Abstract
The tick-borne bacterium “Candidatus Neoehrlichia mikurensis” has recently been recognized as a human pathogen. Together with Borrelia afzelii, it is one of the most common pathogens found in the tick Ixodes ricinus. Here, we compared the epidemiologies of “Ca. Neoehrlichia mikurensis” and B. afzelii by longitudinal sampling from May to September in one of their most abundant vertebrate hosts, the bank vole (Myodes glareolus), using real-time PCR for detection and quantification. The prevalences of “Ca. Neoehrlichia mikurensis” and B. afzelii were determined to be 19% (50/261) and 22% (56/261), respectively. The prevalence of “Ca. Neoehrlichia mikurensis” increased significantly during the sampling season. The clearance rate of “Ca. Neoehrlichia mikurensis” was significantly higher than that of B. afzelii. We found a high frequency of double infections; 46% of all samples infected with “Ca. Neoehrlichia mikurensis” also had a coinfection with B. afzelii. The frequency of coinfections was significantly higher than expected from the prevalence of each pathogen. The high level of coinfections can be caused by interactions between the pathogens or might reflect variation in general susceptibility among voles.
INTRODUCTION
The bacterium “Candidatus Neoehrlichia mikurensis” is a newly discovered tick-borne zoonotic pathogen with several recent human cases in Europe (1–4). It belongs to the family Anaplasmataceae, together with other known human pathogens, such as Anaplasma phagocytophilum, and has been found in Ixodes ricinus ticks from several European countries at a prevalence of 6 to 7% (5–7). Hence, “Ca. Neoehrlichia mikurensis” has emerged as one of the most common potential human pathogens in I. ricinus in Europe and perhaps is second only to Borrelia afzelii (5).
Many tick-borne zoonotic pathogens are maintained in nature by reservoir hosts that are the source of infection in ticks. Knowledge about the epidemiology and ecology of zoonotic pathogens in their vertebrate hosts is necessary to understand their spatial and temporal distribution and can potentially help manage the risk of transmission to humans. We have previously shown that four common species of rodents in southern Sweden are infected with “Ca. Neoehrlichia mikurensis” at a prevalence ca. 9% (8), but little is as yet known about the epidemiology of “Ca. Neoehrlichia mikurensis” in its vertebrate hosts. Basic knowledge about, for example, seasonal variation in prevalence, age-related patterns, the duration of the infection, and interactions with other pathogens would aid in our understanding of this pathogen.
One potentially important factor that can influence a pathogen's epidemiology is coinfection of the host with other pathogens (9, 10). The risk of acquiring a particular infection can be strongly influenced by prior infection with another pathogen. For example, Telfer et al. (11) showed that the risk for field voles to acquire infections by cowpox virus or the protozoan parasite Babesia microti was substantially increased if the animal had a prior infection with A. phagocytophilum. In contrast, A. phagocytophilum infection reduced the risk of Bartonella infection, demonstrating the complicated interplay between different pathogens. Interactions between pathogens can create patterns of positive or negative associations; for example, competition would lead to a lower frequency of coinfections than expected from the prevalence of each pathogen. However, demonstrating interactions between coinfecting pathogens is not easy since positive and negative associations can also be caused by a number of other factors, for example, if some host individuals are generally susceptible to infection.
Coinfections with tick-borne pathogens are commonly reported in the literature; infections with B. burgdorferi sensu lato and A. phagocytophilum have been of considerable interest because A. phagocytophilum can have a severe negative effect on the immune defense by affecting the activity and life span of granulocytes (12), thereby making infected hosts more susceptible to secondary infections. Consistent with this scenario, coinfections with B. burgdorferi sensu lato and A. phagocytophilum in vertebrate hosts, as well as in ticks, are typically more common than expected from just random co-occurrence (reviewed by Nieto and Foley in 2009 [13]). The reason for the higher incidence of coinfections might be that A. phagocytophilum modifies the immune responses and thereby causes sensitivity to B. burgdorferi infection, as has been experimentally demonstrated (14, 15).
We investigate here the epidemiology of “Ca. Neoehrlichia mikurensis” in its natural hosts by focusing on the most abundant rodent species, the bank vole (Myodes glareolus). We used longitudinal sampling of a population of bank voles in Southern Sweden to investigate seasonal and age-related patterns in infection status, as well as the duration of “Ca. Neoehrlichia mikurensis” infection. Moreover, we investigated the occurrence and potential causes of coinfection with “Ca. Neoehrlichia mikurensis” and B. afzelii.
MATERIALS AND METHODS
Study species.
“Ca. Neoehrlichia mikurensis” is named after the Japanese island Mikura, where it was detected in ticks and rodents. Human cases have been described from several European countries (1–4), and it is found in I. ricinus ticks over large parts of Europe (5–7). I. ricinus, like other Ixodes ticks, has a three-host life cycle, and each developmental stage takes a blood meal from a vertebrate host (except adult males, which do not feed). Small mammals, such as bank voles (Myodes glareolus), are the main hosts for larvae, and to some extent nymphs. B. afzelii is not transmitted from female ticks to their offspring (16). Similarly, “Ca. Neoehrlichia mikurensis” seems to lack the capacity for transovarial transmission (7), similar to other tick-borne diseases, such as those associated with A. phagocytophilum (17). Hence, nymphs are the main infective tick life stage for rodents. The bank vole is the most common rodent species over large parts of Europe.
Field work.
From May to September 2008, we trapped bank voles at Kalvs mosse, Revingehed, in southern Sweden (55°42.470′N, 13°29.216′E) in a habitat consisting of open forest with abundant low vegetation. The animals were trapped with live traps (Ugglan Special; Grahn AB, Sweden) baited with grains and apple. A blood sample from the tail (for PCR detection of “Ca. Neoehrlichia mikurensis”) and a skin biopsy specimen from the ear (for PCR detection of B. afzelii) were taken from each animal. The number of I. ricinus (as identified by morphology) larvae was counted on the ears where they are clearly visible. The numbers of ticks on the ears are strongly correlated with the total larval tick load (as determined by keeping animals in captivity for 48 h and counting all tick larvae that drop off; Spearman r = 0.76, P < 0.001, n = 190). Nymphs, the infective stage for both B. afzelii and “Ca. Neoehrlichia mikurensis,” are comparatively rare on rodents (in our population, 8% of bank voles had one or more nymphs at a given point in time from June to August; M. Andersson and L. Råberg, unpublished data) and are therefore difficult to quantify accurately. Instead, we used the numbers of larvae on ears as a proxy for the exposure to nymphs. The number of larvae on an animal is correlated with the number of nymphs (as determined by keeping animals in captivity for 48 h and counting all ticks that drop off; Spearman r = 0.33, P < 0.001, n = 190). Each animal was also marked with a microchip (“transponder”) inserted subcutaneously on the back, which allowed us to identify individuals upon recapture. The animals were thereafter released on the capture site. The handling of animals complied with animal welfare laws and guidelines and was approved by the ethical committee for animal experiments in Malmö/Lund (M101-06 and M141-10).
Detection and quantification of “Ca. Neoehrlichia mikurensis.”
Blood samples were extracted with phenol-chloroform according to a standard protocol (18). The concentration of total DNA was measured with a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE), and the samples were diluted to a concentration of 10 ng of DNA/μl. Detection of “Ca. Neoehrlichia mikurensis” was performed with a quantitative PCR (qPCR) assay targeting the groEL gene (19) using the forward primer NeogroELQ_f (ACA GCC AAT ACT ACC TAT CCT TGA) and the reverse primer NeogroELQ_3r (ACA TGY AAT CCA CCA CGY AAC T).
Real-time PCR was performed with a Stratagene Mx3000p (Stratagene) using Platinum SYBR green qPCR SuperMix-UDG (Invitrogen). Each 25-μl reaction mixture contained 12.5 μl of SYBR green, 1 μl of each primer (10 pmol/μl), 0.1 μl of ROX reference dye (Invitrogen), and 4 μl of template (40 ng of total DNA). The PCR was initially incubated for 1 min at 50°C, followed by 2 min at 95°C. Thereafter, 43 cycles consisting of 15 s with 95°C, an annealing step with 60°C for 30 s, and then 72°C for 30 s. The final melting temperature analysis was performed at between 55 and 95°C. Samples with a CT value lower than 40 and a Tm between 77.25 and 77.80°C were considered positive for “Ca. Neoehrlichia mikurensis.” Only samples identified in two independent PCRs were scored as positive. All positive samples were put on a separate plate and run in duplicates for quantification in order to avoid variation between plates.
To generate a standard curve for quantification, we amplified a longer fragment of the groEL gene with a nested PCR assay (3) (the complete groEL gene is identical to a German isolate under accession number EU810406). The PCR product was purified with a MinElutePCR purification kit (Qiagen) and included as a serially diluted standard (in steps of 1:5) in each run. The most concentrated standard had a CT value of 22. The efficiency of the PCR was between 92.4 and 94.5%. In each run, we also included seven negative controls to check for contamination.
To compare the sensitivity of the real-time PCR method used in the present study with the nested PCR methods used in Andersson et al. (8), we analyzed 72 samples with both methods: 51 samples were negative with both methods, 8 were positive with both methods, 1 was positive with the nested PCR method but negative by real-time PCR, and 12 were positive with real-time PCR but negative with the nested PCR assay. Hence, the real-time PCR method is considerably more sensitive.
Detection and quantification of B. afzelii.
DNA from the biopsy specimens was extracted according to a protocol by Laird et al. (20) and then diluted to a concentration of about 10 ng/μl for PCR assays.
B. afzelii was detected and quantified with a qPCR assay targeting the flaB gene as previously described (21). To check for contamination, eight negative controls were included on each plate. To calibrate values between plates, a standard was prepared by amplifying a longer part of flaB (22). The obtained product was purified, diluted, and run as a serially diluted standard (in steps of 1:5) on each plate. Each sample was run in duplicates on separate plates. The repeatability of infection intensity (bacterial load) between different analyses of the same sample was 0.92, whereas the repeatability between different samples from an individual was 0.70 (21).
Statistical analyses.
Animals were divided into age categories based on their weight (23), with the following cutoffs: voles weighing <15 g were considered juveniles, voles weighing between 15 and 20 g were considered subadults, and finally voles weighing ≥20 g were considered adults. To test whether the prevalence differed between months and age classes, we performed a generalized linear mixed model with binomial error, with month and age and the interaction between them as fixed factors and individuals as the random effect. Infection intensity in single and coinfected infected animals was analyzed by general linear models with infection intensity (log transformed) of the focal pathogen as the dependent variable and infection status with the heterologous pathogen, age, and month as fixed factors.
To test whether the degree of coinfection was affected by seasonal or age-related variations in prevalence, or heterogeneity in exposure among individuals, we performed a generalized linear mixed model with infection status with B. afzelii against infection status with “Ca. Neoehrlichia mikurensis,” month, host age, tick load (log transformed), and their interactions as the fixed factors and the individual as the random effect, using proc glimmix in SAS 9.2 with binomial error and the logit link function.
RESULTS
Epidemiology.
In total, we obtained 340 samples from 261 individual bank voles. One hundred ninety-four individuals were caught once, 57 individuals were caught twice, 9 were caught three times, and a single vole was recaptured four times. Details regarding the numbers of caught and infected animals are presented in Table 1. The overall prevalences (across all samples) were 22.1% (75/340) for “Ca. Neoehrlichia mikurensis” and 29.7% (101/340) for B. afzelii. When only considering samples from the first capture of each animal (so that each animal only occurred once in the data set), the prevalences were 19.2% (50/261) for “Ca. Neoehrlichia mikurensis” and 21.5% (56/261) for B. afzelii. The overall prevalences in different months and age categories are shown in Fig. 1A for B. afzelii and in Fig. 1B for “Ca. Neoehrlichia mikurensis.” The prevalence of “Ca. Neoehrlichia mikurensis” increased during the season, from 8.7% in May to 53.8% in September [F(3, 73) = 6.25, P = 0.0008]. The prevalence also increased with age, from 0% in juveniles to 15.8% in subadults and 25.5% in adults [F(2, 73) = 3.53, P = 0.035]. The interaction between age and month was not significant (P = 0.23). The prevalence of B. afzelii infections differed between months [F(3, 73) = 10.97, P < 0.0001] and age classes [juveniles, 0%; subadults, 2.0%; adults, 42.9%; F(1, 73) = 18.54, P < 0.0001], whereas the interaction between age and month was not significant (P = 0.069). The number of tick larvae counted on the voles are shown in Fig. 2.
TABLE 1.
Numbers of caught and infected bank voles during 2008
| Vole group | No. of voles (% infected or coinfected) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First capture |
All samples |
|||||||||
| May | June | August | September | Total | May | June | August | September | Total | |
| All voles testeda | 45 (0, 0, 45) | 114 (8, 46, 60) | 95 (0, 40, 55) | 7 (0, 5, 2) | 261 (8, 91, 162) | 46 (0, 0, 46) | 137 (8, 47, 82) | 144 (0, 46, 98) | 13 (0, 8, 5) | 340 (8, 101, 231) |
| “Ca. Neoehrlichia mikurensis” positive | 4 (8.9) | 11 (9.6) | 30 (32) | 5 (71) | 50 (19) | 4 (8.7) | 18 (13) | 46 (32) | 7 (54) | 75 (22) |
| B. afzelii positive | 10 (22) | 24 (21) | 22 (23) | 0 (0) | 56 (21) | 10 (22) | 38 (28) | 51 (35) | 2 (15) | 101 (30) |
| Coinfected | 3 (6.7) | 6 (5.3) | 14 (15) | 0 (0) | 23 (8.9) | 3 (6.5) | 10 (7.3) | 28 (19) | 1 (7.7) | 42 (12) |
The numbers in parentheses indicate the numbers of juveniles, subadults, and adults, respectively.
FIG 1.
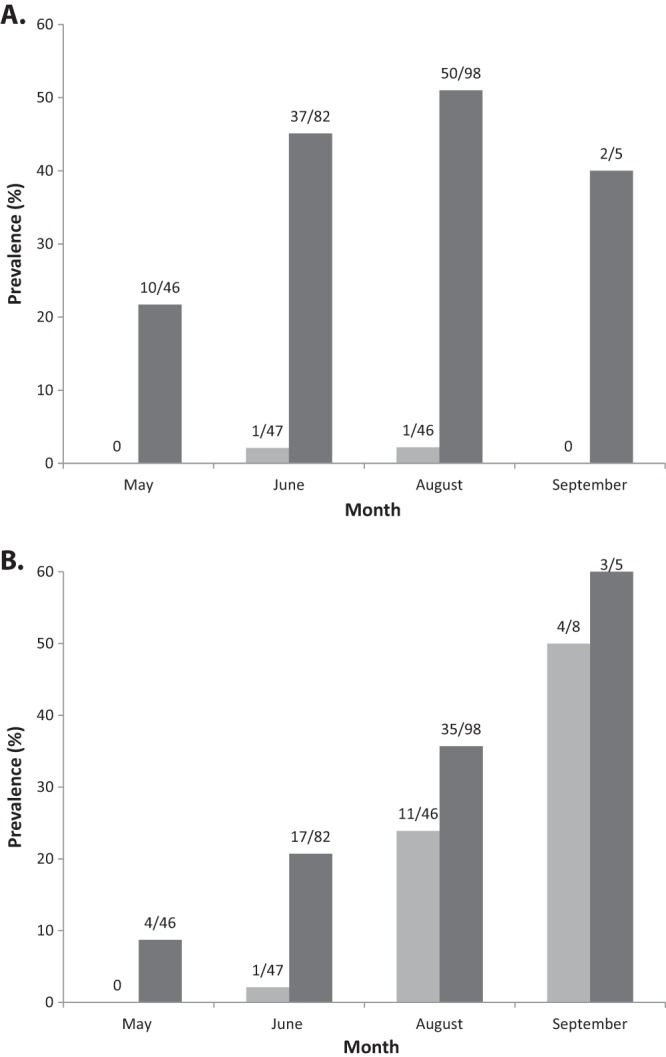
(A) Prevalence of B. afzelii in adults (n = 231; dark bars) and subadults (n = 101; light gray bars). None of the sampled juveniles (n = 8) were infected. All animals are represented in the graph. (B) Prevalence of “Ca. Neoehrlichia mikurensis” in adults (n = 231; dark bars) and subadults (n = 101; light gray bars). None of the juveniles (n = 8) were infected. All animals are represented in the graph.
FIG 2.
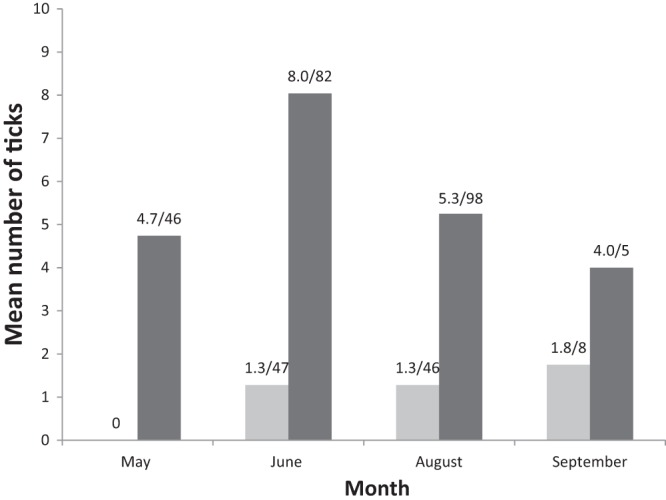
Mean number of tick larvae on voles (on ears). Light gray bars represent subadult voles, and dark bars represent adult voles. Numbers above the bars indicate mean numbers of ticks/sampled voles. All animals are represented in the graph.
Clearance rate.
To compare the infection dynamics of “Ca. Neoehrlichia mikurensis” and B. afzelii, we calculated the rate of clearance of each pathogen in individual voles (the proportion of infected bank voles that were uninfected at a later capture event). Of the eight bank voles that were positive for “Ca. Neoehrlichia mikurensis” the first time they were captured, four were negative at the second (or later) capture. In contrast, only 3 of 24 voles lost B. afzelii infection from first to second capture. The difference in clearance rate (50 versus 12.5%) was statistically significant (Fisher exact test, df = 1, P = 0.047).
Coinfections.
To test whether infections with “Ca. Neoehrlichia mikurensis” and B. afzelii were associated, we performed a χ2 test, using data from the first capture event of each vole. There were significantly more animals with both infections than expected under random co-occurrence (χ2 = 22.1, P < 0.001, Table 2). To test whether this association could be explained by variations in prevalence between age classes or months, or heterogeneity in exposure to ticks, we performed a generalized linear mixed model, including all samples, with B. afzelii infection status against “Ca. Neoehrlichia mikurensis” infection status, age, month, tick load (log transformed), and their interactions. Infection status with “Ca. Neoehrlichia mikurensis” was a significant predictor of B. afzelii infection status (F = 4.81, P = 0.0321), while controlling for month (F = 5.79 P = 0.0015), age (F = 16.30, P = 0.0002), tick load (F = 5.32, P = 0.0245), and the interaction between month and “Ca. Neoehrlichia mikurensis” infection status (F = 4.72, P = 0.005).
TABLE 2.
Numbers of individual voles (each animal occurring only once in the data set) infected with “Ca. Neoehrlichia mikurensis” and/or B. afzelii
| B. afzelii | “Ca. Neoehrlichia mikurensis” (no. of voles) |
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 23 | 33 | 56 |
| Negative | 27 | 178 | 205 |
| Total | 50 | 211 | 261 |
Intensity of infections.
Infection intensity of B. afzelii was slightly higher in animals uninfected with “Ca. Neoehrlichia mikurensis” compared to animals infected with “Ca. Neoehrlichia mikurensis” (F = 8.73, df = 1, 100, P = 0.004), while controlling for month (F = 8.69, df = 1, 100, P = 0.004). Age and interactions between fixed factors were not significant and therefore removed from the model (P ≥ 0.074).
Infection intensity of “Ca. Neoehrlichia mikurensis” differed between age classes (F = 9.90, df = 1, 74, P = 0.002) but was not affected by B. afzelii infection status (F = 1.80, df = 1, 74, P = 0.183). Month and interactions were not significant and were therefore removed from the model (P ≥ 0.667).
DISCUSSION
We compared the infection dynamics of “Ca. Neoehrlichia mikurensis” and B. afzelii and found that they differ both at the level of individual hosts and at the population level. Moreover, coinfections with “Ca. Neoehrlichia mikurensis” and B. afzelii are more frequent than expected by chance.
The prevalence of “Ca. Neoehrlichia mikurensis” in the present study of 19% is substantially higher than in a previous study of rodents (8). The most plausible explanation for this is the difference in sensitivity between the detection methods used in these studies (nested PCR, followed by Sanger sequencing versus quantitative real-time PCR). We compared the two methods and found substantially more positive samples with the real-time PCR method. The former method is also not specific for “Ca. Neoehrlichia mikurensis” since it also amplifies Bartonella spp., which are frequently occurring in rodent populations. Thus, the Bartonella sp. infections likely concealed a number of “Ca. Neoehrlichia mikurensis” infections in the earlier study. The high prevalence in bank voles indicates that they are an important reservoir host species for “Ca. Neoehrlichia mikurensis,” although actual reservoir host capacity (i.e., if they can transmit “Ca. Neoehrlichia mikurensis” to ticks) remains to be demonstrated. The prevalence of B. afzelii in the present study of 22% is well in line with a previous study in the same and adjacent areas (24), reinforcing the notion that the bank vole is an important reservoir for B. afzelii in Sweden.
B. afzelii infections displayed their highly chronic nature with only few cases of clearance of the infection in individual voles between capture events. In contrast, “Ca. Neoehrlichia mikurensis” seems to result in infections of shorter duration. This suggests that ticks might be more important for maintaining “Ca. Neoehrlichia mikurensis” over the winter than vertebrate hosts. This fits well with the quite remarkable difference in the prevalences of the two diseases over the summer months; the prevalence of “Ca. Neoehrlichia mikurensis” increased from <10% in May to almost 60% in September (although the sample size from this month was low), whereas the prevalence of B. afzelii varied less dramatically.
Juvenile animals were always uninfected by both pathogens (although the sample size for juveniles was low). The absence of infection in juveniles could possibly be explained by the lack of exposure to ticks, since juvenile animals have been less exposed to ticks than older animals. In subadults, “Ca. Neoehrlichia mikurensis” had higher prevalence than B. afzelii, This pattern is surprising because subadults should be more exposed to ticks infected with B. afzelii than to “Ca. Neoehrlichia mikurensis” since the prevalence of B. afzelii in ticks is about twice as high as that of “Ca. Neoehrlichia mikurensis” (5, 19). This suggests that the efficiency of transmission from tick to vole might be higher for “Ca. Neoehrlichia mikurensis” than for B. afzelii or that an infection with B. afzelii has a longer prepatent period and develops slower. Overall, “Ca. Neoehrlichia mikurensis” seems to have a more epidemic infection dynamic than B. afzelii, starting at a lower prevalence in early summer but then spreading more rapidly in the host population during the transmission season.
The frequency of coinfections was significantly higher than expected from random co-occurrence of B. afzelii and “Ca. Neoehrlichia mikurensis”—that is, these two pathogens had an aggregated distribution in the host population. The frequent coinfections in the present study are analogous to the situation between the related pathogens A. phagocytophilum and B. burgdorferi sensu lato, where coinfections in general are more frequent than expected solely by chance (13, 25). The aggregation could be a result of seasonal variation in prevalence, differences between age classes, or heterogeneity in exposure to infected ticks (e.g., if some vole individuals are more susceptible to ticks) (26). However, “Ca. Neoehrlichia mikurensis” and B. afzelii were still significantly associated in an analysis where we controlled for these factors, indicating that there must also be other causes of aggregation. The frequent occurrence of double infected voles could be a result of positive interactions between these pathogens. Such facilitation can, for example, be caused by immunosuppression by one pathogen that makes it easier for a second pathogen to infect an already infected host. The commonly observed association between A. phagocytophilum and B. burgdorferi is often explained by that A. phagocytophilum has negative effects on the host's immune system and thereby predisposes for Borrelia infection (12). Alternatively, the aggregation can be caused by a general sensitivity to disease in certain vole individuals that are more susceptible to diseases in general. Unfortunately, it is very difficult to disentangle the relative importance of interactions and variation in general susceptibility for aggregation of pathogens in observational data. In any case, the positive association in reservoir hosts demonstrates the potential for cotransmission of these pathogens to ticks.
Interactions between pathogens could affect not only their distribution across hosts but potentially also infection intensities (27). A study of A. phagocytophilum and B. burgdorferi showed that coinfection resulted in an increased bacterial burden in experimentally infected lab mice, which in turn resulted in higher numbers of B. burgdorferi bacteria in Ixodes scapularis ticks that fed on these mice (14), whereas in another study coinfection did not affect the acquisition of the two agents, and they were transmitted independently and with equal efficiency in coinfections and single infections (28). We did not detect any increased bacterial burden in coinfected compared to singly infected animals in the present study. In fact, voles infected by “Ca. Neoehrlichia mikurensis” had lower infection intensities of B. afzelii, suggesting that, if anything, there might be competition between these pathogens affecting the number of bacteria. This is in contrast to the high rate of coinfection, which rather indicates facilitation between these pathogens. Perhaps the dynamics of coinfections in this system are best described as facilitation during the early phase of infections, where secondary infections are more likely to be established in already infected animals, and then competition during the later phase, reducing the total bacterial load in coinfected animals.
In conclusion, our results point toward the importance of coinfections with these two pathogens, and they might be best viewed as ecologically linked. Other Anaplasmataceae occurring in reservoir hosts have been shown to greatly affect infection risk both in positive and negative manners (11). We suggest positive interactions between “Ca. Neoehrlichia mikurensis” and B. afzelii as an explanation for the observed pattern, although the immunological and/or ecological mechanism for this remains to be further explored.
ACKNOWLEDGMENTS
This study was funded by the Swedish Research Council (L.R.), the Royal Physiographic Society (M.A.), and Lunds Djurskyddsfond (M.A.).
We thank Staffan Bensch, Rawana Alkhalili, and three anonymous reviewers for comments that greatly improved the manuscript and Sandra Chiriac, Mimi Lannefors, Pär Söderquist, and Barbara Tschirren for field assistance.
Footnotes
Published ahead of print 27 December 2013
REFERENCES
- 1.Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C. 2010. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 48:1956–1959. 10.1128/JCM.02423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehr JS, Bloemberg GV, Ritter C, Hombach M, Lüscher TF, Weber R, Keller PM. 2010. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg. Infect. Dis. 16:1127–1129. 10.3201/eid1607.091907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Loewenich FD, Geissdörfer W, Disqué C, Matten J, Schett G, Sakka SG, Bogdan C. 2010. Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: evidence for a European sequence variant. J. Clin. Microbiol. 48:2630–2635. 10.1128/JCM.00588-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pekova S, Vydra J, Kabickova H, Frankova S, Haugvicova R, Mazal O, Cmejla R, Hardekopf DW, Jancuskova T, Kozak T. 2011. Candidatus Neoehrlichia mikurensis infection identified in two hemato-oncologic patients: benefit of molecular techniques for rare pathogen detection. Diagn. Microbiol. Infect. Dis. 69:266–270. 10.1016/j.diagmicrobio.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 5.Richter D, Matuschka F-R. 2012. “Candidatus Neoehrlichia mikurensis,” Anaplasma phagocytophilum, and Lyme disease spirochetes in questing European vector ticks and in feeding ticks removed from people. J. Clin. Microbiol. 50:943–947. 10.1128/JCM.05802-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lommano E, Bertaiola L, Dupasquier C, Gern L. 2012. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in western Switzerland. Appl. Environ. Microbiol. 78:4606–4612. 10.1128/AEM.07961-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte E-J, Takken W, Heyman P, Medlock J, Heylen D, Kleve J, Sprong H. 2012. Prevalence of Neoehrlichia mikurensis in ticks and rodents from northwest Europe. Parasit. Vectors 5:74. 10.1186/1756-3305-5-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson M, Råberg L. 2011. Wild rodents and novel human pathogen Candidatus Neoehrlichia mikurensis, Southern Sweden. Emerg. Infect. Dis. 17:1716–1718. 10.3201/eid1709.101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg HS. 2008. Potential effects of mixed infections in ticks on transmission dynamics of pathogens: comparative analysis of published records. Exp. Appl. Acarol. 46:29–41. 10.1007/s10493-008-9175-5 [DOI] [PubMed] [Google Scholar]
- 10.Herrmann C, Gern L, Voordouw MJ. 2013. Species co-occurrence patterns among Lyme borreliosis pathogens in the tick vector Ixodes ricinus. Appl. Environ. Microbiol. 79:7273–7280. 10.1128/AEM.02158-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330:243–246. 10.1126/science.1190333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumler J, Choi K. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11:1828–1833. 10.3201/eid1112.050898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto NC, Foley JE. 2009. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife, and Ixodes ricinus complex ticks. Vector-Borne Zoonotic Dis. 9:93–102. 10.1089/vbz.2008.0072 [DOI] [PubMed] [Google Scholar]
- 14.Thomas V, Anguita J, Barthold SW. 2001. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect. Immun. 69:3359–3371. 10.1128/IAI.69.5.3359-3371.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden K, Hodzic E, Feng S, Freet KJ, Lefebvre RB, Barthold SW. 2005. Coinfection with Anaplasma phagocytophilum alters Borrelia burgdorferi population distribution in C3H /HeN mice. Infect. Immun. 73:3440–3444. 10.1128/IAI.73.6.3440-3444.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piesman J. 1991. Experimental acquisition of the Lyme disease spirochete, Borrelia burgdorferi, by larval Ixodes dammini (Acari, Ixodidae) during partial blood meals. J. Med. Entomol. 28:259–262 [DOI] [PubMed] [Google Scholar]
- 17.Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145–2165. 10.1099/00207713-51-6-2145 [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 19.Andersson M, Bartkova S, Lindestad O, Råberg L. 2013. Co-infection with “Candidatus Neoehrlichia mikurensis” and Borrelia afzelii in Ixodes ricinus ticks in Southern Sweden. Vector-Borne Zoonotic Dis. 13:438–442. 10.1089/vbz.2012.1118 [DOI] [PubMed] [Google Scholar]
- 20.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. 1991. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 19:4293. 10.1093/nar/19.15.4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Råberg L. 2012. Infection intensity and infectivity of the tick-borne pathogen Borrelia afzelii. J. Evol. Biol. 25:1448–1453. 10.1111/j.1420-9101.2012.02515.x [DOI] [PubMed] [Google Scholar]
- 22.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403–409. 10.1093/infdis/173.2.403 [DOI] [PubMed] [Google Scholar]
- 23.Gliwicz J. 1988. Seasonal dispersal in noncyclic populations of Clethrionomys glareolus and Apodemus flavicollis. Acta Theriol. 33:263–272 [Google Scholar]
- 24.Hellgren O, Andersson M, Råberg L. 2011. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J. Evol. Biol. 24:159–167. 10.1111/j.1420-9101.2010.02148.x [DOI] [PubMed] [Google Scholar]
- 25.Belongia EA. 2002. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector-Borne Zoonotic Dis. 2:265–273. 10.1089/153036602321653851 [DOI] [PubMed] [Google Scholar]
- 26.Lord CC, Barnard B, Day K, Hargrove JW, McNamara JJ, Paul REL, Trenholme K, Woolhouse MEJ. 1999. Aggregation and distribution of strains in microparasites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:799–807. 10.1098/rstb.1999.0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lello J, Boag B, Fenton A, Stevenson I, Hudson P. 2004. Competition and mutualism among the gut helminths of a mammalian host. Nature 428:20–24. 10.1038/428020a [DOI] [PubMed] [Google Scholar]
- 28.Levin ML, Fish D. 2000. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect. Immun. 68:2183–2186. 10.1128/IAI.68.4.2183-2186.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]


