. 6A; see also Fig. S2 in the supplemental material). Interestingly, when purified peroxidase was added to the NrdH/TrxR/NADPH reaction system, the consumption of NADPH was also observed, though it was slightly lower than in the Trx/TrxR/NADPH system. The reaction is dependent on the presence of NrdH, TrxR, and peroxidase, because there was no activity when each was omitted from the assay system. These results suggest that similar to Grx and Trx (11, 56–58), NrdH also acts as a peroxidase cofactor to facilitate the reduction of H2O2 and other alkyl hydroperoxides. The role of NrdH in facilitating peroxidase activity was further corroborated by monitoring the consumption of H2O2 and CHP in a different assay system (FOX assay; see Fig. S3 in the supplemental material).
After the above-described characterization, we found that the catalytic cycle begins with recognition of H2O2-CHP and ends with the regeneration of Prx by NrdH. Thus, enzymatic kinetic assays were performed to analyze this bisubstrate reaction and the concentration optimization (see Fig. S4 in the supplemental material). Plots of reaction velocity versus H2O2-CHP substrate concentration or NrdH fitted well to the nonlinear Hill equation (r2 = 0.987 and 0.979 for H2O2 and CHP, or r2 = 0.992 for NrdH) (see Fig. S4). The Km(app)values of Prx for NrdH (determined at 200 μM peroxide substrate) were 1.77 ± 0.27 and 4.86 ± 0.89 μM, and the kcat(app) values were 8.34 ± 0.32 and 6.34 ± 0.61 s−1, given catalytic efficiencies of 4.71 × 106 M−1 · s−1 and 1.30 × 106 M−1 · s−1. The Michaelis-Menten plots of Prx activity revealed that apparent Km values of Prx for H2O2 and CHP (determined at 5 μM NrdH) were 80.69 ± 9.59 and 64.26 ± 4.07 μM, and the kcat(app) values were 9.29 ± 3.05 and 5.19 ± 4.11 s−1. Therefore, these data indicate that combination of C. glutamicum Prx and NrdH is more efficient in reducing H2O2 [kcat(app)/Km(app) of 1.15 μM−1 · s−1] than CHP [kcat(app)/Km(app) of 0.81 μM−1 · s−1], the optimal concentrations of H2O2 and CHP were 400 μM and 350 μM, respectively, and the optimal concentration of NrdH was 10 μM and 20 μM for H2O2 and CHP in the Prx/NrdH/TrxR couple assay.
The ability of the NrdH::CXXS, NrdH::SXXC, and NrdH::SXXS mutants to reduce H2O2 and CHP was also investigated by following the oxidation of NADPH in the presence of the peroxidase/TrxR/NADPH system. As shown in Fig. 6B and Fig. S5 in the supplemental material, no activity exceeding the background level was observed with the mutant proteins in the reduction of H2O2 and CHP, indicating that both cysteines in the CXXC motif of NrdH were essential for aiding peroxidase in reduction of H2O2 and CHP.
To further investigate whether NrdH facilitates Prx activity by direct interaction like Trx and Grx (53, 56–59), we performed bacterial two-hybrid and GST pulldown assays. As shown in Fig. 6C, although the wild-type NrdH showed relatively weak interaction with Prx in the bacterial two-hybrid assay, the NrdH::CXXS mutant showed very strong interaction with Prx, similar to the positive control, but the NrdH::SXXS mutant showed no interaction with Prx. These results are consistent with the early reports that the intermediate complex formed between a target and Trx or Grx can be stabilized by removing an internal cysteine at the CXXC active sites (56, 59). We speculated the reason for this is that NrdH and its target protein may form a mixed-disulfide intermediate at the first reactive cysteine of the N-terminal cysteinyl residue. This intermolecular disulfide bond is assaulted by the second cysteine of NrdH, so the reduced form NrdH is oxidized and target protein is released. The substitution of the second cysteine to serine can interrupt this reduction process at the stage of the formation of the mixed-disulfide intermediate, thus obtaining steady mixed disulfides between the respective NrdH and Prx when interactions did occur.
To substantiate the NrdH-Prx interaction seen in the bacterial two-hybrid assay, we produced recombinant His6-NrdH::CXXS/His6-NrdH::SXXS and tested their interactions with GST-Prx by using the GST pulldown assay in vitro. As shown in Fig. 6D, we again observed the specific interaction between GST-Prx and His6-NrdH::CXXS but not His6-NrdH::SXXS. Consistent with the role of NrdH acting as a cofactor for peroxidase, the increased resistance to H2O2 and CHP mediated by overexpression of nrdH was largely abrogated in the prx mutant (Fig. 6E). Taken together, these data clearly demonstrate that NrdH is able to facilitate the Prx peroxidase in reducing H2O2 and other alkyl hydroperoxides, by acting as a cofactor to Prx.
DISCUSSION
In this study, we described the biochemical and physiological characterization of the NrdH redoxin from C. glutamicum, a high-G+C-content Gram-positive bacterium that produces mycothiol as its dominant low-molecular-weight thiol instead of glutathione. We demonstrated the protective role of the C. glutamicum NrdH redoxin against various oxidative stresses induced by H2O2, CHP, CDNB, antibiotics, and heavy metal ions. We also demonstrated that the protective effect of NrdH is attributed to its ability in detoxification of ROS by serving as the electron donor for a novel protein substrate, the thioredoxin peroxidase Prx. In addition, we showed that NrdH reduces disulfide bonds in the small-molecule artificial disulfide compound DTNB and protein substrates such as class Ib ribonucleotide reductase, Prx, and possibly other protein substrates, by receiving electrons exclusively from the TrxR/NADPH but not the MSH/Mtr/NADPH pathway. These results are consistent with the finding that NrdH possesses a TrxR binding hydrophobic pocket (22, 24) but lacks the MSH binding pocket as found in Mrx1 (20).
Although NrdH redoxins have long been demonstrated biochemically in multiple bacteria (20–24, 27), their physiological functions remain enigmatic. In C. glutamicum, the nrdH gene seems to be essential, as many attempts to construct an nrdH deletion mutant were unsuccessful. In contrast, although an nrdH deletion mutant was obtained in S. aureus, it did not appear to have a role in coping with oxidative stress (23). We thus overexpressed nrdH in C. glutamicum to examine whether it functions and protects cells against oxidative stresses induced by various exogenous stimuli, by comparing with that of C. glutamicum harboring vector alone. Two distinct types of oxidative stress in bacteria have recently been proposed (60): the primary oxidative stress, occurring when cells are exposed to oxidative agents such as hydrogen peroxide, sodium hypochlorite, and nitric oxide (NO), and the secondary oxidative stress, occurring when cells are exposed to environmental stresses and toxic agents such as bactericidal antibiotics, heavy metal ions, heat, and acid, causing the formation of superoxide (O2•−) and hydrogen peroxide (H2O2) and subsequently leading to a response similar to the response to primary oxidative stress. The primary oxidative stress leads to oxidized sulfhydryl groups, lipid peroxidation, the generation of ROS, and a disturbed NAD+/NADH balance, whereas the secondary oxidative stress leads to ROS production particularly (60). ROS can cause a wide range of biological molecule damages, i.e., protein and lipid oxidation, disulfide bond formation, iron-sulfur cluster disassembly, and DNA fragmentation, and eventually lead to cell death (52–54). Interestingly, we found the overexpressed nrdH protects the cells not only against oxidants that induce primary oxidative stress but also against a broad range of poisonous chemicals that induce secondary oxidative stress, such as CDNB, antibiotics, and heavy metal ions. These results suggest that overexpression of nrdH in C. glutamicum seems to affect the redox status of the host cells by detoxification of ROS produced under different oxidative stresses.
To test this hypothesis, we analyzed the cellular ROS levels and protein oxidation caused by ROS. The increase of the intracellular ROS level and protein oxidation by oxidants, alkylating agents, antibiotics, and heavy metal ion stress was blocked in the nrdH-expressing cells compared to in the control cells harboring vector alone to a certain extent. Although yeast Grx and rice Grx have been reported to detoxify ROS by serving as peroxidase directly (54, 55), this is not the case for NrdH, as no peroxidase activity was observed for NrdH in an in vitro assay (Fig. 6A; see Fig. S2 in the supplemental material). Besides acting as disulfide bond oxidoreductases generally, in vitro studies have shown that both the glutaredoxin and thioredoxin systems can serve as electron donors for other antioxidant enzymes, such as Prx (11, 56–58). Therefore, it is very likely that NrdH, characterized by a glutaredoxin-like amino acid sequence and a thioredoxin-like activity, detoxifies ROS also by recruiting and serving as the electron donor for peroxidases. This assumption was supported by at least three lines of independent evidences. First, as shown in Fig. 6A and Fig. S2 in the supplemental material, although NrdH did not directly eliminate hydrogen peroxide and CHP, it is able to aid the Prx in the reduction of H2O2 and other alkyl hydroperoxides by serving as an electron donor in in vitro assay systems containing NADPH and thioredoxin reductase, albeit at a lower efficiency than thioredoxin. Second, the NrdH variant NrdH::CXXS was shown to interact with Prx directly (Fig. 6C and D). Finally, disruption of the Prx gene almost abolished the antioxidant effect caused by the overexpression of nrdH in C. glutamicum, suggesting that the antioxidant activity of NrdH is mediated mainly by Prx (Fig. 6E). Thus, our results unambiguously revealed that NrdH detoxifies ROS by recruiting and serving as the electron donor to a novel target protein, Prx. Although it cannot be ruled out that NrdH could also act as a cofactor for other antioxidant enzymes, Prx accounts largely for most ROS detoxification activity in nrdH-overexpressing C. glutamicum (Fig. 6E).
The physiological roles of NrdH in resistance to multiple oxidative stresses were also corroborated by the induced expression of nrdH in C. glutamicum under various oxidative stresses (Fig. 5). Induced expression of nrdH by oxidative stresses was also observed in E. coli (51). These results strongly suggest that nrdH expression is important for bacteria growth under oxidative stress conditions. Of particular note is the vast upregulation of the nrdHIEF operon observed in E. coli mutants deficient in peroxide/superoxide scavenging enzymes, such as catalase hydroperoxidase I and alkyl hydroperoxide reductase (51), indicating that the increased expression of nrdHIEF genes may compensate for the lost ROS scavenging activities in these mutants. The induction of the nrdHIEF operon during H2O2 stress in E. coli was mediated by the inactivation of Fur, an iron-dependent repressor (58). It is interesting to reveal whether the E. coli NrdH also acts as an electron donor for a specific peroxidase to dispose ROS, as in C. glutamicum.
While we were preparing the manuscript, Van Laer et al. (20) coincidentally solved the X-ray structure of oxidized NrdH redoxin from C. glutamicum at a 1.5-Å resolution and biochemically characterized this protein. In contrast to our study, which focused on the physiological function of NrdH, they carried out a detailed structural analysis of the protein. But both their study and ours demonstrated that NrdH catalytically reduces disulfide bonds in DTNB and exclusively receives electrons from thioredoxin reductase (TrxR) but not from mycothiol (20). Although NrdH is more similar to Mrx1 in sequence, it cannot reduce the mixed disulfides by either the MSH/Mtr/NADPH pathway or the TrxR/NADPH pathway. In addition, we found that the C. glutamicum NrdH not only reduces disulfide bonds in its native substrate class Ib ribonucleotide reductase (NrdEF) but also in Prx and insulin, with activities comparable to that of Trx through the TrxR/NADPH pathway. Despite their apparent function overlapping, both NrdH and thioredoxin are essential in C. glutamicum, indicating that they must have additional unique functions (10).
In conclusion, our results demonstrate that NrdH plays important roles in resistance to multiple oxidative stresses induced by oxidants, alkylating agents, antibiotics, and heavy metals in C. glutamicum. In addition, we present evidences that NrdH can protect against the damaging effects of ROS by acting as a Prx cofactor. It should be noted that, besides Prx, alternative NrdH target proteins may also account for its function in oxidative stress resistance. Further studies to identify novel targets will allow for a more complete understanding of NrdH function.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National High Technology Research and Development Program of China (863 program, grant 2013AA102802), National Natural Science Foundation of China (no. 31270078, 31100001, and 31170100), and the Opening Project of State Key Laboratory of Microbial Resource, Institute of Microbiology, Chinese Academy of Sciences (no. SKLMR-20120601).
Footnotes
Published ahead of print 27 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03654-13.
REFERENCES
- 1.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. 2008. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 10:445–473. 10.1089/ars.2007.1716 [DOI] [PubMed] [Google Scholar]
- 2.Daily D, Vlamis-Gardikas A, Offen D, Mittelman L, Melamed E, Holmgren A, Barzilai A. 2001. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by activating NF-kappa B via Ref-1. J. Biol. Chem. 276:1335–1344. 10.1074/jbc.M008121200 [DOI] [PubMed] [Google Scholar]
- 3.Rouhier N, Vlamis-Gardikas A, Lillig CH, Berndt C, Schwenn JD, Holmgren A, Jacquot JP. 2003. Characterization of the redox properties of poplar glutaredoxin. Antioxid. Redox Signal. 5:15–22. 10.1089/152308603321223504 [DOI] [PubMed] [Google Scholar]
- 4.Laurent TC, Moore EC, Reichard P. 1964. Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 239:3436–3444 [PubMed] [Google Scholar]
- 5.Holmgren A. 1979. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J. Biol. Chem. 254:3672–3678 [PubMed] [Google Scholar]
- 6.Lillig CH, Prior A, Schwenn JD, Aslund F, Ritz D, Vlamis-Gardikas A, Holmgren A. 1999. New thioredoxins and glutaredoxins as electron donors of 3′-phosphoadenylylsulfate reductase. J. Biol. Chem. 274:7695–7698. 10.1074/jbc.274.12.7695 [DOI] [PubMed] [Google Scholar]
- 7.Lillig CH, Potamitou A, Schwenn JD, Vlamis-Gardikas A, Holmgren A. 2003. Redox regulation of 3′-phosphoadenylylsulfate reductase from Escherichia coli by glutathione and glutaredoxins. J. Biol. Chem. 278:22325–22330. 10.1074/jbc.M302304200 [DOI] [PubMed] [Google Scholar]
- 8.Tarrago L, Laugier E, Zaffagnini M, Marchand C, Le Marechal P, Rouhier N, Lemaire SD, Rey P. 2009. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases B by glutaredoxins and thioredoxins. J. Biol. Chem. 284:18963–18971. 10.1074/jbc.M109.015487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couturier J, Vignols F, Jacquot JP, Rouhier N. 2012. Glutathione- and glutaredoxin-dependent reduction of methionine sulfoxide reductase A. FEBS Lett. 586:3894–3899. 10.1016/j.febslet.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 10.Ordóñez E, Van Belle K, Roos G, De Galan S, Letek M, Gil JA, Wyns L, Mateos LM, Messens J. 2009. Arsenate reductase, mycothiol, and mycoredoxin concert thiol/disulfide exchange. J. Biol. Chem. 284:15107–15116. 10.1074/jbc.M900877200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedrajas JR, Padilla CA, McDonagh B, Barcena JA. 2010. Glutaredoxin participates in the reduction of peroxides by the mitochondrial 1-Cys peroxiredoxin in Saccharomyces cerevisiae. Antioxid. Redox Signal. 13:249–258. 10.1089/ars.2009.2950 [DOI] [PubMed] [Google Scholar]
- 12.Anderson ME. 1998. Glutathione: an overview of biosynthesis and modulation. Chem. Biol. Interact. 111–112:1–14 [DOI] [PubMed] [Google Scholar]
- 13.Kim IS, Shin SY, Kim YS, Kim HY, Yoon HS. 2009. Expression of a glutathione reductase from Brassica rapa subsp. pekinensis enhanced cellular redox homeostasis by modulating antioxidant proteins in Escherichia coli. Mol. Cells 28:479–487. 10.1007/s10059-009-0168-y [DOI] [PubMed] [Google Scholar]
- 14.Perez-Perez ME, Florencio FJ, Lindahl M. 2006. Selecting thioredoxins for disulphide proteomics: target proteomes of three thioredoxins from the cyanobacterium Synechocystis sp. PCC 6803. Proteomics 6(Suppl 1):S186–S195 [DOI] [PubMed] [Google Scholar]
- 15.Newton GL, Bewley CA, Dwyer TJ, Horn R, Aharonowitz Y, Cohen G, Davies J, Faulkner DJ, Fahey RC. 1995. The structure of U17 isolated from Streptomyces clavuligerus and its properties as an antioxidant thiol. Eur. J. Biochem. 230:821–825. 10.1111/j.1432-1033.1995.0821h.x [DOI] [PubMed] [Google Scholar]
- 16.Newton GL, Arnold K, Price MS, Sherrill C, Delcardayre SB, Aharonowitz Y, Cohen G, Davies J, Fahey RC, Davis C. 1996. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YB, Long MX, Yin YJ, Si MR, Zhang L, Lu ZQ, Wang Y, Shen XH. 2013. Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch. Microbiol. 195:419–429. 10.1007/s00203-013-0889-3 [DOI] [PubMed] [Google Scholar]
- 18.Newton GL, Buchmeier N, Fahey RC. 2008. Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol. Mol. Biol. Rev. 72:471–494. 10.1128/MMBR.00008-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Laer K, Buts L, Foloppe N, Vertommen D, Van Belle K, Wahni K, Roos G, Nilsson L, Mateos LM, Rawat M, van Nuland NA, Messens J. 2012. Mycoredoxin-1 is one of the missing links in the oxidative stress defence mechanism of Mycobacteria. Mol. Microbiol. 86:787–804. 10.1111/mmi.12030 [DOI] [PubMed] [Google Scholar]
- 20.Van Laer K, Dziewulska AM, Fislage M, Wahni K, Hbeddou A, Collet JF, Versees W, Mateos LM, Tamu Dufe V, Messens J. 2013. NrdH-redoxin of Mycobacterium tuberculosis and Corynebacterium glutamicum dimerizes at high protein concentration and exclusively receives electrons from thioredoxin reductase. J. Biol. Chem. 288:7942–7955. 10.1074/jbc.M112.392688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan A. 1997. Characterization of Escherichia coli NrdH. A glutaredoxin-like protein with a thioredoxin-like activity profile. J. Biol. Chem. 272:18044–18050 [DOI] [PubMed] [Google Scholar]
- 22.Stehr M, Schneider G, Aslund F, Holmgren A, Lindqvist Y. 2001. Structural basis for the thioredoxin-like activity profile of the glutaredoxin-like NrdH-redoxin from Escherichia coli. J. Biol. Chem. 276:35836–35841. 10.1074/jbc.M105094200 [DOI] [PubMed] [Google Scholar]
- 23.Rabinovitch I, Yanku M, Yeheskel A, Cohen G, Borovok I, Aharonowitz Y. 2010. Staphylococcus aureus NrdH redoxin is a reductant of the class Ib ribonucleotide reductase. J. Bacteriol. 192:4963–4972. 10.1128/JB.00539-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stehr M, Lindqvist Y. 2004. NrdH-redoxin of Corynebacterium ammoniagenes forms a domain-swapped dimer. Proteins 55:613–619. 10.1002/prot.20126 [DOI] [PubMed] [Google Scholar]
- 25.Leiting W, Jianping X. 2010. Comparative genomics analysis of Mycobacterium NrdH-redoxins. Microb. Pathog. 48:97–102. 10.1016/j.micpath.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 26.Jordan A, Pontis E, Aslund F, Hellman U, Gibert I, Reichard P. 1996. The ribonucleotide reductase system of Lactococcus lactis. Characterization of an NrdEF enzyme and a new electron transport protein. J. Biol. Chem. 271:8779–8785 [DOI] [PubMed] [Google Scholar]
- 27.Gustafsson TN, Sahlin M, Lu J, Sjöberg BM, Holmgren A. 2012. Bacillus anthracis thioredoxin systems, characterization and role as electron donors for ribonucleotide reductase. J. Biol. Chem. 287:39686–39697. 10.1074/jbc.M112.413427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gon S, Faulkner MJ, Beckwith J. 2006. In vivo requirement for glutaredoxins and thioredoxins in the reduction of the ribonucleotide reductases of Escherichia coli. Antioxid. Redox Signal. 8:735–742. 10.1089/ars.2006.8.735 [DOI] [PubMed] [Google Scholar]
- 29.Shen XH, Jiang CY, Huang Y, Liu ZP, Liu SJ. 2005. Functional identification of novel genes involved in the glutathione-independent gentisate pathway in Corynebacterium glutamicum. Appl. Environ. Microbiol. 71:3442–3452. 10.1128/AEM.71.7.3442-3452.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Wang Y, Song Y, Wang T, Xu S, Peng Z, Lin X, Zhang L, Shen X. 2013. A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ. Microbiol. 15:557–569. 10.1111/1462-2920.12005 [DOI] [PubMed] [Google Scholar]
- 31.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756. 10.1073/pnas.95.10.5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimova G, Dautin N, Ladant D. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233–2243. 10.1128/JB.187.7.2233-2243.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria, vol 1 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 34.Hisabori T, Hara S, Fujii T, Yamazaki D, Hosoya-Matsuda N, Motohashi K. 2005. Thioredoxin affinity chromatography: a useful method for further understanding the thioredoxin network. J. Exp. Bot. 56:1463–1468. 10.1093/jxb/eri170 [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Shen X, Bryan A, Banga S, Swanson MS, Luo ZQ. 2010. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 6:e1000822. 10.1371/journal.ppat.1000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng J, Che Y, Milse J, Yin Y-J, Liu L, Rückert C, Shen X-H, Qi S-W, Kalinowski J, Liu S-J. 2006. The gene ncgl2918 encodes a novel maleylpyruvate isomerase that needs mycothiol as cofactor and links mycothiol biosynthesis and gentisate assimilation in Corynebacterium glutamicum. J. Biol. Chem. 281:10778–10785. 10.1074/jbc.M513192200 [DOI] [PubMed] [Google Scholar]
- 37.Ceylan S, Seidel V, Ziebart N, Berndt C, Dirdjaja N, Krauth-Siegel RL. 2010. The dithiol glutaredoxins of African trypanosomes have distinct roles and are closely linked to the unique trypanothione metabolism. J. Biol. Chem. 285:35224–35237. 10.1074/jbc.M110.165860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du Y, Zhang H, Lu J, Holmgren A. 2012. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J. Biol. Chem. 287:38210–38219. 10.1074/jbc.M112.392225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SJ, Jung HJ, Choi H, Lim CJ. 2012. Glutaredoxin 2a, a mitochondrial isoform, plays a protective role in a human cell line under serum deprivation. Mol. Biol. Rep. 39:3755–3765. 10.1007/s11033-011-1152-0 [DOI] [PubMed] [Google Scholar]
- 40.Latifi A, Ruiz M, Jeanjean R, Zhang CC. 2007. PrxQ-A, a member of the peroxiredoxin Q family, plays a major role in defense against oxidative stress in the cyanobacterium Anabaena sp. strain PCC7120. Free Radic. Biol. Med. 42:424–431. 10.1016/j.freeradbiomed.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 41.Wolff SP. 1994. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 233:182–189 [Google Scholar]
- 42.Helbig K, Bleuel C, Krauss GJ, Nies DH. 2008. Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol. 190:5431–5438. 10.1128/JB.00271-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schurig-Briccio LA, Farías RN, Rodríguez-Montelongo L, Rintoul MR, Rapisarda VA. 2009. Protection against oxidative stress in Escherichia coli stationary phase. Arch. Biochem. Biophys. 483:106–110. 10.1016/j.abb.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 44.Vinckx T, Wei Q, Matthijs S, Noben JP, Daniels R, Cornelis P. 2011. A proteome analysis of the response of a Pseudomonas aeruginosa OxyR mutant to iron limitation. Biometals 24:523–532. 10.1007/s10534-010-9403-4 [DOI] [PubMed] [Google Scholar]
- 45.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. 10.1016/j.cell.2007.06.049 [DOI] [PubMed] [Google Scholar]
- 46.Carmel-Harel O, Storz G. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439–461. 10.1146/annurev.micro.54.1.439 [DOI] [PubMed] [Google Scholar]
- 47.Stohs SJ, Bagchi D. 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18:321–336. 10.1016/0891-5849(94)00159-H [DOI] [PubMed] [Google Scholar]
- 48.Fortuniak A, Zadzinski R, Bilinski T, Bartosz G. 1996. Glutathione depletion in the yeast Saccharomyces cerevisiae. Biochem. Mol. Biol. Int. 38:901–910 [PubMed] [Google Scholar]
- 49.Gharieb MM, Gadd GM. 2004. Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. Biometals 17:183–188. 10.1023/B:BIOM.0000018402.22057.62 [DOI] [PubMed] [Google Scholar]
- 50.Stohs SJ, Bagchi D, Hassoun E, Bagchi M. 2001. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 20:77–88 [PubMed] [Google Scholar]
- 51.Monje-Casas F, Jurado J, Prieto-Alamo MJ, Holmgren A, Pueyo C. 2001. Expression analysis of the nrdHIEF operon from Escherichia coli. Conditions that trigger the transcript level in vivo. J. Biol. Chem. 276:18031–18037 [DOI] [PubMed] [Google Scholar]
- 52.Nystrom T. 2005. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24:1311–1317. 10.1038/sj.emboj.7600599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA. 2007. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic. Biol. Med. 43:1099–1108. 10.1016/j.freeradbiomed.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collinson EJ, Wheeler GL, Garrido EO, Avery AM, Avery SV, Grant CM. 2002. The yeast glutaredoxins are active as glutathione peroxidases. J. Biol. Chem. 277:16712–16717. 10.1074/jbc.M111686200 [DOI] [PubMed] [Google Scholar]
- 55.Lee KO, Lee JR, Yoo JY, Jang HH, Moon JC, Jung BG, Chi YH, Park SK, Lee SS, Lim CO, Yun DJ, Cho MJ, Lee SY. 2002. GSH-dependent peroxidase activity of the rice (Oryza sativa) glutaredoxin, a thioltransferase. Biochem. Biophys. Res. Commun. 296:1152–1156. 10.1016/S0006-291X(02)02047-8 [DOI] [PubMed] [Google Scholar]
- 56.Goyer A, Haslekås C, Miginiac-Maslow M, Klein U, Le Marechal P, Jacquot JP, Decottignies P. 2002. Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur. J. Biochem. 269:272–282. 10.1046/j.0014-2956.2001.02648.x [DOI] [PubMed] [Google Scholar]
- 57.Chi BK, Busche T, Laer KV, Bäsell K, Becher D, Clermont L, Seibold GM, Persicke M, Kalinowski J, Messens J. 18 September 2013. Protein S-mycothiolation functions as redox-switch and thiol protection mechanism in Corynebacterium glutamicum under hypochlorite stress. Antioxid. Redox Signal. 10.1089/ars.2013.5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin JE, Imlay JA. 2011. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol. Microbiol. 80:319–334. 10.1111/j.1365-2958.2011.07593.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M, Yang Q, Zhang L, Li H, Cui Y, Wu Q. 2007. Identification of novel targets of cyanobacterial glutaredoxin. Arch. Biochem. Biophys. 458:220–228. 10.1016/j.abb.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 60.Mols M, Abee T. 2011. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 13:1387–1394. 10.1111/j.1462-2920.2011.02433.x [DOI] [PubMed] [Google Scholar]
- 61.Tauch A, Kirchner O, Löffler B, Götker S, Pühler A, Kalinowski J. 2002. Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr. Microbiol. 45:362–367. 10.1007/s00284-002-3728-3 [DOI] [PubMed] [Google Scholar]
- 62.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. 10.1016/0378-1119(94)90324-7 [DOI] [PubMed] [Google Scholar]
- 63.Jakoby M, Ngouoto-Nkili C-E, Burkovski A. 1999. Construction and application of new Corynebacterium glutamicum vectors. Biotechnol. Tech. 13:437–441. 10.1023/A:1008968419217 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.